Introduction
Cataracts are the leading cause of blindness among
the elderly population, and are enormous social and economic
burdens for many countries (1–3).
Diet, smoking, heritability, and corticosteroids are known risk
factors for age-related cataract formation (4–6).
Since the very detailed studies of Duncan and Bushell in 1975, it
has been well-accepted that nuclear (NUC) cataracts show normal
levels of sodium, potassium, calcium, magnesium and chloride
(7). Thus, NUC cataracts are not
considered to be of the osmotic type. In contrast, cortical
cataracts show grossly increased levels of sodium and calcium with
very low potassium levels, and are thus considered to be osmotic
cataracts (7).
The ion balance in cortical cataractous lenses is
unregulated, and the increase in the level of sodium ion
(Na+) that occurs in age-related cortical cataracts
points to a relationship between the degree of Na+
increase and the severity of cortical opacification (COR) (8). In human age-related cortical
cataractous lenses, Na+ levels are abnormally elevated,
while potassium ion (K+) levels are reduced. It has been
reported that transparent human lenses contain approximately 17 mM
Na+ and 130 mM K+ (9), while the Na+ level is
greater than 100 mM, and the K+ level is about 80 mM in
cortical cataractous lenses (data as mmol/kg lens water) (7,10).
These changes cause an osmotic disturbance so that water
accumulates in cortical lens cells, and this leads to cell lysis
and the appearance of fluid droplets that scatter light and impair
transparency. In addition, the calcium ion (Ca2+) level
is also enhanced as cataracts develop in human lenses (transparent
lens, 1.93 mM; cataractous lens, 33.3 mM) (11), and excessive Ca2+ levels
accompany a derangement in lens Na+ and K+
levels in age-related human cortical cataracts (7). The accumulation of intracellular
Ca2+ also induces the activation of apoptosis, a loss of
cellular integrity, and the stimulation of proteolytic enzymes, all
of which lead to COR (12). As a
cause for these disturbances in the cortical cataractous lens, it
is known that dysfunctions in ATPases, such as
Na+/K+-ATPase and Ca2+-ATPase,
play a role. The levels of Na+/K+-ATPase
activity in the lenses of normal and cortical cataract patients are
6.6 Pi µmol/hr/g protein and 1.9 Pi µmol/hr/g protein, respectively
(13). In addition, we have
reported that the Ca2+-ATPase activity in lenses
decreases with the onset of lens opacification in the hereditary
cataract model ICR rats, where the levels of Ca2+-ATPase
activity in the transparent and opaque lens were 3.1 Pi mmol/hr/g
protein and 1.4 Pi mmol/hr/g protein, respectively (14). The inhibition of
Na+/K+-ATPase results in high intracellular
Na+ levels, and subsequent increases in intracellular
Ca2+ ion through the Na+/Ca2+
exchanger (15). The enhanced
intracellular Ca2+ levels are regulated by
Ca2+-ATPase. These previous reports show that the
regulation of ion balance by ATPases is needed to maintain lens
transparency in cortical cataracts. On the other hand, the cellular
changes that lead to the formation of posterior subcapsular
cataracts (PSC) have been the least studied of all types of
cataracts. The major risk factors associated with PSC are severe
myopia, diabetes, and exposure to therapeutic doses of steroids and
ionizing radiation; however, to our knowledge, there are no
sufficient reports of biochemical analyses of human PSC (16).
Many researchers have reported on the mechanisms of
ATPase dysfunction. We have also reported our findings using the
lenses of hereditary cataract rats as models for human senile
cataracts (UPLR and ICR rats) and cultured human lens epithelial
(HLE) cells (14,17–19),
and found 2 pathways leading to the disintegration of ion balance.
One pathway involves oxidative stress via reactive oxygen species
(ROS) that results in enhanced lipid peroxidation, leading to the
inhibition of ATPase, and an increase in membrane permeability to
Na+ and Ca2+ in ICR lenses (14). The second pathway is one in which
ROS injure the mitochondria, resulting in the collapse of ATP
production. The decrease in ATP content causes ATPase dysfunction,
resulting in an elevation in Na+ and Ca2+ in
UPLR lenses (18,20). The elevation in Ca2+
levels via the 2 pathways leads to lens opacification. Therefore,
it is very important to elucidate the changes in ATP production and
ATP content during cataract development.
It is known that cytochrome c oxidase (CCO), which
reduces oxygen to water and pumps protons across the inner
mitochondrial membrane, plays an important role in ATP production.
The three subtypes of mitochondrial cytochrome c oxidase (MTCO)1, 2
and 3 are the largest of the CCO subunits (21). Therefore, we investigated changes
in the gene expressions of MTCO1-3 and ATP contents according to
the type and severity of cataracts using lens epithelium collected
from patients with 3 different types of opacification (COR, NUC
opacification and PSC.
