Introduction
Glucose is the main physiological stimulator of
insulin release, and the mechanism by which glucose regulates
insulin secretion has been investigated for several decades. It is
known that the regulation of insulin secretion is mediated by
signals involved in glucose metabolism. Glucose enters β-cells via
the glucose transporter 2 (GLUT2) and it is metabolized via the
glycolytic pathway, whose downstream signaling pathway induces
insulin secretion (1). A recent
study identified that glucose-sensing receptors in β-cells are
involved in regulating the effect of glucose on insulin secretion
(2). The sweet taste receptors
(STRs) are heterodimers formed by two members of the class C G
protein-coupled receptor family, taste 1 receptor member (T1R) 2
and T1R3 (3). STRs have been
identified to function as a glucose-sensing receptor in the taste
cells of the tongue (3). When STRs
are activated by sugar molecules, the signal is transduced to a
taste-cell-specific trimeric G protein, gustducin, and the
subsequent activation of phospholipase C-β2 (PLCβ2) leads to the
inositol 1,4,5-trisphosphate-mediated release of Ca2+
from intracellular stores (4,5). The
increase in intracellular Ca2+ activates the monovalent
cation-specific transient receptor potential cation channel
subfamily M member 5 (TRPM5), leading to Na+ influx and
depolarization of the cell (4,5). In
addition to the taste cells of the tongue, STR subunits are also
found in mouse and human β-cells (6,7). It
was demonstrated that STRs are associated with the PLCβ2/TRPM5
cascade in β-cells (6). However,
compared with T1R3, the expression level of T1R2 is lower in islets
and in the pancreatic β-cell line MIN6 (7). Knockdown of T1R3 attenuates the
effect of the sweetener acesulfame K, whereas knockdown of T1R2
does not affect the action of acesulfame K (8). T1R3 knockdown in MIN6 cells also
decreases the amount of intracellular ATP and the insulin secretion
following stimulation with 25 mM glucose (9). Therefore, these previous findings
indicated that T1R3 may be important for glucose sensing and for
the regulation of insulin secretion induced by high concentrations
of glucose.
3-Deoxyglucosone (3DG), a highly reactive
α-dicarbonyl compound, is formed from carbohydrates during food
processing and storage, and it is also produced by living organisms
(10,11). The clinical significance of 3DG is
associated with its ability to react with certain proteins to form
advanced glycation end products that are involved in the
pathogenesis of diabetic complications (12). Accumulating evidence demonstrated
that the plasma levels of 3DG in diabetic patients are increased by
~2 folds compared with healthy subjects (13,14).
Collectively, these previous studies indicate the role of 3DG in
the pathogenesis of diabetic complications. Our previous animal and
clinical studies have demonstrated that 3DG is an independent
factor associated with the development of prediabetes (15–19).
Additionally, our previous studies showed that an abnormal increase
of 3DG leads to the impairment of pancreatic β-cell function
(15,18). In addition, increased plasma levels
of 3DG after glucose stimulation was observed in normal subjects
and patients with impaired glucose metabolism (20). In our previous study,
pathologically relevant plasma levels of 3DG were induced in normal
rats with a single intravenous (i.v.) injection of 50 mg/kg 3DG,
and an acute rise in the circulating levels of 3DG induced glucose
intolerance, thus impairing the function of pancreatic β-cells
(19), suggesting the potential
involvement of 3DG in the increased risk of glucose intolerance in
patients with postprandial glucose excursions. However, the role of
STRs on the deleterious effects of pathologically relevant plasma
levels of 3DG on β-cells function at high physiological glucose
levels remains unknown.
A previous study identified that the expression of
the STR subunit T1R3, α-gustducin and TRPM5 are downregulated in
the ileum of Zucker diabetic fa/fa rats (21). In line with this previous study,
our previous study identified that the protein expression levels of
STR and its downstream factor TRPM5 are downregulated in duodenal
and colon tissues following a 2-week administration of 50 mg/kg 3DG
intragastric (i.g.) in rats exhibiting a prediabetic condition
(16). Additionally, in the
pancreatic islets of diabetic and diet-induced obese mice, the
protein expression levels of the STR subunits were downregulated
(22,23). Furthermore, in vitro
experiments suggested that the downregulation of the expression
level of STR genes is involved in the impairment of the
STR-mediated insulin secretion by β-cells (22,23).
Our previous study identified that acute exposure of STC-1 L-cells
to 3DG in the presence of 25 mM glucose for 1 h was able to
downregulate the expression level of STR, decreasing STR-mediated
zinc finger GATA like protein 1 (GLP-1) secretion (24). Therefore, 3DG may exhibit
deleterious effects on the STR signaling pathways. Altogether, it
is possible that the deleterious effects on glucose tolerance
caused by an acute increase in circulating 3DG may be associated
with the impairment of β-cell function mediated by the
downregulation of the STR signaling pathway in β-cells.
Accumulating evidence has demonstrated that
stimulation of insulin secretion by high concentrations of glucose
involves STRs (2). Therefore, the
present study aimed to investigate the in vivo and in
vitro effects of pathologically relevant plasma levels of 3DG
on insulin secretion and STR signaling in β-cells at high
physiological glucose levels. After acute exposure of INS-1 cells
to 3DG (1.85, 30.84 and 61.68 mM) in the presence of 25.5 mM
glucose for 1 h, insulin secretion and the expression levels of
components of the STR signaling pathways were examined. Insulin
secretion at glucose concentrations of 5.6 and 25.5 mM and the
expression levels of components of the STR signaling pathways were
also examined in pancreatic islets collected from rats after a
single i.v. injection of 50 mg/kg 3DG + 0.5 g/kg glucose.
Materials and methods
Reagents
3DG was synthesized following a modified method
reported by Kato et al (25), as previously described (26). Lactisole purchased from
Sigma-Aldrich (Merck KGaA) was dissolved in DMSO, and the final
concentration of DMSO was adjusted to 0.05% for all conditions
tested.
