Introduction
Peripheral arterial disease (PAD) is an
atherosclerotic disease, which affects the arteries of the limbs.
It occurs via a complex process including endothelial dysfunction
in animals (1) and humans
(2), lipid metabolic disturbance,
thrombosis and inflammation (3).
It has been reported that the incidence of PAD is as high as 15–20%
in people >70 years old (4,5),
with the prevalence of intermittent claudication (IC) rising to ~6%
in people >60 years old (6).
PAD is usually associated with atherosclerosis in the whole-body
vasculature and is considered to be a principal cause of mortality
worldwide. Although there have been multiple studies conducted
regarding relevant risk factors in PAD, the underlying epigenetic
mechanism is poorly characterized (7–9).
MicroRNAs (miRNAs) are short noncoding RNAs that are
involved in the mediation of human gene expression by binding to
mRNA, in addition to suppressing protein synthesis (7). There are no fewer than 1,500 human
miRNAs in the miRBase database, which serve a critical role in the
post-transcriptional modification of gene expression via targeting
of the 3′-untranslated region (UTR) of specific mRNAs (8), thereby affecting various cellular
processes in embryonic development and disease conditions (9–11).
Previous studies have indicated that the determination of miRNA
expression in patients with PAD may serve a prognostic and
diagnostic role in the future (12,13).
Recently, regarding the study of tumor and hind limb
ischemia, miRNA (miR)-93 has been considered to be able to mediate
angiogenesis in various molecular pathways (14,15).
miR-93 is a member of the miRNA-106b~25 cluster, and has been
demonstrated to serve a key oncogenic role by regulating cell
proliferation, migration, the cell cycle and tube formation
(16–18). However, the association between
miR-93 and PAD remains unknown and the target gene of miR-93 in PAD
has not been fully characterized.
The present study determined the expression levels
of miR-93 in the serum of patients with PAD and investigated the
function of miR-93 in angiogenesis in a cell model and in an
ischemic hind-limb mouse model. In addition, the study aimed to
identify the underlying downstream targets involved in PAD.
Materials and methods
Clinical samples
A total of 146 patients with PAD (79 male and 67
female) with a mean age of 58.9±9.8 years were admitted to the 1st
Hospital of Lanzhou University (Lanzhou, China) to receive drug
therapy and other ancillary treatment. In addition, 32 normal
control subjects (18 male and 14 female) with a mean age of
57.6±8.1 years were recruited. The ankle brachial index (ABI) was
used to assess the severity of PAD (19). The blood samples from the PAD
patients at the time of admission and from the controls were
collected and centrifuged at 200 × g for 10 min at 4°C to harvest
the separated serum, and stored at −80°C for further analysis.
Written informed consent was obtained from all patients and the
experiment was approved by the ethics committee of 1st Hospital of
Lanzhou University (Lanzhou, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR analyses of miR-93 were performed on patient
serum using specific TaqMan assays (Life Technologies; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) as previously described
(20). In addition, RNA was
isolated from EA.hy926 cells to analyze the cyclin dependent kinase
inhibitor 1A (CDKN1A) mRNA expression and the miRNA-93 expression
using TaqMan assays (Life Technologies; Thermo Fisher Scientific,
Inc.) as previously described (21). PCR primer sequences were obtained
from previously published studies (20,21).
Cell culture and transfection
EA.hy926 endothelial cells (American Type Culture
Collection, Manassas, VA, USA) were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.), 10% fetal
calf serum (Gibco; Thermo Fisher Scientific, Inc.), 1% glutamine,
1% nonessential amino acids, and penicillin (100 U/ml) at 37°C and
5% CO2 under humidified conditions. The cells were
cultured in hypoxic conditions when the oxygen concentration of the
incubator was adjusted to 1%. EA.hy926 cells (2×106/ml)
cultured in the normal and hypoxic conditions were respectively
transfected with miRNA-93 mimics using RNAiMAX Reagent (Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
instructions. Oligonucleotide transfection RNA oligos were
chemically synthesized and purified by Shanghai Genepharma Co. Ltd.