Materials and methods
Reagents
The ATP Bioluminescent Assay kit was obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The RNase-Free
DNase set, RNA later® Solution and RNeasy Min kit were
provided by Qiagen (Tokyo, Japan). The RNA PCR kit was purchased
from Takara Bio Inc. (Shiga, Japan), and LightCycler FastStart DNA
Master SYBR-Green I was provided by Roche Diagnostics Applied
Science (Mannheim, Germany). All other chemicals used were of the
highest purity commercially available.
Human lens epithelium samples
The human lens epitheliums were provided from
patients agreed to the use of their samples in scientific research,
and their samples were collected during November 20, 2014 from Jun
26, 2014. During surgery, the central part of the anterior lens
capsule (5 mm diameter) containing attached epithelial cells was
isolated by the continuous curvilinear capsulorrhexis (CCC) method
using forceps. Samples of nucleated lens epithelium were obtained
from non-Alzheimer's and non-diabetes patients during cataract
surgery at Kanazawa Medical University (Ishikawa, Japan). The type
and severity of cataract (grade) were determined according to the
WHO classification (22), and
patients providing samples of enucleated lens epithelium with COR,
NUC and/or PSC were screened for visual acuity in the clinic prior
to surgery. Table I shows the
number of cataract patients in each cataract type group. Lens
epithelium from normal patients (transparent lens) was collected
during cataract surgery combined with vitrectomy for macular holes
or epiretinal membrane, and used as non cataractous controls
(clear, normal lens, control). In this study, 18 samples were
collected from patients with transparent lenses (age 63.1±3.8
years; male n=9; female n=9), and 85 samples from cataract patients
(age 69.2±1.7 years; male n=46; female n=39) were collected as
cataractous lenses. The samples were divided in half at random,
with one half used to measure mitochondrial gene expression (MTCO
mRNA), and the other half to measure ATP content; Table II shows the donor (patient)
characteristics of the two sample halves [measurement of MTCO mRNA;
normal lenses 64.2±7.1 years (male n=3, female n=3), cataractous
lenses 67.0±3.2 years (male n=17, female n=19); measurement of ATP:
normal lenses 62.5±4.4 y (male n=6, female n=6), cataractous lenses
70.8±1.7 years (male n=29, female n=20)], and Fig. 1 shows an image of lens
opacification monitored using an EAS and slit lamp microscope. MTCO
mRNA levels and ATP contents were measured by the revers
transcription-quantitative polymerase chain reaction (RT-qPCR)
method and the luciferin-luciferase assay method, respectively.
Samples for PCR were stored in RNA later® solution or
liquid nitrogen, and samples for ATP measurement were placed in
liquid nitrogen immediately after removal. All experiments were
carried out in accordance with the ARVO guiding principles, and
approved by the Kanazawa Medical University Research Ethics
Committee (project identification code 96, 28 May 2014), and the
Kindai University School of Pharmacy Committee for Research Ethics
(project identification code 13–046, 7 September 2013).
 | Table I.Number of patients in each cataract
group. |
Table I.
Number of patients in each cataract
group.
| Type | n | Type
(mixed-cataract) | n |
|---|
| COR | 35 | COR+NUC | 7 |
| NUC | 22 | COR+PSC | 2 |
| PSC | 8 | COR+NUC+PSC | 3 |
|
|
| NUC+PSC | 8 |
 | Table II.Characteristics of patients in this
study. |
Table II.
Characteristics of patients in this
study.
|
| Measurement of MTCO
mRNA | Measurement of
ATP |
|---|
|
|
|
|
|---|
|
| n | n |
|---|
|
|
|
|
|---|
| Type | Grade-1 | Grade-2 | Grade-3 | Grade-1 | Grade-2 | Grade-3 |
|---|
| COR | 5 | 6 | 6 | 11 | 10 | 9 |
| NUC | 10 | 4 | 3 | 12 | 6 | 3 |
| PSC | 3 | 4 | 7 | 3 | 3 | 4 |
RT-qPCR method
Gene expression was measured using a LightCycler DX
400 according to the manufacturer's instructions and our previous
report (23). Briefly, total RNA
was extracted from the enucleated lens epithelium samples, and
purified using an RNase-Free DNase Set and RNeasy Min Kit. Reverse
transcription (RT) was carried out with an RNA PCR kit, and the PCR
reaction was performed using LightCycler FastStart DNA Master
SYBR-Green I. The RT reaction was carried out at 42ºC for 15 min,
followed by 5 min at 95ºC, and the conditions for PCR were as
follows: 95ºC for 10 min (Hot start), 60 cycles of 95ºC for 10 sec
(denaturing), 63ºC for 10 sec (annealing), and 72ºC for 5 sec
(extension). In addition, the following specific primers (final
concentration 10 pmol) were used: 5′-CCGTCCTAATCACAGCAGTCCTA-3′ and
5′-TGAGGTTGCGGTCTGTTAGTAGT-3′ for MTCO1;
5′-CCGCCATCATCCTAGTCCTCAT-3′ and 5′-GATCGTTGACCTCGTCTGTTATGT-3′ for
MTCO2; 5′-ACGGCATCTACGGCTCAACA-3′ and 5′-TGGCGGATGAAGCAGATAGTGA-3′
for MTCO3; 5′-GTGGCATCCACGAAACTACC-3′ and
5′-CAGGGCAGTGATCTCCTTCT-3′ for β-actin. The differences in the
threshold cycles for target groups (MTCO1, 2 and 3) and β-actin
were used to calculate the levels of mRNA expression in the human
lens epithelium samples (18,19,23).
