Introduction
Breast cancer, considered heterogeneous cancer, both
biologically and molecularly, is the most common malignancy in
women worldwide (1). Breast cancer
still remains the first cause of cancer-related deaths among women,
especially in developing countries in which 60% of breast
cancer-related deaths are reported (2). In spite of being treated by different
therapeutic approaches including chemotherapy, radiotherapy,
surgery, hormone therapy and other targeted therapies, breast
cancer is facing important issues such as high long-term mortality,
drug-resistance, and metastases (3). Thus, search for effective alternative
therapies with fewer side effects has become necessary.
In Africa, up to 80% of the population uses
traditional medicine for primary health care (4). Algeria, the biggest African country
with a large variety of soils (littoral, steppe, mountains, and
desert) and climates, possesses a rich flora (more than 3,000
species and 1,000 genders) (5). In
Algeria, many patients use medicinal plants as a treatment for many
ailments and serious diseases, such as cancer, diabetes and
arterial hypertension, for several considerations: Historical,
cultural and economic (6–8). Although few studies have been
published on ethnobotanical and pharmacological properties of
Algerian medicinal plants, several species have been found to be
used by Algerian breast cancer patients such as Aristolochia
longa L., Berberis vulgaris L., Thymus vulgaris
L., Prunus persica (L.) Batsch, and Artemisia
herba-alba L. (9). Previously,
we have demonstrated that aqueous extracts of Aristolochia
longa roots, an Algerian medicinal plant widely used in cancer
therapy by local populations, induced apoptosis of Burkitt's
lymphoma BL41 cell line by targeting the mitochondrial pathway
(10). An aqueous extract of
Aristolochia longa induced cell growth inhibition in HBL100
and MDA-MB-231 triple negative breast cancer cells in a
dose-dependent manner (11). Other
extracts of Aristolochia longa aerial parts exhibited
promising antioxidant and anticancer activities in different cell
lines (data not published).
Bryonia dioica Jacq. (white bryony) a
climbing perennial herb with tuberous roots is locally named in
Algeria ‘Fachira’ and ‘queriou'aa’ by the locals. The species grows
in North Africa, temperate Europe, and Western Asia (12). The roots of B. dioica are
characterized by the presence of cucurbitacins, oxygenated
tetra-cyclic triterpenoids possessing anti-inflammatory,
anti-infectious, cytotoxic and apoptogenic effects (9,13).
The plant is used to treat several ailments such as asthma,
bronchitis, hypertension, gastric ulcers, and diabetes (14). Important biological and therapeutic
effects of B. dioica were demonstrated such as antidiabetic
(15), antibacterial (16) and antioxidant activities (17). The plant possesses also important
anticancer activities. Indeed, we have previously shown that B.
dioica aqueous extract induced apoptosis in Burkitt's lymphoma
BL41 cell line by triggering the intrinsic pathway (18). Besides, B. dioica aqueous
extracts exhibited promising cytotoxic activities against different
blood and breast cancer cell lines (data not published).
As part of our continuing work to evaluate the
anticancer activity of Algerian medicinal plants used in cancer
treatment, the present study aimed to evaluate apoptogenic activity
and identify the major bioactive compound of B. dioica.
Materials and methods
Reagents
Cell culture media,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and Propidium iodide were purchased from Sigma-Aldrich (St. Louis,
MO, USA). Annexin V-FITC was from BD Biosciences (Franklin Lakes,
NJ, USA). The other generic chemicals were from Sigma-Aldrich,
Roche Applied Science (Mannheim, Germany) or Merck KGaA, Darmstadt,
Germany.
Cells and culture conditions
The human triple-negative breast cancer MDA-MB-231
cell line was obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The cells were cultured in DMEM medium
with Glutamax supplemented with 10% FCS, 100 U/ml penicillin and
100 µg/ml streptomycin, in a humidified atmosphere with 5%
CO2 in air at 37°C. The experiments were performed three
times using cells in the exponential growth phase.