(Shanghai, China). The following rno-mir-93 agomir sequences were
used: Sense, 5′-CAAAGUGCUGUUCGUGAGGUAG-3′ and antisense,
5′-ACCUGCACGAACAGCACUUUGUU-3′. The sequences of the rno-mir-93
agomir negative control used were as follows: Sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. The final concentration of miRNA
mimics used for transfection was 100 nM, and the cells were
harvested at 12 h for subsequent experimentation.
Cell proliferation, migration and tube
formation
In order to assess the functions of transfected
cells under hypoxic conditions, cell proliferation, migration and
tube formation assays were performed. Cell proliferation was
determined using the MTT method (12). Transfected cells were seeded into
96-well plates at 10,000 cells/well and viability was detected. In
order to identify transfected cell migration ability, a wound
healing assay was performed. Transfected cells were cultured in
serum-free medium for 24 h, and an artificial wound was created
using a 100-µl sterile pipette tip. A total of 24 h post-wound
infliction, the width of the scratch gap was measured using an
inverted microscope (magnification, ×10). In the tube formation
assay, 10 µl growth factor-reduced Matrigel was seeded into each
well and permitted to polymerize for 30 min at 37°C. Subsequently,
10,000 cells were placed on the Matrigel for 24 h at 37°C and
images were viewed using an inverted microscope (magnification,
×10). A total of three independent detections were performed for
each assay, with analysis of five random visual fields for each
chamber.
Luciferase reporter assays
The miRNA body map web tool, including EIMMO
(http://www.mirz.unibas.ch/ElMMo3/)
and miRanda-mirSVR (http://microRNA.org/), was employed to identify
potential target genes of miRNA-93. EA.hy926 cells were
co-transfected with the wild-type (WT) or mutant (Mut) CDKN1A
3′-UTR reporter genes or negative control miRNA mimics
(pMIR-Control; Genscript Cor--p., Piscataway, NJ, USA). Following
culture for 36 h, the luciferase activity was determined via
comparison with Renilla luciferase activity when the cells
had been lysed with a passive lysis buffer, using the
Dual-Luciferase Reporter Assay System (Promega Corporation,
Madison, WI, USA).
Western blotting
Western blot analysis using EA.hy926 cells was
performed as previously described (21,22).
The total proteins were incubated with primary antibodies against
CDKN1A (1:1,000; cat. no. SAB4300419; Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany), and subsequently incubated with a secondary
antibody (1:2,000; cat. no. ab6721; Abcam, Cambridge, UK). GAPDH
(1:2,000; cat. no. G5262; Sigma-Aldrich; Merck KGaA) was used as an
internal control. The quantification of the western blotting
results was performed using ImageJ software version 1.43 (National
Institutes of Health, Bethesda, MD, USA).
Hind-limb ischemic model
C57Bl/6J mice (n=12) weighing 230±20 g were
purchased from the Institute of Laboratory Animal Sciences, Peking
Union Medical College (Beijing, China). All the procedures were
approved by the Animal Experimental Ethics Committee, 1st Hospital
of Lanzhou University. A critical hind limb ischemia model was
created as previously described (23). Prior to surgery, the mice were
anesthetized via injection of ketamine 90 mg/kg and xylazine 10
mg/kg. The ligation and division of the left femoral artery and
vein were conducted to surgically create severe unilateral hind
limb ischemia. At the time of surgery, mice were randomly divided
into two groups (n=6 per group): The negative control group (NC
intramuscular injection); and the miR-93 group (premiR-93
intramuscular injection). PremiR-93 (PM10951) or miR-mimic NC
(Genscript Corp.) were dissolved in PBS and intramuscularly
injected into the gastrocnemius muscle (100 µM in 25 µl), as
previously described (24). Laser
Doppler perfusion imaging (LDPI) was conducted 2 weeks post-surgery
to detect the blood flow of the ischemic and normal limbs, as
previously stated (25). The
scores for muscle necrosis and ambulatory impairment were assessed
respectively.