β-actin was detected in 24.6±0.5 cycles, and the expression levels
were similar among all normal and cataract patients with COR, NUC
and PSC. On the other hand, the β-actin mRNA expression level was
lower than that in widely used HLE cells (12.3±0.8 cycles, 23)
Measurement of ATP
ATP was measured by the luciferin-luciferase assay
method (18,19). Briefly, human lens epithelium
samples were homogenized in 100 µl of saline, and centrifuged at
20,400 g for 15 min. The resultant supernatant was assayed using an
ATP Bioluminescent Assay Kit and a luminometer AB-2200 (Atto
Corporation, Tokyo, Japan) according to the manufacturer's
instructions. The protein levels were determined using a Bio-Rad
Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA) with
bovine serum albumin as the standard, and ATP levels are expressed
as nmol/mg protein. The protein levels were 5.9±0.4 mg with no
significant differences among the normal and cataract patients with
COR, NUC and PSC. There are previous reports that ATP levels in the
total lens of humans is approximately 0.45 mmol/g lens (Iwata and
Takehana, 1982), and the lens weight is approximately 200 mg. In
this study, we used the central part of anterior capsule (5 mm
diameter) with lens epithelial cells (approximately 10 mg) for the
measurement of ATP levels, and found total ATP levels of
approximately 4.13 nmol/sample (82.6 nmol/200 mg, 0.41 mmol/g) in
agreement with the previous report.
Statistical analysis
All data are expressed as the mean ± S.E.M, and
unpaired Student's t-test, Aspin-Welch's t-test, or one-way ANOVA
followed by Dunnett's multiple comparison were used for statistical
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of age and gender on MTCO gene
expression in lens epithelium of cataract patients
Before we investigated whether mitochondrial
function changes in lenses according to the type and severity of
cataract, we demonstrated the effects of age and gender on the MTCO
mRNA and ATP contents of lens epithelium from cataract patients.
Fig. 2A-C shows the correlation
between MTCO gene expression in lens epithelium and age, confirming
that MTCO mRNA levels in cataractous lens epithelium do not change