Preparation of B. dioica aqueous
extract
The roots of B. dioica were collected in
March 2012 in Mascara, Algeria. Botanical identification and
authentication were done by Dr Kada Righi (Department of
Agriculture, Faculty of Nature and Life sciences, Mascara
University, Mascara, Algeria). A voucher specimen of the plant
(voucher no. LRSBG/B-2012/003) was deposited in the herbarium of
the laboratory, Department of Biology, Faculty of Nature and Life
sciences, Mascara University, Mascara, Algeria. The collected roots
were dried at room temperature, pulverized and finely sieved. The
B. dioica aqueous extract was prepared as follows: the dried
roots were boiled for 20 min at 100°C, cooled to room temperature,
and then filtered. The filtered solution was collected,
concentrated, lyophilized and stored in a desiccator at +4°C until
used.
MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
tetrazolium reduction assay
The effects of the B. dioica aqueous extract
on MDA-MB-231 cells viability were determined by the colorimetric
MTT assay. Briefly, MDA-MB-231 cells were seeded at a density of
8×103 cells/well in 96-well plates and incubated for 24
h at 37°C. Thereafter, cells were treated with increasing
concentrations (from 0 to 500 µg/ml) of B. dioica aqueous
extract for 24, 48, and 72 h. At the end of the treatment, 50 µl of
MTT (0.5 mg/ml) was added and the cells were incubated at 37°C, 5%
CO2 for 1 h. After medium removal, 500 µl of DMSO was
added to each well to dissolve the formazan formed during the
reaction and the plate was then shaken for 10 min under obscurity.
The absorbance was recorded at 570 nm using a 96-well plate reader
(ASYS-UVM-340). All the experiments were performed in
triplicate.
Detection of apoptosis-Annexin
V-FITC/PI staining
Apoptosis induction was assessed using the Annexin
V-FITC assay. Briefly, MDA-MB-231 were treated with 50 and 250
µg/ml of B. dioica aqueous extract for 48 h. Cells were
harvested, re-suspended in the ice-cold 1× binding buffer, and then
incubated with Annexin V-FITC and PI solutions for 15 min at room
temperature in the dark. After incubation, the cells were analyzed
using FACSCalibur, BD Biosciences (19).
Cell cycle analysis
Following exposure to B. dioica aqueous
extract (50 and 250 µg/ml) for 48 h, MDA-MB-231 cells were
collected and fixed with cold 70% ethanol and stored overnight at
−20°C. Cells were washed, re-suspended in PBS and incubated at 37°C
for 30 min with 10 mg/ml RNase and 1 mg/ml propidium iodide (PI).
DNA content was then determined using a FACSCalibur flow cytometer
(BD Biosciences) (20).
UV-vis analysis
UV-vis analysis of B. dioica aqueous extract
was performed on a Shimadzu spectrophotometer (λ=200–800 nm) as
described by (21). The absorption
peak values were recorded.
Chromatographic analyses
To determine the major compounds in the B.
dioica aqueous extract, we performed liquid chromatography-mass
spectrometric analyses using HPLC Agilent 1100 (Agilent
Technologies GmbH, Waldbronn, Germany) coupled to an ultraviolet
detector and to an Agilent Trap XCT mass spectrometer equipped with
an electrospray (ESI) source with a nebulizer spacer as previously
described (22).
Statistical analysis
Mean data values are presented, with their standard
deviations (mean ± SD). The statistical comparisons were made by
one-way analysis of variance followed by Bonferroni's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
B. dioica aqueous extract induced cell
growth inhibition of MDA-MB-231
In the present study, we investigated the effects of
an aqueous extract of B. dioica roots on cell viability
in vitro, by incubating MDA-MB-231 cells with increasing
concentrations (from 0 to 500 µg/ml) of the extract. After 24, 48
and 72 h, cell viability was determined by the colorimetric MTT
assay. We determined survival as a percentage of that for untreated
cells. As shown in Fig. 1, B.
dioica aqueous extract induced cell growth inhibition in a
time-dependent manner. At 50 µg/ml, B. dioica aqueous
extract resulted in 50.36, 72.39 and 91.15% inhibition of cell
viability of MDA-MB-231 cells after 24, 48 and 72 h,
respectively.
The highest inhibitory effect was produced at
concentrations of 50 µg/ml or higher after 72 h of treatment
(91.15±0.71%). Interestingly, this effect remains unchanged at
higher concentrations (100, 250 and 500 µg/ml).