Histology
Alterations in muscle tissue morphology were
examined with hematoxylin and eosin (H&E) and platelet
endothelial cell adhesion molecule (CD31) staining. The hind limb
tissues were fixed in 4% paraformaldehyde for 48 h at room
temperature, dehydrated, paraffin-embedded and sliced into tissue
sections (4 mm). All the slices were stained using H&E and
anti-CD31 for histological analysis, according to the
manufacturer's specific instructions (26).
Statistical analysis
Descriptive statistics were calculated and are
presented as the mean ± standard error of the mean in the figures.
One-way analysis of variance was performed for multiple group
comparisons followed by the Student-Newman-Keuls test for
group-wise comparisons. The Student's t-test was used for
comparison between two groups. P<0.05 was considered to indicate
a statistically significant difference. The statistical analysis
was performed using SPSS software (SPSS for Windows 17.0; SPSS,
Inc., Chicago, IL, USA). A minimum of three repeats were performed
per assay.
Results
Expression levels of miR-93 in
patients with PAD
In order to detect the importance of miR-93 in PAD,
the expression of miR-93 was analyzed using RT-qPCR. miR-93
expression in patients with PAD was significantly upregulated when
compared with the controls (Fig.
1A). It has been demonstrated that PAD severity, as determined
by the ABI, is also correlated with the degree of functional
impairment (27,28). Among participants able to walk for
6 min without stopping at baseline, the diagnosis of PAD degree was
made in accordance with baseline ABI categories (<0.50;
0.50<0.70; 0.70<0.90; and 0.90<1.10) (29). Furthermore, to validate the
fold-change in miR-93 expression in patients with differing degrees
of PAD, corresponding expression of miR-93 was determined, and the
results demonstrated that there was a positive association between
miR-93 expression and PAD severity (Fig. 1B). Collectively, these findings
indicated that miR-93 was involved in the development and
progression of PAD.
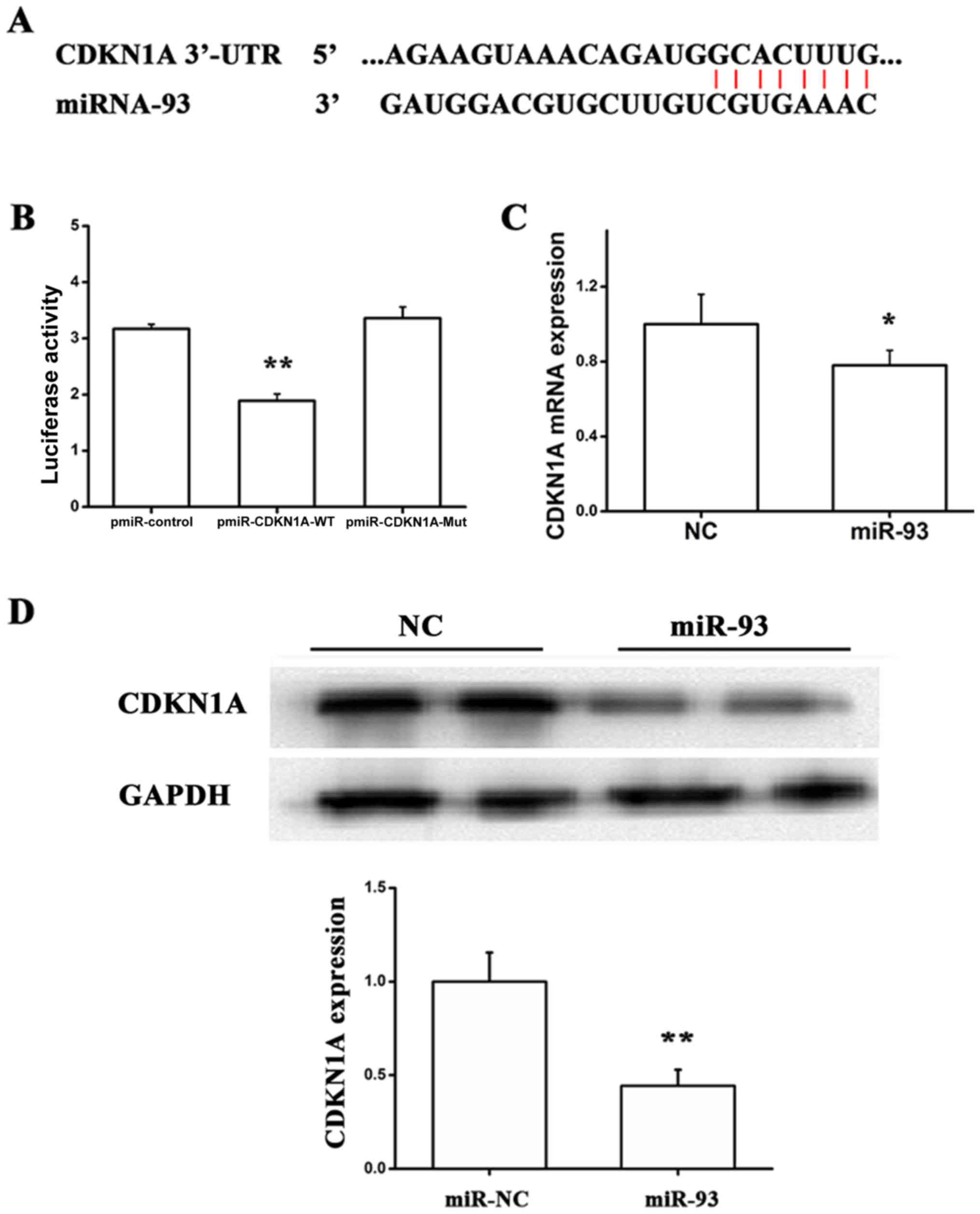