with age (r values for MTCO1, 2, 3 vs. age: 0.192, 0.238 and
0.1975, respectively). In addition, age had no effect on the ATP
content of cataractous lens epithelium (Fig. 2D, r=0.13). Fig. 3 shows no differences in the MTCO
mRNA levels and ATP contents of lens epithelium between male and
female cataract patients.
 | Figure 2.Association between age and MTCO (A)
[MTCO1/β-actin, (B) MTCO2/β-actin and (C) MTCO3/β-actin] mRNA
expression levels and (D) ATP content in normal and opaque lens
epithelium of Japanese patients. The patient characteristics are
shown in Tables I and II (the data for Figs. 2A, B and C are all from the same
lens epithelium samples, and include normal, COR, NUC, PSC,
COR+NUC, COR+PSC, COR+NUC+PSC, and NUC+PSC. Open circles,
transparent lens epithelium (clear, control); closed circles,
opaque lens epithelium. MTCO, mitochondrial cytochrome c oxidase;
COR, cortical opacification; NUC, nuclear opacification; PSC,
posterior subcapsular opacification. |
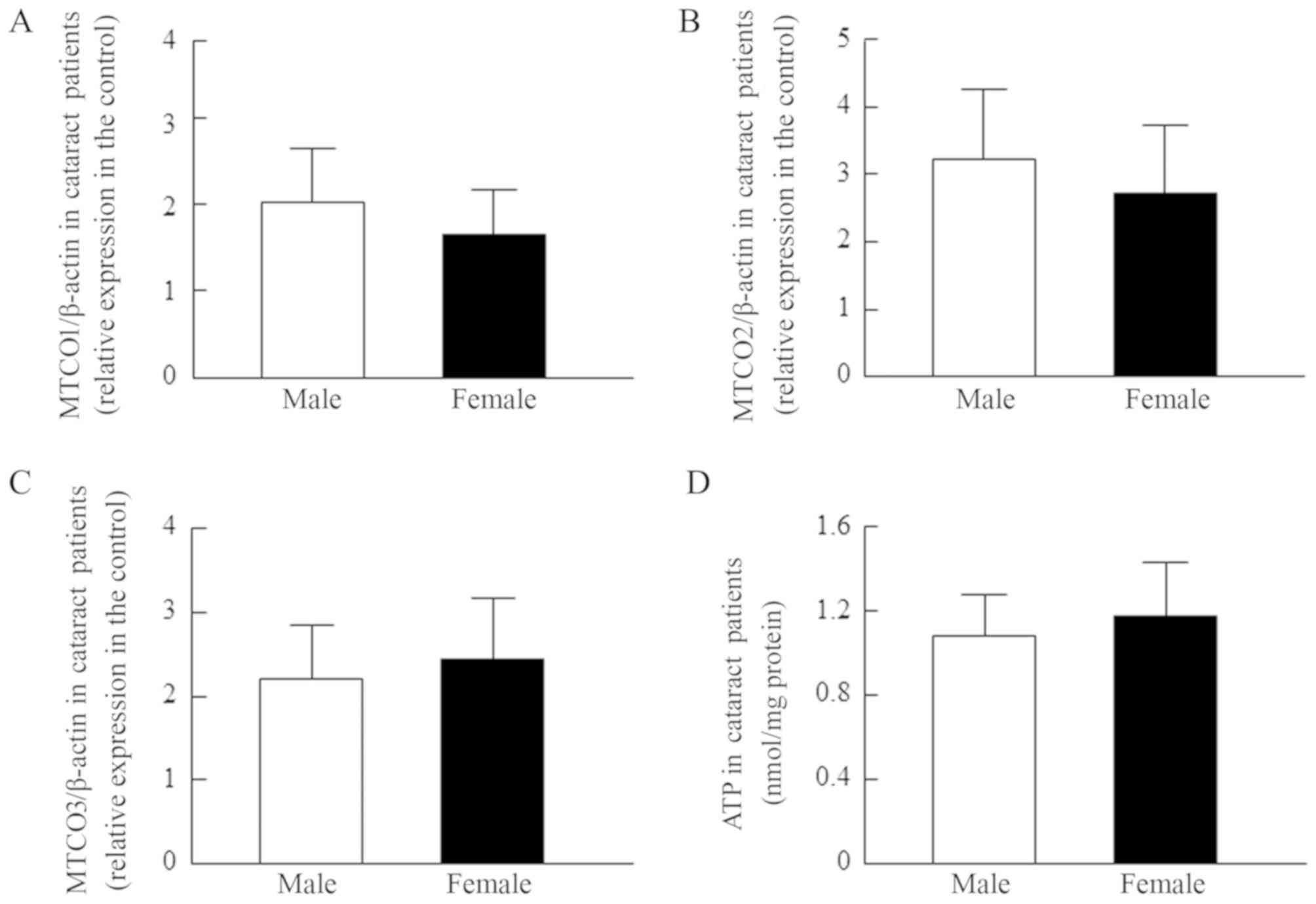 | Figure 3.MTCO [(A) MTCO1/β-actin, (B)
MTCO2/β-actin and (C) MTCO3/β-actin] mRNA expression levels and (D)
ATP content in the lens epithelium of male and female patients with
cataracts. The patient characteristics are shown in Tables I and II (the data for Figs. 3A, B and C are all from the same
lens epithelium samples, and include COR, NUC, PSC, COR+NUC,
COR+PSC, COR+NUC+PSC, and NUC+PSC). The MTCO mRNAs were expressed
as the relative expression in the control. The findings indicated
that sex is unrelated to the MTCO mRNA levels or ATP content of
lens epithelium. MTCO, mitochondrial cytochrome c oxidase; COR,
cortical opacification; NUC, nuclear opacification; PSC, posterior
subcapsular opacification. |
Changes in mitochondrial function in
lens epithelium in relation to cataract severity in patients with
COR, NUC and PSC
Figs. 4–6 show the MTCO1, MTCO2, and MTCO3 mRNA
levels and ATP contents in relation to the grade of COR (Fig. 4), NUC (Fig. 5) and PSC (Fig. 6) in cataract patients. For patients
with COR, the MTCO1-3 mRNA levels are significantly enhanced in
grade-1 cataracts, while the ATP content is increased in grades-1
and 2. At more advanced stages, the levels appear to normalize.