B. dioica aqueous extract induced
apoptosis of MDA-MB-231
We then investigated whether this decrease in cell
viability was associated with the induction of apoptosis by
incubating MDA-MB-231 cells with 50 and 250 µg/ml of B.
dioica aqueous extract for 48 h. The apoptotic cell death rate
was estimated with Annexin V-FITC and PI double staining by flow
cytometry. The results showed that B. dioica aqueous extract
induced MDA-MB-231 apoptosis in a dose-dependent manner (Fig. 2). Indeed, apoptotic cells elevated
significantly from 38.95 to 64.61% of the MDA-MB-231 cells exposed
to 50 and 250 µg/ml, respectively. However, the apoptosis rate in
untreated cells was only 0.68%.
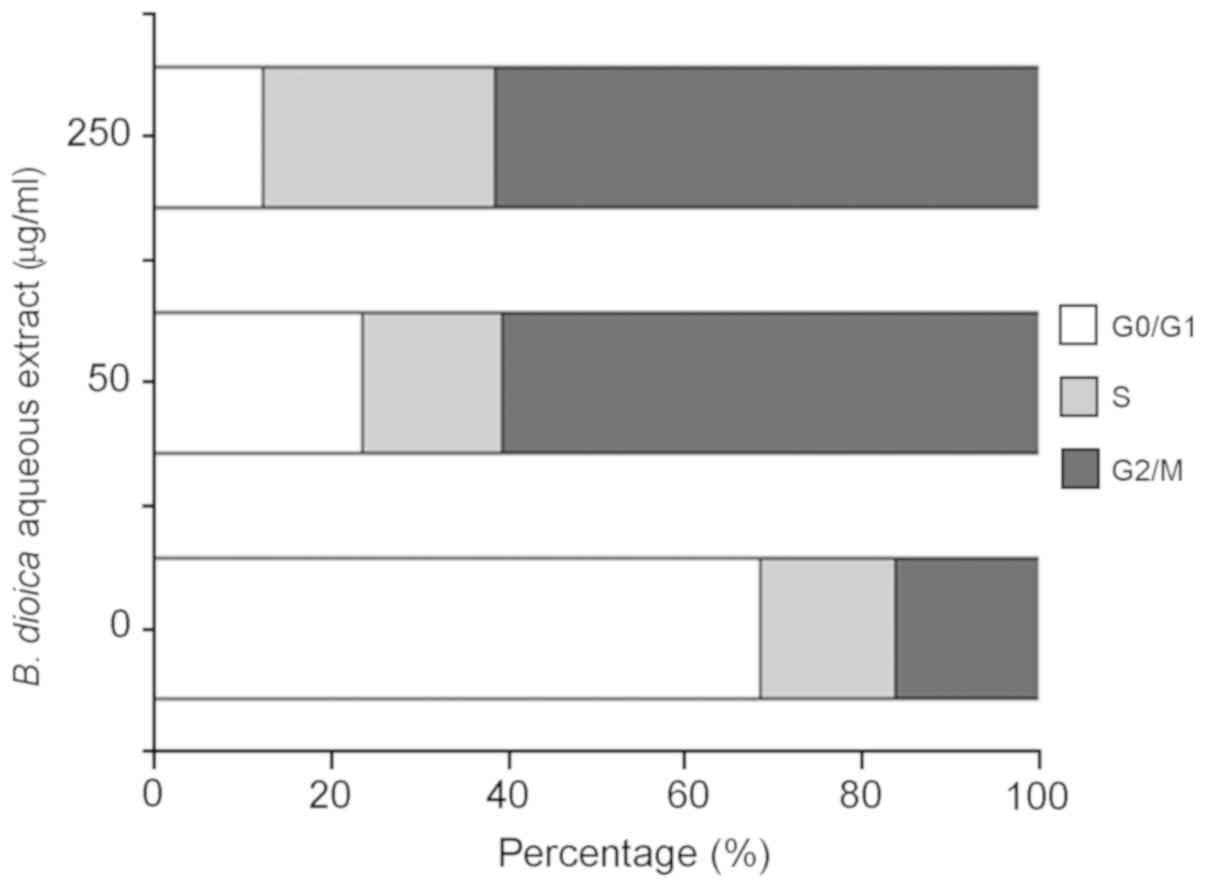
B. dioica aqueous extract induced cell
cycle arrest of MDA-MB-231
To examine the effect of B. dioica aqueous
extract on the cell cycle progression, MDA-MB-231 cells were
incubated for 48 h in the absence or presence of 50 and 250 µg/ml
of the extract. Cell nuclei were stained with PI and the percentage
of cells in each phase of the cell cycle were determined by flow
cytometry. As shown in Fig. 3, our
data revealed that treatment with B. dioica aqueous extract
caused cell cycle arrest of MDA-MB-231 cells at G2/M phase. In
fact, the percentage of cells in G2/M increased from 15.7%
(untreated cells) to 59.13% (50 µg/ml) and 58.51% (250 µg/ml).