Animals and cell culture
Male Sprague-Dawley rats (age, 11 weeks; weight ~200
g) purchased from the JOINN Laboratories were housed at 20–23°C
with a relative humidity of 50–60%) and under a 12-h light/dark
cycle, in accordance with the European Union Directive 2010/63/EU
for animal experiments (27). All
the animal protocols were approved by The Local Committee on Ethics
of Animal Experiments of Suzhou TCM Hospital Affiliated to Nanjing
University of Chinese Medicine (Suzhou, China). The rats were
allowed free access to food and water, and were fed a standard rat
diet (Shuangshi Laboratory Animal Feed Science Co., Ltd.). After 1
week of acclimatization, eighteen rats were randomly divided into
the experimental groups (n=6 in each group). The rats were fasted
overnight before the experiments. A single i.v. injection of 0.9%
saline (control), 0.5 g/kg glucose or 50 mg/kg 3DG + 0.5 g/kg
glucose was administered to the rats. The insulin-secreting INS-1
rat β-cells were obtained from Soochow University (Suzhou, China)
and were cultured as previously described (18). INS-1 cells were cultured in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) containing 11.2
mM glucose, 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 50
µM β-mercaptoethanol (Sigma-Aldrich; Merck KGaA) in a humidified
incubator with 5% CO2 at 37°C.
Rat islet isolation and culture
After overnight fasting, rats were anesthetized by
intraperitoneal injection of 100 mg/kg pentobarbital sodium
(Sigma-Aldrich; Merck KGaA) and were administered a single i.v.
injection of 0.5 g/kg glucose or 50 mg/kg 3DG + 0.5 g/kg glucose.
After 2 h, the anesthetized rats were sacrificed by CO2
exposure (displacement rate, 20%/min). If no breath was observed
for ≥60 sec, the sacrificed rodent was removed from the chamber and
pancreatic islets were isolated using the collagenase method, as
previously described (19). The
pancreas was removed and digested by incubation in DMEM
(Sigma-Aldrich; Merck KGaA) supplemented with collagenase (1.5
mg/ml). The digested pancreas was filtered and centrifuged at 1,000
× g at 4°C for 3 min. The isolated islets were cultured in
RPMI-1640 culture medium containing 11.2 mM glucose and 10% fetal
calf serum (Gibco; Thermo Fisher Scientific, Inc.) in a humidified
incubator with 5% CO2 at 37°C. The islets were
subsequently used for the measurement of insulin secretion and
western blot analysis.
Measurement of insulin concentration
in INS-1 cells and rat islets
INS-1 cells (4×103 cells/well) were
seeded in 96-well plates in RPMI-1640 culture medium containing
11.2 mM glucose and cultured overnight. The cells were incubated in
RPMI-1640 containing 25.5 mM glucose for 1 h in the presence or
absence of 3DG (1.85, 30.84 and 61.68 mM) or lactisole (5 mM), and
the supernatant from the medium was collected for the measurement
of insulin concentration. Islets were harvested from fasting rats 2
h after a single i.v. injection of 0.5 g/kg glucose or 50 mg/kg 3DG
+ 0.5 g/kg glucose. In total, 30 islets were isolated from
3DG-treated rats and were immediately cultured in RMPI-1640
containing 5.6 mM glucose for 1.5 h. Subsequently, insulin
secretion under different conditions were assessed.
Following the indicated treatment, INS-1 cells or
islets from 3DG-treated rats were transferred to RMPI-1640
containing 5.6 mM glucose for 1.5 h. For basal insulin secretion
measurement, INS-1 cells or 30 islets were incubated with 5.6 mM
glucose in RPMI-1640 for 1.5 h. For glucose-stimulated insulin
secretion measurement, INS-1 cells or islets were incubated with
25.5 mM glucose in RPMI-1640 for 1 h. The insulin concentration in
the supernatant was measured using a rat insulin ELISA kit (cat.
no. EZRMI-13K; Linco Research, Inc.; EMD Millipore) according to
the manufacturer's protocol.
Western blot analysis
INS-1 cells grown to confluence in 6-well dishes
were incubated with various concentrations of 3DG (1.85, 30.84 and
61.68 mM) in RPMI-1640 culture medium containing 25.5 mM glucose
for 1 h. The cells were subsequently transferred to RPMI-1640
culture medium containing 5.6 mM glucose for 1.5 h. Then, 2 h after
a single i.v. injection of vehicle (0.9% saline, control group),
0.5 g/kg glucose or 50 mg/kg 3DG + 0.5 g/kg glucose, the pancreatic
islets were isolated from rats. In total, 100 islets were then
transferred to RPMI-1640 culture medium containing 5.6 mM glucose
for 1.5 h. INS-1 cells and islets were homogenized in a lysis
buffer containing 1% Triton X-100, protease inhibitors and
phosphatase inhibitors (Beyotime Institute of Biotechnology).
Western blot analysis was performed using INS-1 cells and isolated
pancreatic islets as previously described (18,19).
The total protein concentration was determined using a
bicinchoninic acid protein assay kit (cat. no. P0012; Beyotime
Institute of Biotechnology). In total, 50 µg protein from each
sample was separated by SDS-PAGE. Proteins were transferred onto
PVDF membranes (Merck KGaA). The membranes were blocked for 1 h at
room temperature in Tris-buffered saline with 1% Tween (Beijing
Solarbio Science & Technology Co., Ltd.) containing 5% dry
milk. The membranes were incubated with the appropriate primary
antibody at 4°C overnight. Subsequently, membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit (1:5,000;
cat. no. AT0097; CMCTAG, Inc.), goat anti-mouse (1:1,500; cat. no.
AT0098; CMCTAG, Inc.) or rabbit anti-goat (1:1,500; cat. no.
AT0101; CMCTAG, Inc.) secondary antibodies at room temperature for
2 h. The blots were detected with an enhanced chemiluminescence-kit
(Beyotime Institute of Biotechnology) followed by autoradiography.
The amount of protein loaded was confirmed by stripping the blots
using restore western blot stripping buffer (cat. no. 21509; Thermo
Scientific, Inc.) and reprobing them using an anti-β-actin antibody
(dilution, 1:1,000; cat. no. AT0001; CMCTAG, Inc.). The primary
antibodies used for western blot were as follows: Anti-T1R3 (1:200;
cat. no. sc-22458; Santa Cruz Biotechnology, Inc.), anti-TRPM5
(1:167; cat. no. ab87642; Abcam) and anti-GLUT2 (1:100; cat. no.
ab54460; Abcam). The intensity of the bands was analyzed using the
SpotDenso tool of the built-in software of the detection instrument
(Alphaimager 2200; ProteinSimple).