Similar to the results for patients with COR, the MTCO1-3 mRNA
levels in the lens epithelium of patients with NUC, were
significantly higher in grade-1, and the ATP content tended to be
enhanced in grades-1 and 2; however, the MTCO3 mRNA level was
obviously decreased in grade-3 NUC, and the ATP content in grade-3
NUC was significantly lower than in normal lens. In the lens
epithelium of patients with grade-1 PSC, both the MTCO1-3 mRNA
levels and ATP content were significantly enhanced, but
subsequently, the levels decreased. In grade-3 PSC, the MTCO1-3
mRNA levels and ATP content of the lens epithelium were
significantly lower than in normal lens.
 | Figure 4.Changes in MTCO [(A) MTCO1/β-actin,
(B) MTCO2/β-actin and (C) MTCO3/β-actin] mRNA expression levels and
(D) ATP content according to the opacity grade of patients with
COR. MTCO and ATP levels were measured in the epithelium of COR
patients. The patient characteristics (grade-1, 2, and 3) are shown
in Tables I and II, and the MTCO mRNAs were expressed as
the relative expression in the control. Open columns, transparent
lens epithelium (clear, control); closed columns, opaque (grade-1,
2, and 3) lens epithelium. n=5-12. *P<0.05, vs. control. The
MTCO1-3 mRNA levels were significantly enhanced in grade-1 COR, and
ATP content was significantly elevated in grade-2 COR;
subsequently, the levels normalized. MTCO, mitochondrial cytochrome
c oxidase; COR, cortical opacification; NUC, nuclear opacification;
PSC, posterior subcapsular opacification. |
 | Figure 6.Changes in [(A) MTCO1/β-actin, (B)
MTCO2/β-actin and (C) MTCO3/β-actin] mRNA expression levels and (D)
ATP content according to the opacity grade of patients with PSC.
MTCO and ATP levels were measured in the epithelium of PSC
patients. The patient characteristics (grade-1, 2, and 3) are shown
in Tables I and II, and the MTCO mRNA were expressed as
the relative expression in the control. Open columns, transparent
lens epithelium (clear, control); closed columns (grade-1, 2, and
3), opaque lens epithelium. n=3-12. *P<0.05, vs. control. The
MTCO1-3 mRNA expression levels and ATP content in grade-1 PSC were
enhanced. On the other hand, in grade-3 PSC, the MTCO1-3 mRNA
levels and ATP content were significantly lower than in normal
patients. MTCO, mitochondrial cytochrome c oxidase; PSC, posterior
subcapsular opacification. |
 | Figure 5.Changes in [(A) MTCO1/β-actin, (B)
MTCO2/β-actin and (C) MTCO3/β-actin] mRNA expression levels and (D)
ATP content according to the opacity grade of patients with NUC.
MTCO and ATP levels were measured in the epithelium of NUC
patients. The patient characteristics (grade-1, 2, and 3) are shown
in Tables I and II, and the MTCO mRNAs were expressed as
the relative expression in the control. Open columns, transparent
lens epithelium (clear, control); closed columns (grade-1, 2, and
3), opaque lens epithelium. n=3-12. *P<0.05, vs. control. The
MTCO1-3 mRNA levels in grade-1 NUC were significantly enhanced, and
the ATP content also tended to be increased in grade-1 and grade-2
NUC. However, in grade-3 NUC, the MTCO3 mRNA levels and ATP content
were significantly lower than in normal patients. MTCO,
mitochondrial cytochrome c oxidase; NUC, nuclear opacification;
PSC, posterior subcapsular opacification. |
Discussion
The disintegration of ion balance in the lens causes
opacification, resulting in blurred vision and blindness. We
previously reported that decreases in ATPase activity and function
leads to ion balance disintegration in the lens of models for human
senile cataracts and in HLE cells, and that the decreases in ATPase
activity and function are caused by lipid peroxidation and the
collapse of ATP production via mitochondrial damage by the ROS
stimulation, respectively (14,17–19,23).
However, there is no report on mitochondrial function in the lens
as it relates to cataract severity. In the present study, we
demonstrate the changes of MTCO mRNA levels and ATP content
according to the type and severity of cataracts using lens
epithelium from patients with 3 different types of opacification
(COR, NUC and PSC). We have found that the MTCO and ATP contents
(ATP production) in the lens epithelium is enhanced in early-stage
cataracts (mild cataracts), while ATP production is decreased in
severe cataracts. The decrease in ATP production may precede lens
opacification.
ATP production takes place in the mitochondria, and
CCO, the terminal enzyme in the mitochondrial respiratory chain,
plays an important role in ATP production. CCO contains 13 subunits
per monomer. Of these, CCO-4, 5a, 5b, 6a, 6b, 6c, 7a, 7b, 7c and 8
(10 subunits) are synthesized on cytoplasmic ribosomes and imported
into the mitochondria (24–27).
These 10 subunits are encoded in the nuclear DNA. On the other
hand, the remaining three subunits (mitochondrial subunits, MTCO)
are encoded in the mitochondrial genome, and these catalytic
subunits are the largest of the CCO subunits (mitochondrial CCO,
MTCO). The three MTCO subunits (MTCO1, 2 and 3) are down-regulated
earlier and more severely than the 10 subunits synthesized on
cytoplasmic ribosomes (nuclear subunits) in response to functional
inactivation (21). Based on these
previous reports, we measured the gene expression of mitochondrial
genome (MTCO1, 2 and 3) as an indicator of ATP production in this
study. In addition, we determined the ATP contents in the lens
epithelium of Japanese patients.