Moreover, B. dioica aqueous extract resulted in a
dose-dependent decrease in the population of cells in the G0/G1
phase from 65.82% (untreated cells) to 23.00, and 11.80% after
treatment with 50 and 250 µg/ml, respectively.
Major compounds of B. dioica aqueous
extract
The UV-vis spectrum of B. dioica aqueous
extract was characterized by two major absorption bands: 350–385 nm
(Band I, cinnamoyl system) and 250–280 nm (Band II, benzoyl
system), corresponding to flavonol structure.
As shown in Fig. 4,
peak 2 corresponded to the major compound found in the B.
dioica extract. This compound was detected at 2.3 min of
retention time and represented 75.3% of the extract.
The MS spectra (Fig.
5) of the major compound (peak 2) showed a molecular ion of
m/z=318.5 and two main fragments (m/z=230.4 and 274.5). According
to the obtained data (Table I) and
in comparison with literature, we suggest that the major compound
in B. dioica aqueous extract could be myricetin (Fig. 6).
 | Table I.Compounds identified in B.
dioica aqueous extract in positive-ion mode. |
Table I.
Compounds identified in B.
dioica aqueous extract in positive-ion mode.
| Peak number | RT, min | Area fraction
percentage | (M-H)+(m/z) | MS2
Characteristic ions (m/z) | Compound |
|---|
| 1 | 1.3 | 24.7 | – | – | – |
| 2 | 2.3 | 75.3 | 318.5 | 318.5, 274.5,
230.4 | Myrecitin |
Discussion
Natural products provide an appreciable percentage
of new active lead molecules, and drugs despite competition from
different methods of drug discovery (23). Medicinal plants constitute a common
alternative for cancer treatment by providing cytotoxic and
apoptogenic agents (24–27). We have previously found that the
aqueous extract of B. dioica roots exerted a promising
anticancer activity against Burkitt's lymphoma BL41 cells. This
cytotoxic effect was accompanied by the induction of apoptosis
(18). Likewise, we have
demonstrated that the extract was able to inhibit different cancer
cell lines growth including those of multiple myeloma, lymphoma and
triple negative breast cancer (data not published). In the current
study, we showed that B. dioica aqueous extract induced cell
growth inhibition of breast cancer MDA-MB-231 cells in a
time-dependent manner. At 50 µg/ml, the extract suppressed
effectively the proliferation of MDA-MB-231 cells (91.15%
inhibition of proliferation) after 72 h. Recently, Sahpazidou et
al (28) evaluated the
cytotoxic effects of polyphenolic extracts from grape stems against
various cancer cell lines (breast, kidney, colon, and thyroid).
After 72 h of treatment, MDA-MB-231 cells were the most sensitive
to all tested extracts, with IC50 values of 120 and 184
µg/ml. We then investigated the apoptotic effects of B.
dioica aqueous extract in MDA-MB-231 cells.
Apoptosis is one of the main types of programmed
cell death and can be triggered by a variety of stimuli received by
the cells (29). Induction of
apoptosis in the activated cancer cells may be an effective
strategic approach for cancer therapy (30). We examined the induction of
apoptosis with Annexin V-FITC and PI double staining by flow
cytometry. Our results showed that the proportion of apoptotic
cells significantly elevated in MDA-MB-231 (50 and 250
µg/ml)-treated cells from 0.68% in untreated cells to 35.95 and
64.61%, respectively. These results are in agreement with our
previous study demonstrating that the B. dioica aqueous
extract was able to induce apoptosis of Burkitt's lymphoma BL41
cells in a dose-dependent manner. Apoptosis induction was
accompanied by triggering the intrinsic pathway (activation of
caspase-3 and −9, cleavage of PARP and loss of mitochondria
membrane potential) (18).