Statistical analysis
All presented data are representative of ≥3
independent experiments. Data are presented as the mean ± SD.
Comparisons were performed using Student's t-test or one-way ANOVA
followed by Bonferroni's post-hoc test. Statistical analyses were
performed using Prism (version 5; GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Treatment with the STR inhibitor
lactisole impairs insulin secretion in INS-1 cells
The role of STRs on insulin secretion in response to
high concentrations of glucose in INS-1 β-cells was examined
(Fig. 1). INS-1 cells were treated
with the STR inhibitor lactisole at a concentration of 5 mM in the
presence of 25.5 mM glucose for 1 h, and the concentration of
insulin in the supernatant was subsequently measured. Insulin
secretion in response to 25.5 mM glucose stimulation was reduced to
81% in the presence of lactisole (Fig.
1A). Subsequently, INS-1 cells treated with lactisole were
incubated at different concentrations of glucose to examine basal
and glucose-stimulated insulin secretions. INS-1 cells pretreated
with lactisole and 25.5 mM glucose showed an increased basal
insulin secretion in response to 5.6 mM glucose, whereas
glucose-stimulated insulin secretion was significantly lower in
INS-1 exposed to 25.5 mM glucose, and insulin secretion was reduced
to 83% (Fig. 1B). The present
findings suggested that the pharmacologic inhibition of STRs in
INS-1 rat β-cells impaired insulin secretion in response to 25.5 mM
glucose stimulation.
Acute exposure of INS-1 cells to 3DG
and 25.5 mM glucose for 1 h impairs insulin secretion
Following the same experimental conditions used for
the lactisole treatment, INS-1 cells were exposed to pathologically
relevant concentrations of 3DG at high concentrations of glucose,
and insulin secretion was subsequently examined. INS-1 cells were
treated with various concentrations of 3DG (1.85, 30.84 and 61.68
mM) at 25.5 mM glucose for 1 h and the insulin concentration in the
supernatant was measured. Following treatment with 1.85, 30.84, and
61.68 mM 3DG, the insulin secretion in response to 25.5 mM glucose
stimulation was reduced to 89, 83 and 84%, respectively (Fig. 2A). Subsequently, INS-1 cells were
pretreated with 3DG for 1 h, and insulin secretion was measured
following exposure to 5.6 mM glucose for 1.5 h or 25.5 mM glucose
for 1 h. Exposure to 30.84 and 61.68 mM 3DG increased basal insulin
secretion in response to 5.6 mM glucose by 22 and 26%, respectively
(Fig. 2B). Following treatment
with 30.84 and 61.68 mM 3DG, glucose-stimulated insulin secretion
in response to 25.5 mM glucose was reduced to 71 and 73%,
respectively (Fig. 2B). The
present results were consistent with the aforementioned lactisole
treatment results (Fig. 1).
Collectively, the present results suggested that acute exposure of
INS-1 cells to pathologically relevant concentrations of 3DG at
high concentrations of glucose impaired insulin secretion from
β-cells in vitro.
Acute exposure of INS-1 cells to 3DG
at 25.5 mM glucose for 1 h decreases the activity of the STR
signaling pathway
The effects of pathologically relevant plasma levels
of 3DG for 1 h at 25.5 mM glucose on the protein expression levels
of members of the STR pathway were investigated in INS-1 cells.
INS-1 cells were exposed to various concentration of 3DG (1.85,
30.84 and 61.68 mM) in the presence of 25.5 mM glucose for 1 h, and
the protein expression levels of T1R3 were measured after a 1.5-h
incubation with 5.6 mM glucose. Treatment with 3DG at various
concentrations significantly reduced the protein expression levels
of T1R3, TRPM5 and GLUT2 (Fig. 3).
The present results suggested that acute exposure of INS-1 cells to
pathologically relevant concentrations of 3DG for 1 h at 25.5 mM
glucose was sufficient to reduce the protein expression levels of
members of the STR signaling pathway.
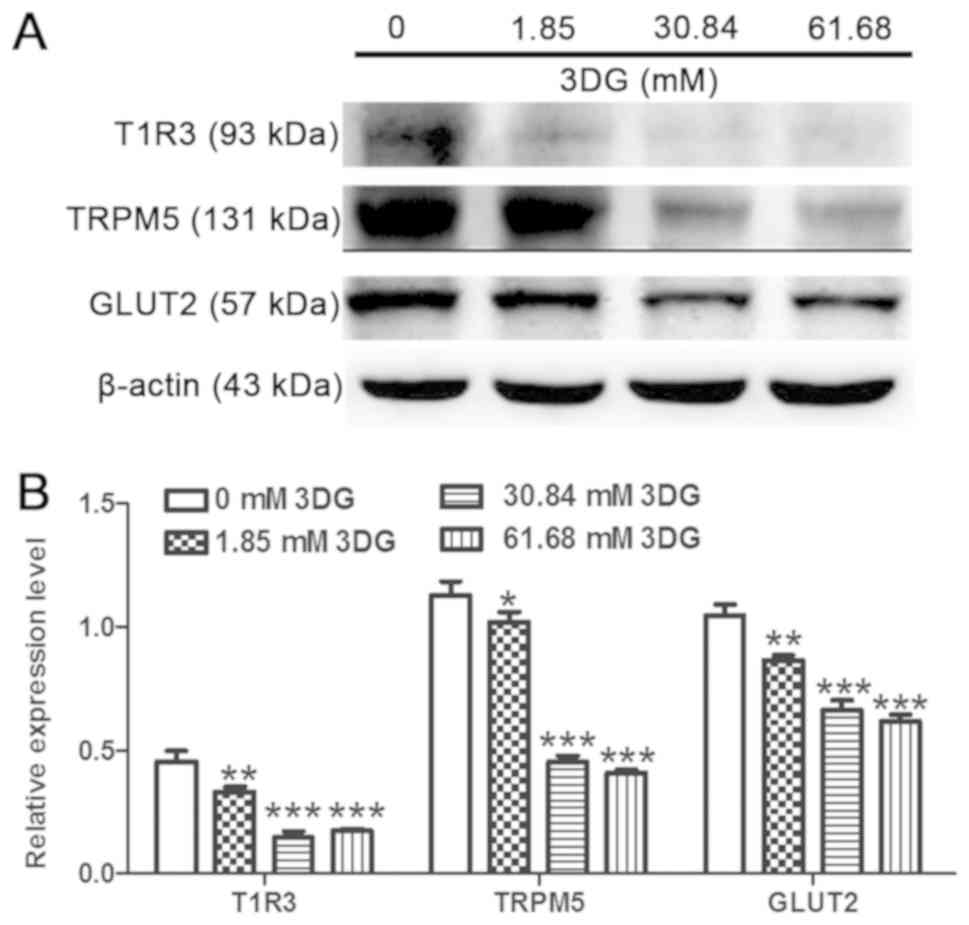 | Figure 3.Acute exposure of INS-1 cells to 3DG
at 25.5 mM glucose for 1 h downregulates the sweet taste receptor
signaling pathway. INS-1 cells were incubated for 1 h with 25.5 mM
glucose in the absence or presence of 3DG at 1.85, 30.84 or 61.68
mM. (A) Cells were incubated with 5.6 mM glucose for 1.5 h and the
protein expression levels of T1R3, TRPM5 and GLUT2 were measured.