CDKN1A is a potential target of
miR-93
To further identify the underlying mechanism by
which miR-93 facilities proliferation, migration and tube formation
of EA.hy 926 cells, we verified CDKN1A as a direct target of miR-93
using accessible databases (30,31).
The luciferase reporter was constructed, which contained the
wild-type or mutant miR-93 target sequences of CDKN1A 3′-UTR. The
luciferase reporter assay was performed to demonstrate whether the
CDKN1A 3′-UTR is a direct target of miR-93 (Fig. 2A). As indicated in Fig. 2B, compared with the mutant reporter
gene, the luciferase activity of the wild-type 3′-UTR reporter gene
was significantly downregulated, suggesting that miR-93 is able to
bind to the CDKN1A 3′-UTR. It was additionally observed that miR-93
significantly inhibited CDKN1A expression at the mRNA and protein
levels, using RT-qPCR and western blotting (Fig. 2C and D). Taken together, these
findings demonstrated that miR-93 may directly suppress CDKN1A
expression in EA.hy926 cells by targeting the CDKN1A 3′-UTR.
Effects of miR-93 in EA.hy926 cells in
response to hypoxia
To examine the effect of miR-93 on endothelial cells
under hypoxic conditions, the expression of miR-93 was analyzed
using RT-qPCR, and the results demonstrated that miR-93 expression
was upregulated in response to hypoxia when compared with normal
conditions (Fig. 3A).
Additionally, cells were transfected with miR-93, in which the
miR-93 level was quantified by RT-qPCR at 48 h post-transfection.
miR-93 was successfully overexpressed in EA.hy926 cells (Fig. 3B), and a number of experiments were
performed in vitro, including the MTT assay, wound healing
assay and tube formation assay. The data demonstrated that the
proliferation, migration and tube formation of cells that were
transfected with miR-93 and cultured in hypoxic conditions were
markedly enhanced (Fig. 3C-E).
Thus, the aforementioned data suggested that miR-93 maintained
endothelial cell activity by promoting proliferation, migration and
tube formation in response to hypoxia.