Moreover, it has been demonstrated that several herbal extracts
caused MDA-MB-231 cells growth inhibition through apoptosis
induction at high concentrations (31,32).
Our data showed that B. dioica aqueous extract was able to
induce marked apoptosis at a lower concentration (50 µg/ml) in
MDA-MB-231 cells, which are known to be resistant to apoptosis
(33).
Generally, cell cycle arrest and induction of
apoptosis are connected, an occurrence of cell cycle arrest leads
to cell apoptosis (34). In the
present study, the progression of cell cycle was assessed by
propidium iodide (PI) staining of cell DNA after incubation of
cells with B. dioica aqueous extract. Besides apoptosis
induction, the cytotoxic effect caused by B. dioica aqueous
extract was further due to a cell cycle arrest of MDA-MB-231 cells
at G2/M phase. In fact, treatment with B. dioica aqueous
extract resulted in an accumulation of MDA-MB-231 cells in the G2/M
phase (15.7% of untreated cells vs. 59.1% of treated cells with 50
µg/ml). This effect may be attributed to the presence of flavonols
(major compounds in the extract). It has been demonstrated that
flavonols such as quercetin or kaempferol induce G2/M phase cell
cycle arrest in different cancer cell lines (35). Zhang et al (36) demonstrated that three flavonols
(Kaempferol, quercetin, and myricetin) exerted cytotoxic effects on
a human oesophageal adenocarcinoma cell line (OE33) by inducing
G2/M arrest. Similarly, quercetin, myricetin, laricitrin, and
syringetin were capable of inhibiting the proliferation of
colorectal epithelial adenocarcinoma cells via cell cycle arrest in
the G2/M phase (37).
Taken together, our data demonstrate that B.
dioica aqueous extract-mediated inhibition of MDA-MB-231 cell
growth may be the result of apoptosis induction and cell-cycle
arrest in the G2/M phase.
The UV-vis absorption spectra of B. dioica
aqueous extract showed two absorption bands characteristic of
flavonol skeleton, 350–385 nm (Band I) and 250–280 nm (Band II)
indicating that the major compounds in the extract are flavonols
(38). Flavonols are characterized
by fully unsaturated C-rings that connect the A and B rings in a
single conjugated system. All flavonoids have aromatic
chromophores, as indicated by UV absorptions in the 250 nm region
of their UV spectra (Band B) (39). Band A lies in the 350–385 nm range
for flavonols (40). Flavonols,
considered as the strongest antioxidant flavonoids, exhibit
important antioxidant activity, mainly based on scavenging of
oxygen radicals (41). Moreover,
their increased intake has been correlated with a reduced risk of
various cancers such as ovarian, breast, prostate, lung and liver
cancers (42). These cancers were
found to be of the most frequent malignancies in Algeria.
Previously, Barros et al (2011) (43) reported the presence of one flavonol
in B. dioica roots: Kaempferol 3, 7-di-O-rhamnoside.
Kaempferol has been demonstrated to possess anticancer and
apoptogenic activity against a variety of cancer cell lines such as
myelogenous leukemia cell line K562, promyelocytic human leukemia
U937 (44), Human lung non-small
carcinoma H460 cell line (45),
breast cancer MDA-MB-231 cell line (46) and oral cancer cell lines (SCC-1483,
SCC-25 and SCC-QLL1) (47).
In the present study, we identified Myricetin
(2,5,7,3,4,5-pentahydroxylflavonol) as the major compound in B.
dioica aqueous extract. Myricetin the most common flavonoid
found in herbs, vegetables, and fruits, has been shown to possess
important biological activities including antioxidant,
antimicrobial, antidiabetic and anticarcinogenic effects (48). Anticancer and apoptogenic
activities of myricetin have been demonstrated against several
cancers such as colorectal cancer (49), ovarian cancer (50,51),
leukemia (52) or lung cancer
(53). Furthermore, it has been
reported that myricetin was able to cause cell death in different
cancer cell lines by arresting the cell cycle at different phases
(54). In fact, Myricetin arrested
the cell cycle of cancer cells by triggering CDKs and cyclins
(55,56).