(B) Protein expression levels of T1R3, TRPM5 and GLUT2, as assessed
by densitometry. *P<0.05, **P<0.01, ***P<0.001 vs. 0 mM
3DG. n=3. 3DG, 3-deoxyglucosone; T1R3, taste 1 receptor member 3;
TRPM5, transient receptor potential cation channel subfamily M
member 5; GLUT2, glucose transporter 2. |
Impairment of insulin secretion and
downregulation of the STR signaling pathway are induced in islets
exhibiting glucose intolerance induced by acute exposure to
3DG
The effects of glucose intolerance induced by acute
exposure to 3DG on insulin secretion and on the expression levels
of members of the STR signaling pathway were investigated. Islets
from fasting rats were collected 2 h after i.v. administration of
50 mg/kg 3DG + 0.5 g/kg glucose. Subsequently, islets were
incubated with 5.6 mM glucose for 1.5 h, and insulin secretion in
response to 5.6 mM glucose for 1.5 or 25.5 mM glucose for 1 h was
assessed. Islets isolated from rats treated with 3DG exhibited a
21% increase in basal insulin secretion, at 5.6 mM glucose
(Fig. 4A). By contrast,
glucose-stimulated insulin secretion (25.5 mM glucose) decreased to
90%. The protein expression levels of the members of the STR
signaling pathways in islets isolated from 3DG-treated rats were
subsequently measured. The protein expression levels of T1R3 and
TRPM5 in islets collected from rats treated with 0.5 g/kg glucose
were significantly reduced compared with the control groups,
whereas the protein expression level of GLUT2 was significantly
increased (Fig. 4B and C). The
protein expression levels of T1R3, TRPM5 and GLUT2 in islets
collected from rats treated with 50 mg/kg 3DG + 0.5 g/kg glucose
were significantly reduced compared with rats treated with 0.5 g/kg
glucose and 0.9% saline. The present results suggested that insulin
secretion at 25.5 mM glucose stimulation was impaired, and the
protein expression levels of members of the STR signaling pathway
were downregulated in islets of rats exhibiting acute glucose
intolerance induced by acute exposure to 3DG, consistently with our
aforementioned in vitro results (Figs. 2 and 3).
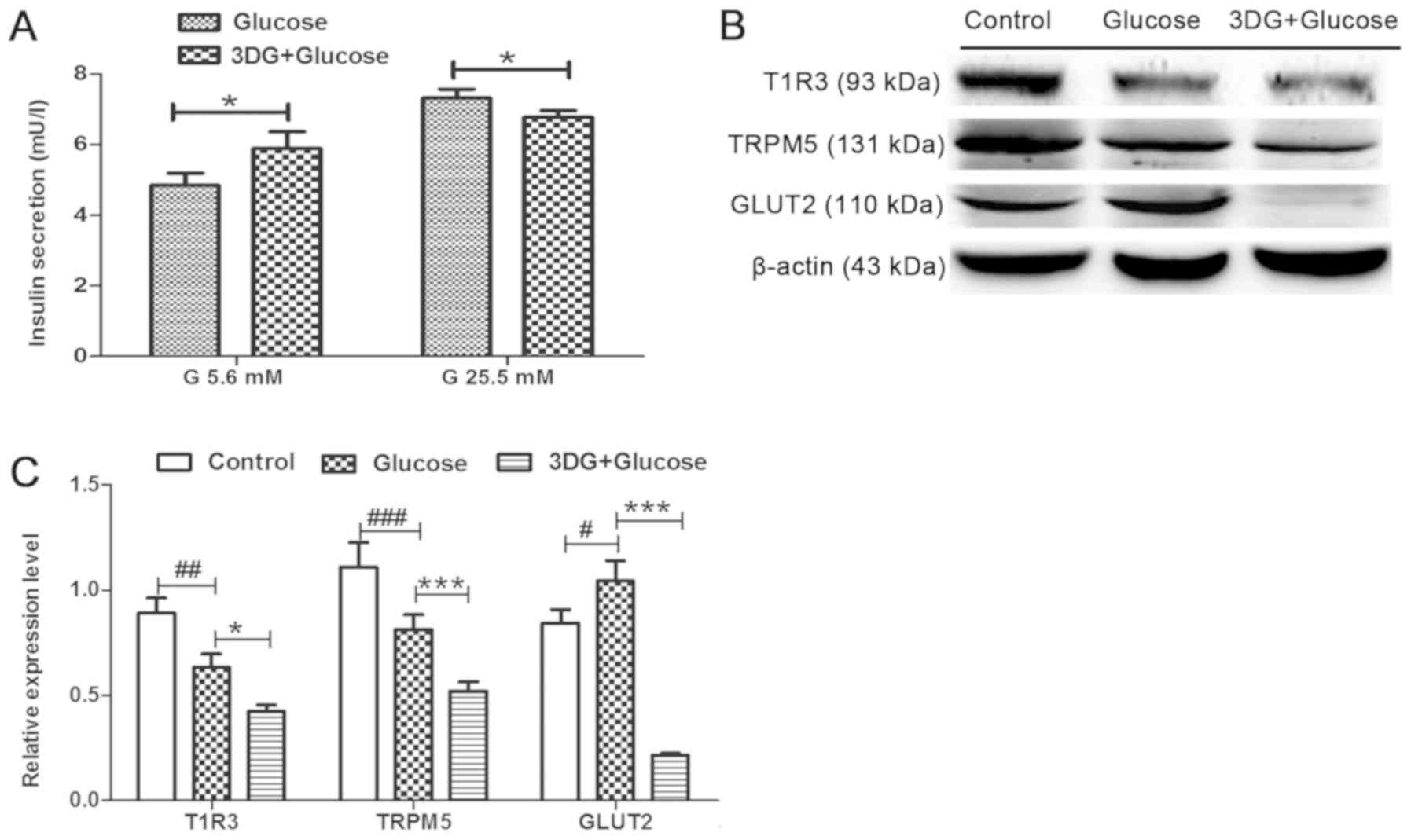 | Figure 4.Impairment of insulin secretion and
downregulation of the sweet taste receptor signaling pathway in
islets of rats with acute glucose intolerance induced by 3DG. After
overnight fasting, rats were treated with physiological saline, 0.5
g/kg glucose i.v. or 50 mg/kg 3DG + 0.5 g/kg glucose i.v. (A)
Pancreatic islets were collected from rats 2 h after treatment and
were incubated in RPMI-1640 containing 11.2 mM glucose. Next,
islets were incubated with 5.6 mM glucose for 1.5 h or with 25.5 mM
glucose for 1 h. Then, the insulin concentration in the supernatant
was measured. n=6. (B) Islets were isolated 2 h after treatment
with physiological saline, 0.5 g/kg glucose i.v. or 50 mg/kg 3DG +
0.5 g/kg glucose i.v. and processed for the analysis of the protein
expression levels of T1R3, TRPM5 and GLUT2 by western blotting. (C)
Protein expression levels of T1R3, TRPM5 and GLUT2, as assessed by
densitometry. *P<0.05, ***P<0.001 as indicated.
#P<0.05, ##P<0.01,
###P<0.001 as indicated (n=3). G, gluocse; i.v.,
intravenously; 3DG, 3-deoxyglucosone; T1R3, taste 1 receptor member
3; TRPM5, transient receptor potential cation channel subfamily M
member 5; GLUT2, glucose transporter 2. |