Overexpression of miR-93 in the
hind-limb ischemic model to improve perfusion recovery
It has been reported that BALB/cJ mice have
decreased expression of miR-93 and exhibit little increase in
miR-93 following hind-limb ischemia when compared with C57B1/6J
mice (32). Thus, local
intramuscular injections of premiR-93 and NC were administered to
detect whether the overexpression of miR-93 improved perfusion in
the hind-limb ischemic model. The LDPI experiment indicated that
miR-93 increased the blood flow ratio and improved tissue necrosis
and ambulatory impairment, as presented in Fig. 4A-C. Consistent with improved
perfusion recovery, ischemic hind limb muscles from the mice
injected with premiR-93 indicated decreased muscle necrosis and a
higher capillary density when compared with NC-treated mice
(Fig. 4D), suggesting that
overexpression of miR-93 may be sufficient to promote angiogenesis
following hind-limb ischemia.
Discussion
The discovery of miRNAs was considered a milestone
in molecular biology, and miRNAs are critical regulators involved
in numerous cellular processes, including proliferation, migration,
apoptosis and differentiation. miRNAs have been closely associated
with the development and progression of tumors (33,34).
In the present study, miR-93 expression in the peripheral blood of
patients with PAD was compared with the control group, and it was
observed that miR-93 was upregulated in patients with PAD. To
validate the molecular mechanisms underlying PAD, EA.hy926
endothelial cells were used to observe miR-93 expression in
response to hypoxia. In vitro analyses demonstrated that
miR-93 promoted EA.hy926 cell proliferation, migration and tube
formation by binding to CDKN1A, which was demonstrated to be a
direct target. The aforementioned findings revealed that miR-93
enhanced the proliferation, migration and tube formation of
EA.hy926 cells by directly targeting CDKN1A.
PAD induces tissue hypoperfusion and eventually
results in critical limb ischemia (35). A number of miRNAs have been
reported to be associated with the development of PAD and involved
in vascular disorders. Liu et al (36) reported that miRNA-15b is associated
with apoptosis and angiogenesis in myocardial infarction.
Kuehbacher et al (37)
demonstrated that miR-27b may be regarded as a pro-angiogenic miRNA
by regulating angiogenesis through the angiogenic inhibitor,
thrombospondin-1. Chamorro-Jorgane et al (38) indicated that miR-16 modulates
angiogenic signaling and vascular integrity, serving a role in
angiogenesis by suppressing the proliferation, migration and
angiogenic capacity of endothelial cells. However, little
information is available pertaining to the association between
miRNA-93 expression and PAD. The results of the present study
revealed that miRNA-93 was upregulated in EA.hy926 endothelial
cells when cultured in hypoxic conditions, and in freshly frozen
muscle tissues. To further verify the angiogenic function of
miRNA-93, the effect of miRNA-93 on endothelial cells was observed
in vitro and the results suggested that miRNA-93 may
facilitate proliferation, migration and tube formation under
hypoxic conditions, indicating that miRNA-93 may contribute to the
progression of PAD by regulating endothelial cell activities
(32).
In previously established models of PAD, a series of
studies (32,39,40)
indicated that C57Bl/6 J mice recover well, while BALB/cJ mice
exhibit poor perfusion recovery following hind-limb ischemia. In
vivo, C57Bl/6 J mice were used as a model and it was observed
that hind-limb blood perfusion was improved, and scores for muscle
necrosis and ambulatory impairment were significantly decreased
following injection of premiR-93, which indicated that
overexpression of miR-93 promoted angiogenesis to improve recovery
from hind-limb ischemia. Furthermore, extensive histological
staining of the hind-limb tissue revealed a high density of CD31,
which is considered to be a key marker of endothelial cells.
Consistent with the results of the in vitro studies
(32,41), these findings further confirmed the
role of miR-93 in ischemia-induced angiogenesis.
CDKN1A is regarded as an important inhibitor of the
cell cycle, mediator of DNA damage and effector of the tumor
suppressor cellular tumor antigen p53, displaying a key role in the
development and progression of various cancer types (42). CDKN1A, an endothelial
dysfunction-associated gene, is associated with the decrease in
human umbilical vein endothelial cell viability caused by sodium
arsenite (43). Yamagata et
al (44) reported that
docosahexaenoic acid regulates the expression of numerous genes
that involve CDKN1A, which is associated with senescence and
dysfunction in endothelial cells.