First, we investigated whether the ATP production
and content in the central lens epithelium differs by age (Fig. 2) or gender (Fig. 3). There was no correlation between
any of the three MTCO mRNA levels and age in lens epithelium of
Japanese patients (MTCO1, r=0.192; MTCO2, r=0.238;
MTCO3, r=0.1975), and no differences were observed between
males and females (Fig. 3). In
addition, the r value for the ATP content vs. age was 0.13,
and the ATP contents in lens epithelium of patients with cataracts
was also similar between males and females. From these results, we
could ignore the differences in age and gender among the samples,
and use them for the following investigation.
Next, we investigated the changes in MTCO mRNA
levels and ATP content in lens epithelium according to the type and
severity of cataract. It is commonly assumed that there are three
major types of cataracts: COR, NUC, and PSC (28). Therefore, we classified the
opacification as one of these three types, and measured the MTCO
mRNA levels and ATP contents according to the grade as shown in
Tables I and II.
For all three types of cataracts examined (COR, NUC
and PSC), the MTCO mRNA levels in the lens epithelium peaked in
grade-1, and subsequently decreased with increasing opacity
(Figs. 4–6). The MTCO mRNA levels in the lens
epithelium of patients with grade-3 PSC were significantly lower
than in normal lens. The MTCO3 mRNA levels in the grade-3 NUC
samples were also decreased in comparison with normal lens, but the
levels in the grade-3 COR lens epithelium were similar to those in
normal lens (Figs. 4–6). Moreover, the same result was found
for the ATP content (the behavior of the MTCO mRNA levels and ATP
content were both similar). These results suggest that ATP
production in the lens epithelium of Japanese patients is enhanced
in early-stage cataracts (mild cataracts), and that ATP production
is decreased in severe cataracts. The peak in the MTCO mRNA levels
was apparent in grade-1 cataracts, while the peak in ATP content
was observed in grades-1,2 COR and grade-2 NUC. CCO is a product of
mitochondrial genomes (MTCO1, 2 and 3) and nuclear genomes (CCO-4,
5a, 5b, 6a, 6b, 6c, 7a, 7b, 7c and 8) (24–27),
and we measured only the mitochondrial genomes by RT-qPCR methods.
Therefore, the expression of MTCO mRNAs for the nuclear genomes and
CCO activity may be related to a time lag. On the other hand, the
decreased ATP levels may be preceded by altered ATP hydrolysis. The
contribution of MTCO to ATP production and ATP hydrolysis in the
lenses of Japanese patients will be examined in a future study.
It is important to discuss the mechanism for the
increase and subsequent decrease in MTCO mRNA levels. The
epithelial mRNA values for stage 1 cortical and nuclear cataracts
are all significantly higher than those for normal epithelium
(Figs. 4 and 5), even though cortical cataracts are
osmotic while nuclear cataracts are not. Multiple factors likely
contribute to the development of age-related cataracts (13,29–31),
and many different factors could have led to the observed changes
in MTCO mRNA expression and ATP content (18,19,23,32).
For example, it has been reported that ATP production in the lens
of UPLR is enhanced by mild oxidative stress, such as by nitric
oxide (protective mechanism), while excessive oxidative stress
causes damage to the mitochondrial genome, resulting in a decrease
in the ATP content of the lens (20,32).
Therefore, both changes (increase and decrease) in lens MTCO mRNA
levels could be caused by ROS, and the differences in ROS levels in
various parts of the lens may be related to the ATP contents as
well as the cataract type and classification. In addition, it is
necessary to discuss the relationship between mitochondrial
dysfunction and the process of cataract development. Previous
reports have shown that a decrease in ATP content via mitochondrial
damage in the lens induces a decrease in
Na+/K+-ATPase and Ca2+-ATPase
function, with the resulting collapse in mineral balance enhancing
the Ca2+ content of the lens. This enhanced
Ca2+ level accelerates lens opacification (11,13,18,19).
In Japanese patients with COR, NUC and PSC, the MTCO mRNA levels
are enhanced in early-stage cataracts, and then, in the case of NUC
and PSC, decrease in severe cataracts (grade-3, Figs. 4–6). It has been reported that neither
doubling the ATP concentration nor halving it has much impact on
the Na+/K+-ATPase or Ca2+-ATPase
activity of most cells (33–35).
That the ATP level in grade-3 NUC is 60.7% that of normal patients
support previous studies in which the development of NUC does not
involve calcium alterations in the lens. Taking these findings
together, we hypothesize that mitochondrial function is enhanced in
early-stage cataracts by multiple factors, such as a protective
mechanism against ROS. This enhanced mitochondrial function
supports the regulation of ion balance in the mildly opaque lens.