Discussion
In the present study, the inhibition of the STRs
pathway with lactisole in INS-1 rat β-cells was identified to
decrease insulin secretion following stimulation with a high
concentration of glucose (25.5 mM). Furthermore, the present
results suggested that acute exposure of INS-1 cells to
pathologically relevant concentrations of 3DG at 25.5 mM glucose
inhibited insulin secretion. Accordingly, islets from rats
collected 2 h after treatment with 50 mg/kg 3DG + 0.5 g/kg glucose
i.v. exhibited a reduction in insulin secretion after stimulation
with 25.5 mM glucose. Importantly, acute exposure of INS-1 cells to
3DG at 25.5 mM glucose for 1 h decreased the protein expression
levels T1R3, a subunit of STRs, and TRPM5 and GLUT2, two factors
downstream of the STRs signaling pathway. Notably, islet tissues
collected from rats treated with 3DG showed a similar
downregulation. The present results suggested that acute exposure
to pathologically relevant concentrations of 3DG inhibited insulin
secretion from β-cells in the presence of 25.5 mM glucose by, at
least in part, downregulating the STR signaling pathway.
A previous study demonstrated that the plasma levels
of 3DG in non-diabetic elderly subjects vary between 0.049 and 4.54
mM with a median value of 0.27 mM and a 95th percentile
of 2.79 mM (15). A previous study
observed that plasma-free 3DG levels in streptozotcin-induced
diabetic rats was increased by ~2-fold (0.92 ± 0.13 mM) compared
with normal rats (0.38±0.069 mM) (28). Our previous study, to the best of
our knowledge, was the first to demonstrate that the plasma levels
of 3DG increased ~2-fold (0.47–0.73 mM) following administration of
50 kg/kg 3DG i.g. for 2 weeks in mice compared with normal mice
(0.22±0.083 mM), and increased circulating levels of 3DG were
identified to be involved in the development of prediabetes
(18). Additionally, our previous
study identified that pathologically relevant plasma levels of 3DG
in rats were induced with a single i.v. injection of 3DG (50 mg/kg)
(19). The plasma levels of 3DG
peaked at 15 min after injection (102.86±19.48 mM) and declined to
11.34±3.6 mM after 1 h. However, the 3DG levels were maintained at
significantly increased levels for ≥2 h (8.7±0.6 mM) (19). INS-1 cells were derived from
X-ray-induced rat insulinoma cells, which were previously used as
an in vitro model of β-cells due to their insulin secretion
capacity following stimulation with glucose (29). The present results suggested that
insulin secretion of INS-1 cells at 25.5 mM glucose stimulation
increased ~1.6-fold compared with basal glucose stimulation (5.6
mM), consistently with previous studies (30,31).
Therefore, insulin secretion and the expression of component of the
STR pathway were investigated in INS-1 β-cells under pathological
levels of 3DG (30.84 and 61.68 mM) at 25.5 mM glucose.
Additionally, the expression levels of components of the STR
pathway were previously identified to be downregulated, and
STR-mediated glucagon-like peptide 1 secretion was identified to be
reduced in STC-1 L-cells in response to acute exposure to 1.85 mM
3DG for 1 h at 25 mM glucose (24). Therefore, in the present study, the
effects of 1.85 mM 3DG at 25.5 mM glucose on insulin secretion and
on the expression levels of members of the STR pathway were
examined in INS-1 cells.