Previous studies have emphasized the functional
implications of CDKN1A during the development of PAD (45,46).
As previously stated, PAD is an atherosclerotic disease, in which
the pathological response contributes to a complex inflammatory
reaction induced by endothelial functional loss (45). In the present study, using a
luciferase reporter assay, CDKN1A was identified as a direct target
of miR-93 in EA.hy926 cells, suggesting that miR-93 exerts an
important effect in the regulation of angiogenesis during PAD via
binding to CDKN1A.
In conclusion, miR-93 contributes to angiogenesis by
enhancing the proliferation, migration and tube formation of
EA.hy926 endothelial cells, which is associated with the reduced
expression of CDKN1A. Overexpression of miR-93 was able to
ameliorate ischemia in the mouse hind-limb, providing a novel
opportunity to elucidate whether miR-93/CDKN1A may be a promising
therapeutic target for PAD.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81770483),
the Jiangsu Provincial Science and Technology Office (grant no.
BL2014043), and the Suzhou Health and Family Planning Commission
Program (grant no. LCZX201504).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS designed the study, performed experiments,
analyzed the data and wrote the manuscript. YM performed a number
of in vitro and in vivo experiments. ZL performed the
animal experiments. WW, YC and SL analyzed the data and drafted the
manuscript. XL designed and supervised the study, and edited the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and the experiment was approved by the ethics committee of
1st Hospital of Lanzhou University (Lanzhou, China). All the
procedures were performed in accordance with national (D.L.n.26,
March 4th, 2014) and international laws and policies (directive
2010/63/EU), and were approved by the Animal Experimental Ethics
Committee, 1st Hospital of Lanzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lentz SR, Sobey CG, Piegors DJ, Bhopatkar
MY, Faraci FM, Malinow MR and Heistad DD: Vascular dysfunction in
monkeys with diet-induced hyperhomocyst(e)inemia. J Clin Invest.
98:24–29. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tawakol A, Omland T, Gerhard M, Wu JT and
Creager MA: Hyperhomocyst(e)inemia is associated with impaired
endothelium-dependent vasodilation in humans. Circulation.
95:1119–1121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faxon DP, Fuster V, Libby P, Beckman JA,
Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi
RP, et al: Atherosclerotic vascular disease conference: Writing
group III: Pathophysiology. Circulation. 109:2617–2625. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Criqui MH, Fronek A, Barrett-Connor E,
Klauber MR, Gabriel S and Goodman D: The prevalence of peripheral
arterial disease in a defined population. Circulation. 71:510–515.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Selvin E and Erlinger TP: Prevalence of
and risk factors for peripheral arterial disease in the United
States: Results from the National health and nutrition examination
survey, 1999–2000. Circulation. 110:738–743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Norgren L, Hiatt WR, Dormandy JA, Nehler
MR, Harris KA, Fowkes FG; TASC II Working Group, ; Bell K,
Caporusso J, Durand-Zaleski I, et al: Inter-society consensus for
the management of peripheral arterial disease (TASC II). Eur J Vasc
Endovasc Surg. 33 (Suppl 1):S1–S75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morisaki K, Yamaoka T and Iwasa K: Risk
factors for wound complications and 30-day mortality after major
lower limb amputations in patients with peripheral arterial
disease. Vascular. 26:12–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weragoda J, Seneviratne R, Weerasinghe MC
and Wijeyaratne SM: Risk factors of peripheral arterial disease: A
case control study in Sri Lanka. BMC Res Notes. 9:5082016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forés R, Alzamora MT, Pera G, Valverde M,
Angla M, Baena-Díez JM and Mundet-Tuduri X: Evolution and degree of
control of cardiovascular risk factors after 5 years of follow-up
and their relationship with the incidence of peripheral arterial
disease: ARTPER cohort. Med Clin (Barc). 148:107–113. 2017.(In
English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trionfini P and Benigni A: MicroRNAs as
master regulators of glomerular function in health and disease. J
Am Soc Nephrol. 28:1686–1696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato M and Natarajan R: MicroRNAs in
diabetic nephropathy: Functions, biomarkers, and therapeutic
targets. Ann N Y Acad Sci. 1353:72–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stather PW, Sylvius N, Wild JB, Choke E,
Sayers RD and Bown MJ: Differential microRNA expression profiles in
peripheral arterial disease. Circ Cardiovasc Genet. 6:490–497.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fichtlscherer S, De Rosa S, Fox H,
Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T,
Müller-Ardogan M, et al: Circulating microRNAs in patients with
coronary artery disease. Circ Res. 107:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savita U and Karunagaran D:
MicroRNA-106b-25 cluster targets β-TRCP2, increases the expression
of Snail and enhances cell migration and invasion in H1299 (non
small cell lung cancer) cells. Biochem Biophys Res Commun.