However, in severe PSC, the decrease in MTCO mRNA levels may
promote the progression of lens opacification via a dysfunction in
ATP production. Further studies are needed to confirm the mRNA and
protein levels of other nuclear subunits of CCO (CCO-4, 5a, 5b, 6a,
6b, 6c, 7a, 7b, 7c and 8), since the lack of the investigation of
the protein expression of all the subunits of CCO as a limitation
of the present study. In addition, it is important to elucidate the
precise relationship between mitochondrial damage and cataract
development. Therefore, we are now investigating the changes in ROS
and CCO activity in the lens epithelium of Japanese patients, and
will demonstrate the protein expression of CCO and localization of
mitochondria in the opaque lens.
In conclusion, we measured the MTCO gene expression
and ATP content in lens epithelium of Japanese patients with
cataracts, and found that ATP production is enhanced in early-stage
cataracts, and is then decreased in severe cataracts in comparison
with transparent lens epithelium. ATP depletion in the case of
grade-3 PSC may promote the development of lens opacification
through a dysfunction in ATPase. However, these data all derive
from Japanese patients; therefore, it is important to determine if
these findings are applicable to other populations as well. These
results provide significant information for the elucidation of the
mechanisms of cataract development.
Acknowledgements
Not applicable.
Funding
The present study was supported by Daiwa Securities
Health Foundation (Grant code 21).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
NN created the concept for the study, designed the
protocol, and wrote the manuscript; YM and HO measured the ATP
contents and mRNA levels; TS, EK and HS collected the enucleated
lens epithelium (samples) during cataract surgery. All authors
contributed to the conception and design of the study, and to the
interpretation of the data.
Ethics approval and consent to
participate
All experiments were carried out in accordance with
the ARVO guiding principles, and approved by the Kanazawa Medical
University Research Ethics Committee (project identification code
96, 28 May 2014) and the Kindai University School of Pharmacy
Committee for Research Ethics (project identification code 13–046,
7 September 2013). Informed consent was obtained from patients
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Ca2+
|
calcium ion
|
|
CCO
|
cytochrome c oxidase
|
|
COR
|
cortical opacification
|
|
HLE
|
human lens epithelial
|
|
K+
|
potassium ion
|
|
Na+
|
sodium ion
|
|
MTCO
|
mitochondrial cytochrome c oxidase
|
|
NUC
|
nuclear opacification
|
|
PCR
|
polymerase chain reaction
|
|
PSC
|
posterior subcapsular
opacification
|
|
ROS
|
reactive oxygen species
|
|
RT
|
reverse transcription
|
References
|
1
|
Angra SK, Murthy GV, Gupta SK and Angra V:
Cataract related blindness in India & its social implications.
Indian J Med Res. 106:312–324. 1997.PubMed/NCBI
|
|
2
|
Ezegwui IR, Aghaji AE, Uche NJ and
Onwasigwe EN: Challenges in the management of paediatric cataract
in a developing country. Int J Ophthalmol. 4:66–68. 2011.PubMed/NCBI
|
|
3
|
Furtado JM, Lansingh VC, Carter MJ,
Milanese MF, Peña BN, Ghersi HA, Bote PL, Nano ME and Silva JC:
Causes of blindness and visual impairment in Latin America. Surv
Ophthalmol. 57:149–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leske MC, Chylack LT Jr and Wu SY: The
lens opacities case-control study. Risk factors for cataract. Arch
Ophthalmol. 109:244–251. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hammond CJ, Snieder H, Spector TD and
Gilbert CE: Genetic and environmental factors in age-related
nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med.
342:1786–1790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iyengar SK, Klein BE, Klein R, Jun G,
Schick JH, Millard C, Liptak R, Russo K, Lee KE and Elston RC:
Identification of a major locus for age-related cortical cataract
on chromosome 6p12-q12 in the beaver dam eye study. Proc Natl Acad
Sci USA. 101:14485–14490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duncan G and Bushell AR: Ion analyses of
human cataractous lenses. Exp Eye Res. 20:223–230. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maraini G and Mangili R: Differences in
protein and in the water balance of the lens in nuclear and
cortical types of senile cataract. The Human Lens in Relation to
Cataract. CIBA Foundation Symposium. Elliott K and Fitzsimons DW:
Elsevier; Amsterdam, Netherlands: pp. 79–95. 1973
|
|
9
|
Patmore L and Duncan G: The physiology of
lens membranes. Mechanisms of Cataract Formation in the Human Lens.
Duncan G: Academic Press; London, England: pp. 193–217. 1981
|
|
10
|
Davies PD, Duncan G, Pynsent PB, Arber DL
and Lucas VA: Aqueous humour glucose concentration in cataract
patients and its effect on the lens. Exp Eye Res. 39:605–609. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwata S and Takehana N: Biochemical
studies on human cataract lens. II. Opacity-related changes of
cations, ATP and GSH in various types of human senile cataracts.
Yakugaku Zasshi. 102:940–945. 1982.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta PD, Johar K and Vasavada A:
Causative and preventive action of calcium in cataracto-genesis.