Previous studies demonstrated that, following
inhibition of the STR signaling pathway, pancreatic β-cells
stimulated by high concentrations of glucose exhibit a reduction of
insulin secretion (7,9). Lactisole is a synthetic compound that
is used to evaluate the function and physiological roles of the STR
pathway in human (32). Previous
studies observed that lactisole may be used for investigating the
role of STRs in rodents (32,33).
The present study suggested that inhibition of STRs with lactisole
significantly suppressed insulin secretion by INS-1 β-cells
following stimulation with 25.5 mM glucose. In fact, after 1 h of
treatment with lactisole, insulin secretion stimulated by 25.5 mM
glucose was decreased compared with the control group. The present
results are consistent with a previous study investigating the
effect of lactisole in reducing insulin secretion from MIN6 β-cells
stimulated by 25 mM glucose (32).
Interestingly, the STRs expressed in β-cells are involved in
inhibiting the basal insulin secretion (23). Pharmacological inhibition of STRs
with lactisole in human islets induces basal insulin hypersecretion
at short-term fasting glucose concentrations (23). Consistently with this previous
study (23), the present study
identified that basal insulin secretion at 5.6 mM glucose increased
in INS-1 rat β-cells after 1 h of treatment with the STR inhibitor
lactisole. The present results suggested that lactisole may be a
useful pharmacological tool to assess the function of STRs in INS-1
cells. The present results suggested that under basal conditions,
the STRs on the surface of β-cells may be able to sense the level
of glucose, leading to a decrease in the basal insulin secretion,
preventing hypoglycemia. Under stimulated conditions, the STRs on
the surface of β-cells are activated by the increase in circulating
glucose, and mediate insulin secretion, restoring physiological
plasma glucose levels (23).
Although the molecular mechanisms underlying STR-mediated
inhibition of basal insulin secretion are not fully understood, it
is possible that basal insulin hypersecretion is a typical
characteristic of the function of STRs in β-cells.
In the present study, INS-1 cells were treated with
3DG following the same experimental conditions used to investigate
the role of lactisole, and acute exposure to 3DG in INS-1 cells or
in islets isolated from rats treated with 50 mg/kg 3DG + 0.5 g/kg
glucose i.v. not only resulted in a reduction in insulin secretion
following stimulation with 25.5 mM glucose, but also induced basal
insulin hypersecretion at 5.6 mM glucose, consistently with the
treatment with the STR inhibitor lactisole. Therefore, the present
results suggested that acute exposure to pathologically relevant
plasma levels of 3DG impaired the STR-mediated regulation of
insulin secretion in pancreatic β-cells. The present results are
consistent with the effects that were previously reported for
methylglyoxal, a highly reactive α-dicarbonyl compound (34). Elmhiri et al (34) hypothesized that the acute effect of
methylglyoxal on insulin secretion may be related to the metabolic
state of β-cells. STRs expressed in β-cells serve distinct roles in
finely tuning insulin secretion under basal and glucose-stimulated
conditions. Therefore, it is possible that STRs may be key
molecules involved in methylglyoxal regulation of insulin
secretion. Accordingly, in the present study, acute exposure to 3DG
showed deleterious effects on the STR-mediated regulation of
insulin secretion in pancreatic β-cells.
The present results suggested that 3DG exhibited
deleterious effects on STR-mediated insulin secretion. In fact,
acute exposure to pathologically relevant concentration of 3DG in
the presence of 25.5 mM glucose downregulated the protein
expression levels of the STR subunit T1R3 and of other components
of the canonical STR signaling pathway (35), such as TRPM5 and GLUT2.
Collectively, acute exposure to pathologically relevant plasma
levels of 3DG at 25.5 mM glucose downregulated the protein
expression level of T1R3, impairing the effects of STRs on the
regulation of insulin secretion by pancreatic β-cells. The present
results are not sufficient to understand the mechanism underlying
the 3DG-mediated T1R3 downregulation; however, the mechanisms
underlying 3DG function may involve several processes. 3DG was
previously reported to react with the N-terminal amino group of
nucleic acids (36), leading to
inhibition of transcription and causing a decrease in gene
expression. Carbonylation of proteins is a permanent modification
that may alter the function or structure of proteins, affecting
their downstream signaling pathway (37). The accumulation of the dicarbonyl
compound 3DG was previously reported to induce carbonyl stress,
increasing the carbonylation of proteins (38). The ability of 3DG to downregulate
the protein expression level of T1R3 may be mediated by the
carbonyl stress caused by 3DG. Therefore, further studies are
required to investigate the mechanism underlying the 3DG-mediated
downregulation of the protein expression level of T1R3 following
exposure to high concentrations of glucose.
Since STR-mediated regulation of insulin secretion
was identified in the present study to be impaired in islets
collected from rats treated with 3DG, the expression levels of
members of the STR signaling pathway were investigated. At 2 h
after administration of 0.5 g/kg glucose i.v., the plasma levels of
glucose declined to levels only slightly higher than the basal
glucose levels (19). GLUT2 is a
glucose-sensitive gene (39), and
GLUT2 expression level in islet tissues from rats 2 h after acute
administration of 0.5 g/kg glucose i.v. increased compared with
islets from fasting rats. Interestingly, the protein expression
levels of T1R3 and TRPM5 in islet tissues harvested 2 h after acute
administration of 0.5 g/kg glucose i.v. were markedly decreased
compared with fasting rats. Possibly, under high glucose levels,
the STR-mediated regulation of basal insulin secretion may not be
immediately detected. Furthermore, the upregulation of T1R3
expression in the islets of fasting rats suggested a role of STRs
in sensing circulating glucose in modulating basal insulin
secretion. Therefore, the downregulated expression levels of T1R3,
TRPM5 and GLUT2 in islet tissues collected from rats 2 h after
acute administration of 50 mg/kg 3DG + 0.5 g/kg glucose i.v. could
be responsible for the impaired STR-mediated insulin secretion in
islets from 3DG-treated rats. Additionally, since GLUT2 expression
can be regulated by insulin (40),
the downregulation of GLUT2 expression may be consistent with our
previous results suggesting that 50 mg/kg 3DG i.v. administered to
rats impaired the insulin signaling pathway (19). GLUT2 is one of the main factors
required for glucose-dependent insulin secretion 4(41). Therefore,
the reduced insulin secretion at 25.5 mM glucose following
treatment with 3DG identified in the present study in INS-1 cells
and islets could be caused, at least in part, by a decrease in
GLUT2 expression. Collectively, the impairment in insulin secretion
and in the STR signaling pathway suggested that elevated
circulating levels of 3DG may be involved in the development of
β-cell dysfunction.