434:841–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang L, Deng Z, Shatseva T, Yang J, Peng
C, Du WW, Yee AJ, Ang LC, He C, Shan SW and Yang BB: MicroRNA
miR-93 promotes tumor growth and angiogenesis by targeting
integrin-β8. Oncogene. 30:806–821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang L, Du WW, Yang W, Rutnam ZJ, Peng C,
Li H, O'Malley YQ, Askeland RW, Sugg S, Liu M, et al: MiR-93
enhances angiogenesis and metastasis by targeting LATS2. Cell
Cycle. 11:4352–4365. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu S, Patel SH, Ginestier C, Ibarra I,
Martin-Trevino R, Bai S, McDermott SP, Shang L, Ke J, Ou SJ, et al:
MicroRNA93 regulates proliferation and differentiation of normal
and malignant breast stem cells. PLoS Genet. 8:e10027512012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li F, Liang X, Chen Y, Li S and Liu J:
Role of microRNA-93 in regulation of angiogenesis. Tumour Biol.
35:10609–10613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu YF, Mao YP, Li YQ, Ren XY, He QM, Tang
XR, Sun Y, Liu N and Ma J: MicroRNA-93 promotes cell growth and
invasion in nasopharyngeal carcinoma by targeting disabled
homolog-2. Cancer Lett. 363:146–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu G, Friggeri A, Yang Y, Park YJ,
Tsuruta Y and Abraham E: miR-147, a microRNA that is induced upon
Toll-like receptor stimulation, regulates murine macrophage
inflammatory responses. Proc Natl Acad Sci USA. 106:15819–15824.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang F, Huang XR, Chung AC, Hou CC, Lai KN
and Lan HY: Essential role for Smad3 in angiotensin II-induced
tubular epithelial-mesenchymal transition. J Pathol. 221:390–401.
2010.PubMed/NCBI
|
|
22
|
Koka V, Huang XR, Chung AC, Wang W, Truong
LD and Lan HY: Angiotensin II up-regulates angiotensin I-converting
enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP
kinase pathway. Am J Pathol. 172:1174–1183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Q, Chen RR, Shen Y, Mooney DJ,
Rajagopalan S and Grossman PM: Sustained vascular endothelial
growth factor delivery enhances angiogenesis and perfusion in
ischemic hind limb. Pharm Res. 22:1110–1116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge Y, Sun Y and Chen J: IGF-II is
regulated by microRNA-125b in skeletal myogenesis. J Cell Biol.
192:69–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Couffinhal T, Silver M, Zheng LP, Kearney
M, Witzenbichler B and Isner JM: Mouse model of angiogenesis. Am J
Pathol. 152:1667–1679. 1998.PubMed/NCBI
|
|
26
|
Bao H, Lv F and Liu T: A pro-angiogenic
degradable Mg-poly(lactic-co-glycolic acid) implant combined with
rhbFGF in a rat limb ischemia model. Acta Biomaterial. 64:279–289.
2017. View Article : Google Scholar
|
|
27
|
McDermott MM, Greenland P, Liu K, Guralnik
JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH,
et al: The ankle brachial index is associated with leg function and
physical activity: The walking and leg circulation study. Ann
Intern Med. 136:873–883. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mcdermott MG, Liu K, Jack M, Guralnik MD,
Mehta S, Criqui MH, Martin GJ and Greenland P: The ankle brachial
index independently predicts walking velocity and walking endurance
in peripheral arterial disease. J Am Geriatr Soc. 46:1355–1362.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McDermott MM, Liu K, Greenland P, Guralnik
JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic
L, et al: Functional decline in peripheral arterial disease:
Associations with the ankle brachial index and leg symptoms. JAMA.