Acta Pharmacol Sin. 25:1250–1256. 2004.PubMed/NCBI
|
|
13
|
Iwata S: Crystalline Lens. Suishotai, in
Japanease. Iwata S: Medical-Aoi Publication Press; Tokyo, Japan:
pp. 355–360. 1986
|
|
14
|
Nagai N, Ito Y and Takeuchi N: Inhibitive
effects of enhanced lipid peroxidation on Ca(2+)-ATPase in lenses
of hereditary cataract ICR/f rats. Toxicology. 247:139–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blaustein MP: Endogenous ouabain: Role in
the pathogenesis of hypertension. Kidney Int. 49:1748–1753. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beebe DC, Holekamp NM and Shui YB:
Oxidative damage and the prevention of age-related cataracts.
Ophthalmic Res. 44:155–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagai N, Ito Y, Takeuchi N Usui S and
Hirano K: Comparison of the mechanisms of cataract development
involving differences in Ca(2+) regulation in lenses among three
hereditary cataract model rats. Biol Pharm Bull. 31:1990–1995.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagai N and Ito Y: Adverse effects of
excessive nitric oxide on cytochrome c oxidase in lenses of
hereditary cataract UPL rats. Toxicology. 242:7–15. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagai N and Ito Y: Dysfunction in
cytochrome c oxidase caused by excessive nitric oxide in human lens
epithelial cells stimulated with interferon-γ and
lipopolysaccharide. Curr Eye Res. 37:889–897. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nabekura T, Tomohiro M, Ito Y and Kitagawa
S: Changes in plasma membrane Ca2+ -ATPase expression and ATP
content in lenses of hereditary cataract UPL rats. Toxicology.
197:177–183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang HL, Ongwijitwat S and Wong-Riley MT:
Bigenomic functional regulation of all 13 cytochrome c oxidase
subunit transcripts in rat neurons in vitro and in vivo.
Neuroscience. 140:177–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thylefors B, Chylack LT Jr, Konyama K,
Sasaki K, Sperduto R, Taylor HR and West S; WHO Cataract Grading
Group, : A simplified cataract grading system. Ophthalmic
Epidemiol. 9:83–95. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagai N, Ito Y, Shibata T, Kubo E and
Sasaki H: A positive feedback loop between nitric oxide and amyloid
β (1–42) accelerates mitochondrial damage in human lens epithelial
cells. Toxicology. 381:19–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kadenbach B, Jarausch J, Hartmann R and
Merle P: Separation of mammalian cytochrome c oxidase into 13
polypeptides by a sodium dodecyl sulfate-gel electrophoretic
procedure. Anal Biochem. 129:517–521. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuhn-Nentwig L and Kadenbach B: Isolation
and properties of cytochrome c oxidase from rat liver and
quantification of immunological differences between isozymes from
various rat tissues with subunit-specific antisera. Eur J Biochem.
149:147–158. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taanman JW: Human cytochrome c oxidase:
Structure, function, and deficiency. J Bioenerg Biomembr.
29:151–163. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lenka N, Vijayasarathy C, Mullick J and
Avadhani NG: Structural organization and transcription regulation
of nuclear genes encoding the mammalian cytochrome c oxidase
complex. Prog Nucleic Acid Res Mol Biol. 61:309–344. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livingston PM, Carson CA and Taylor HR:
The epidemiology of cataract: A review of the literature.
Ophthalmic Epidemiol. 2:151–164. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shearer TR, David LL, Anderson RS and
Azuma M: Reviewof selenite cataract. Curr Eye Res. 11:357–369.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dilsiz N, Olcucu A and Atas M:
Determination of calcium, sodium, potassium and magnesium
concentrations in human senile cataractous lenses. Cell Biochem
Funct. 18:259–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shun Shin GA, Bron AJ, Brown NP and
Sparrow JM: The relationship between central nuclear scatter and
perinuclear retrodots in the human crystalline lens. Eye (Lond).
6:407–410. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fariss MW, Chan CB, Patel M, Van Houten B
and Orrenius S: Role of mitochondria in toxic oxidative stress. Mol
Interv. 5:94–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Skou JC and Esmann M: Effects of ATP and
protons on the Na: K selectivity of the (Na+ + K+)-ATPase studied
by ligand effects on intrinsic and extrinsic fluorescence. Biochim
Biophys Acta. 601:386–402. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fu YF, Schuurmans Stekhoven FM, Swarts HG,
de Pont JJ and Bonting SL: The locus of nucleotide specificity in
the reaction mechanism of (Na+ + K+)-ATPase determined with ATP and
GTP as substrates. Biochim Biophys Acta. 817:7–16. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang YC and Yingst DR: Effects of
intracellular free Ca and rate of Ca influx on the Ca pump. Am J
Physiol. 256:C1138–C1144. 1989. View Article : Google Scholar : PubMed/NCBI
|




