To the best of our knowledge, the present study is
the first to provide evidence that acute exposure to pathologically
relevant levels of 3DG at high physiological glucose levels
downregulated the STR signaling pathway in pancreatic β-cells. The
downregulation of the STR signaling pathway was involved in the
acute effect of 3DG on the regulation of insulin secretion. The
present results suggested a novel mechanism linking the impairment
of β-cell function with the increase in the circulating levels of
3DG via the downregulation of the STR signaling pathway. The
present findings suggested that the STRs expressed in β-cells may
be a promising therapeutic target to prevent and treat diabetes.
Furthermore, the present results suggested that STRs expressed on
β-cell may be a promising therapeutic target to prevent and treat
diabetes.
Acknowledgements
Not applicable.
Funding
The present work was supported by Grants from
Jiangsu Province's Key Provincial Talents Program (grant nos.
QNRC2016259 and QNRC2016252), Suzhou Science and Technology
Department (grant no. SYSD2017191) and Suzhou TCM Hospital
Affiliated to Nanjing University of Chinese Medicine (grant no.
YQN2016006).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
All authors contributed to the concept and design of
the study, and all authors interpreted the data. XDS, LZ, GRJ, MS,
GQL, FW, LRZ and HF collected and analyzed the data. XDS, LZ and
GRJ drafted the manuscript. GRJ reviewed the manuscript for
important intellectual content. All authors revised the article and
approved the final manuscript. GRJ is responsible for the integrity
of the work as a whole.
Ethics approval and consent to
participate
All the animal experimental procedures were
conducted in compliance with The Directive 2010/63/EU. The present
study was approved by The Local Committee on Ethics of Animal
Experiments of Suzhou TCM Hospital Affiliated to Nanjing University
of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schuit FC, Huypens P, Heimberg H and
Pipeleers DG: Glucose sensing in pancreatic beta-cells: A model for
the study of other glucose-regulated cells in gut, pancreas, and
hypothalamus. Diabetes. 50:1–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kojima I, Medina J and Nakagawa Y: Role of
the glucose-sensing receptor in insulin secretion. Diabetes Obes
Metab. 1 (Suppl 19):S54–S62. 2017. View Article : Google Scholar
|
|
3
|
Nelson G, Hoon MA, Chandrashekar J, Zhang
Y, Ryba NJ and Zuker CS: Mammalian sweet taste receptors. Cell.
106:381–390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Margolskee RF: Molecular mechanisms of
bitter and sweet taste transduction. J Biol Chem. 277:1–4. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Depoortere I: Taste receptors of the gut:
Emerging roles in health and disease. Gut. 63:179–190. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kyriazis GA, Soundarapandian MM and
Tyrberg B: Sweet taste receptor signaling in beta cells mediates
fructose-induced potentiation of glucose-stimulated insulin
secretion. Proc Natl Acad Sci USA. 109:E524–E532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakagawa Y, Nagasawa M, Yamada S, Hara A,
Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y and Kojima
I: Sweet taste receptor expressed in pancreatic beta-cells
activates the calcium and cyclic AMP signaling systems and
stimulates insulin secretion. PLoS One. 4:e51062009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakagawa Y, Nagasawa M, Mogami H, Lohse M,
Ninomiya Y and Kojima I: Multimodal function of the sweet taste
receptor expressed in pancreatic β-cells: Generation of diverse
patterns of intracellular signals by sweet agonists. Endocr J.
60:1191–1206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakagawa Y, Ohtsu Y, Nagasawa M, Shibata H
and Kojima I: Glucose promotes its own metabolism by acting on the
cell-surface glucose-sensing receptor T1R3. Endocr J. 61:119–131.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niwa T: 3-Deoxyglucosone: Metabolism,
analysis, biological activity, and clinical implication. J
Chromatogr B Biomed Sci Appl. 731:23–36. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Degen J, Beyer H, Heymann B, Hellwig M and
Henle T: Dietary influence on urinary excretion of 3-deoxyglucosone
and its metabolite 3-deoxyfructose. J Agric Food Chem.
62:2449–2456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brings S, Fleming T, Freichel M,
Muckenthaler MU, Herzig S and Nawroth PP: Dicarbonyls and advanced
glycation end-products in the development of diabetic complications
and targets for intervention. Int J Mol Sci. 18:E9842017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lal S, Kappler F, Walker M, Orchard TJ,
Beisswenger PJ, Szwergold BS and Brown TR: Quantitation of
3-deoxyglucosone levels in human plasma. Arch Biochem Biophys.
342:254–260. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamada Y, Nakamura J, Fujisawa H, Yago H,
Nakashima E, Koh N and Hotta N: Effects of glycemic control on
plasma 3-deoxyglucosone levels in NIDDM patients. Diabetes Care.
20:1466–1469. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang G, Zhang L, Ji Q, Wang F, Xu H,
Huang F and Wang C: Accumulation of plasma 3-deoxyglucosone
impaired glucose regulation in Chinese seniors: Implication for
senile diabetes? Diabetes Metab Syndr. 6:140–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Song X, Zhou L, Liang G, Xu H,
Wang F, Huang F and Jiang G: Accumulation of intestinal tissue
3-deoxyglucosone attenuated GLP-1 secretion and its insulinotropic
effect in rats. Diabetol Metab Syndr. 8:782016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Zhou L, Song X, Liang G, Xu H,
Zhang L and Jiang G: Acute reduction of incretin effect and glucose
intolerance in rats by single intragastric administration of
3-deoxyglucosone. Exp Clin Endocrinol Diabetes. 125:4–11.