292:453–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Correction: Human MicroRNA targets. PLoS
Biol. 2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hazarika S, Farber CR, Dokun AO,
Pitsillides AN, Wang T, Lye RJ and Annex BH: MicroRNA-93 controls
perfusion recovery following hind-limb ischemia by modulating
expression of multiple genes in the cell cycle pathway.
Circulation. 127:1818–1828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Q, Gumireddy K, Schrier M, le Sage
C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, et al:
The microRNAs miR-373 and miR-520c promote tumour invasion and
metastasis. Nat Cell Biol. 10:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Slovut DP and Sullivan TM: Critical limb
ischemia: Medical and surgical management. Vasc Med. 13:281–291.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Z, Yang D, Xie P, Ren G, Sun G, Zeng X
and Sun X: MiR-106b and MiR-15b modulate apoptosis and angiogenesis
in myocardial infarction. Cell Physiol Biochem. 29:851–862. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuehbacher A, Urbich C, Zeiher AM and
Dimmeler S: Role of Dicer and Drosha for endothelial microRNA
expression and angiogenesis. Circ Res. 101:59–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chamorro-Jorganes A, Araldi E, Penalva LO,
Sandhu D, Fernández-Hernando C and Suárez Y: MicroRNA-16 and
microRNA-424 regulate cell-autonomous angiogenic functions in
endothelial cells via targeting vascular endothelial growth factor
receptor-2 and fibroblast growth factor receptor-1. Arterioscler
Thromb Vasc Biol. 31:2595–2606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dokun AO, Keum S, Hazarika S, Li Y,
Lamonte GM, Wheeler F, Marchuk DA and Annex BH: A quantitative
trait locus (LSq-1) on mouse chromosome 7 is linked to the absence
of tissue loss after surgical hindlimb ischemia. Circulation.
117:1207–1215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chalothorn D, Clayton JA, Zhang H, Pomp D
and Faber JE: Collateral density, remodeling and VEGF-A expression
differ widely between mouse strains. Physiol Genomics. 30:179–191.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Long J, Wang Y, Wang W, Chang BH and
Danesh FR: Identification of microRNA-93 as a novel regulator of
vascular endothelial growth factor in hyperglycemic conditions. J
Biol Chem. 285:23457–23465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Negishi M, Wongpalee S P, Sarkar S, Park
J, Lee KY, Shibata Y, Reon BJ, Abounader R, Suzuki Y, Sugano S and
Dutta A: A new lncRNA, APTR, associates with and represses the
CDKN1A/p21 promoter by recruiting polycomb proteins. PLoS One.
9:e952162014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nuntharatanapong N, Chen K, Sinhaseni P
and Keaney JF Jr: EGF receptor-dependent JNK activation is involved
in arsenite-induced p21Cip1/Waf1 upregulation and endothelial
apoptosis. Am J Physiol Heart Circ Physiol. 289:H99–H107. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamagata K, Suzuki S and Tagami M:
Docosahexaenoic acid prevented tumor necrosis factor alpha-induced
endothelial dysfunction and senescence. Prostaglandins Leukot
Essent Fatty Acids. 104:11–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yin DX, Zhao HM, Sun DJ, Yao J and Ding
DY: Identification of candidate target genes for human peripheral
arterial disease using weighted gene co-expression network
analysis. Mol Med Rep. 12:8107–8112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spinetti G, Cordella D, Fortunato O,
Sangalli E, Losa S, Gotti A, Carnelli F, Rosa F, Riboldi S, Sessa
F, et al: Global remodeling of the vascular stem cell niche in bone
marrow of diabetic patients: Implication of the microRNA-155/FOXO3a
signaling pathway. Circ Res. 11:510–522. 2013. View Article : Google Scholar
|