2017.PubMed/NCBI
|
|
18
|
Zhang L, Zhou L, Song X, Liang G, Xu Z,
Wang F, Huang F and Jiang G: Involvement of exogenous
3-deoxyglucosone in β-cell dysfunction induces impaired glucose
regulation. Mol Med Rep. 16:2976–2984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang G, Song X, Xu H, Wang F, Zhang L,
Zhou L and Jiang G: 3-Deoxyglucosone induced acute glucose
intolerance in sprague-dawley rats: Involvement of insulin
resistance and impaired β-cell function. Exp Clin Endocrinol
Diabetes. 124:431–436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maessen DE, Hanssen NM, Scheijen JL, van
der Kallen CJ, van Greevenbroek MM, Stehouwer CD and Schalkwijk CG:
Post-glucose load plasma α-dicarbonyl concentrations are increased
in individuals with impaired glucose metabolism and type 2
diabetes: The CODAM study. Diabetes Care. 38:913–920. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng R, Qian C, Liu Q, Jin Y, Liu L, Li S,
Liao Y, Zhou H, Liu W, Rayner CK and Ma J: Expression of sweet
taste receptor and gut hormone secretion in modeled type 2 diabete.
Gen Comp Endocrinol. 252:142–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Medina A, Nakagawa Y, Ma J, Li L, Hamano
K, Akimoto T, Ninomiya Y and Kojima I: Expression of the
glucose-sensing receptor T1R3 in pancreatic islet: Changes in the
expression levels in various nutritional and metabolic states.
Endocr J. 61:797–805. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kyriazis GA, Smith KR, Tyrberg B, Hussain
T and Pratley RE: Sweet taste receptors regulate basal insulin
secretion and contribute to compensatory insulin hypersecretion
during the development of diabetes in male mice. Endocrinology.
155:2112–2121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang F, Song X, Zhou L, Liang G, Huang F,
Jiang G and Zhang L: The downregulation of sweet taste receptor
signaling in enteroendocrine L-cells mediates
3-deoxyglucosone-induced attenuation of high glucose-stimulated
GLP-1 secretion. Arch Physiol Biochem. 124:430–435. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kato H, van Chuyen N, Shinoda T, Sekiya F
and Hayase F: Metabolism of 3-deoxyglucosone, an intermediate
compound in the maillard reaction, administered orally or
intravenously to rats. Biochim Biophys Acta. 1035:71–76. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang G, Wang F, Song X, Zhang L, Qian Z
and Jiang G: 3-Deoxyglucosone induces insulin resistance by
impairing insulin signaling in HepG2 cells. Mol Med Rep.
13:4506–4512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chlebus M, Guillen J and Prins JB:
Directive 2010/63/EU: Facilitating full and correct implementation.
Lab Anim. 50:1512016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamada H, Miyata S, Igaki N, Yatabe H,
Miyauchi Y, Ohara T, Sakai M, Shoda H, Oimomi M and Kasuga M:
Increase in 3-deoxyglucosone levels in diabetic rat plasma.
Specific in vivo determination of intermediate in advanced Maillard
reaction. J Biol Chem. 269:20275–20280. 1994.PubMed/NCBI
|
|
29
|
Asfari M, Janjic D, Meda P, Li G, Halban
PA and Wollheim CB: Establishment of 2-mercaptoethanol-dependent
differentiated insulin-secreting cell lines. Endocrinology.
130:167–178. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang MY, Oh TJ and Cho YM: Glucagon-like
peptide-1 increases mitochondrial biogenesis and function in INS-1
rat insulinoma cells. Endocrinol Metab (Seoul). 30:216–220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bo J, Xie S, Guo Y, Zhang C, Guan Y, Li C,
Lu J and Meng QH: Methylglyoxal impairs insulin secretion of
pancreatic β-Cells through increased production of ROS and
mitochondrial dysfunction mediated by upregulation of UCP2 and
MAPKs. J Diabetes Res. 2016:20298542016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hamano K, Nakagawa Y, Ohtsu Y, Li L,
Medina J, Tanaka Y, Masuda K, Komatsu M and Kojima I: Lactisole
inhibits the glucose-sensing receptor T1R3 expressed in mouse
pancreatic β-cells. J Endocrinol. 226:57–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou L, Huang W, Xu Y, Gao C, Zhang T, Guo
M, Liu Y, Ding J, Qin L, Xu Z, et al: Sweet taste receptors
mediated ROS-NLRP3 inflammasome signaling activation: Implications
for diabetic nephropathy. J Diabetes Res. 2018:70782142018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elmhiri G, Barella LF, Vieau D, Camous S,
Mathias PC and Abdennebi-Najar L: Acute exposure to a precursor of
advanced glycation end products induces a dual effect on the rat
pancreatic islet function. Int J Endocrinol. 2014:3782842014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mace OJ, Affleck J, Patel N and Kellett
GL: Sweet taste receptors in rat small intestine stimulate glucose
absorption through apical GLUT2. J Physiol. 582:379–392. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ashraf JM, Shahab U, Tabrez S, Lee EJ,
Choi I, Aslam Yusuf M and Ahmad S: DNA glycation from
3-deoxyglucosone leads to the formation of AGEs: Potential role in
cancer auto-antibodies. Cell Biochem Biophys. 74:67–77. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hecker M and Wagner AH: Role of protein
carbonylation in diabetes. J Inherit Metab Dis. 41:29–38. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aldini G, Dalle-Donne I, Facino RM,
Milzani A and Carini M: Intervention strategies to inhibit protein
carbonylation by lipoxidation-derived reactive carbonyls. Med Res
Rev. 27:817–868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thorens B: GLUT2, glucose sensing and
glucose homeostasis. Diabetologia. 58:221–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song Z, Wang H, Zhu L, Han M, Gao Y, Du Y
and Wen Y: Curcumin improves high glucose-induced INS-1 cell
insulin resistance via activation of insulinsignaling. Food Funct.
6:461–469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guillam MT, Hümmler E, Schaerer E, Yeh JI,
Birnbaum MJ, Beermann F, Schmidt A, Dériaz N and Thorens B: Early
diabetes and abnormal postnatal pancreatic islet development in
mice lacking Glut-2. Nat Genet. 17:327–330. 1997. View Article : Google Scholar : PubMed/NCBI
|


