Myricetin was found to be the major component in
different herbal extracts inducing apoptosis and/or cell cycle
arrest in human breast cancer cells (57). In vivo, myricetin was found
to be more effective than vincristine in chemoprevention of
dimethyl benzanthracene-induced-breast cancer in female Wistar rats
(58). Interestingly, myricetin
was not cytotoxic towards normal breast cells, justifying its
ability to be considered for in vivo studies (59).
Recently, a second-generation myricetin analog:
Oncamex has been demonstrated to induce apoptosis and cell cycle
arrest in MCF-7, MDA-MB-231, BT-549 and HBL-100 breast cancer cell
lines. In addition, the myricetin analog exhibited important
anti-breast cancer activity in mice implanted with MDA-MB-231
×enografts (60).
In conclusion, we demonstrate a strong cytotoxic
effect of the B. dioica aqueous extract against breast
cancer MDA-MB-231 cells line. B. dioica aqueous extract was
able to induce marked apoptosis and cell cycle arrest at G2/M phase
of MDA-MB-231 cells at a lower concentration (50 µg/ml). The
phytochemical study (UV-vis and LC-MSD-Trap-XCT) revealed the
presence of myricetin as the major compound that may contribute to
the apoptogenic activity of the B. dioica aqueous extract.
Thus, B. dioica could be considered as a promising source
for developing novel therapeutics against breast cancer.
Acknowledgements
The authors would like to thank Mrs Susana Hernandez
(Centro de Investigación del Cáncer, Salamanca, Spain) for
technical assistance.
Funding
BB received funding for a short-term internship from
the Algerian Ministry of Higher Education and Scientific Research.
The study was supported by CSIC-CIBERONC-Universidad de
Salamanca-Spain.
Availability of data and materials
The datasets used, generated and/or analyzed during
the present study are available from the corresponding author upon
reasonable request.
Authors' contributions
BB and AP designed the study. BB and AE performed
the experiments. BB wrote the manuscript. BB and AP contributed to
the manuscript revisions.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z,
Zhang L, Ji X, Liu W, Huang B, Liu B, et al: Breast cancer
development and progression: Risk factors, cancer stem cells,
signaling pathways, genomics, and molecular pathogenesis. Genes
Dis. 5:77–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahman S and Zayed H: Breast cancer in the
GCC countries: A focus on BRCA1/2 and non-BRCA1/2 genes. Gene.
668:73–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bai X, Ni J, Beretov J, Graham P and Li Y:
Cancer stem cell in breast cancer therapeutic resistance. Cancer
Treat Rev. 69:152–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Assefa B, Glatzel G and Buchmann C:
Ethnomedicinal uses of Hagenia abyssinica (Bruce) J.F. Gmel.
Among rural communities of Ethiopia. J Ethnobiol Ethnomed.
6:202010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bouabdelli F, Djelloul A, Kaid-Omar Z,
Semmoud A and Addou A: Antimicrobial activity of 22 plants used in
urolithiasis medicine in western Algeria. Asian Pac J Trop Dis. 2
(Suppl 1):S530–S535. 2012. View Article : Google Scholar
|
|
6
|
Azzi R, Djaziri R and Lahfa F:
Ethnopharmacological survey of medicinal plants used in the
traditional treatment of diabetes mellitus in the North Western and
South Western Algeria. J Med Plants Res. 6:2041–2050. 2012.
|
|
7
|
Benarba B, Belabid L, Righi K, Bekkar AA,
Elouissi M, Khaldi A and Hammimed A: Ethnobotanical study of
medicinal plants used by traditional healers in Mascara (North West
of Algeria). J Ethnopharmacol. 175:626–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benarba B: Medicinal plants used by
traditional healers from South-West Algeria: An ethnobotanical
study. J Intercult Ethnopharmacol. 5:320–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benarba B: Use of medicinal plants by
breast cancer patients in Algeria. EXCLI J. 14:1164–1166.
2015.PubMed/NCBI
|
|
10
|
Benarba B, Ambroise G, Aoues A, Meddah B
and Vazquez A: Aristolochia longa aqueous extract triggers
the mitochondrial pathway of apoptosis in BL41 Burkitt's lymphoma
cells. Int J Green Pharm. 2012:45–49. 2012. View Article : Google Scholar
|
|
11
|
Benarba B, Pandiella A and Elmallah A:
Anticancer activity, phytochemical screening and acute toxicity
evaluation of an aqueous extract of Aristolochia longa L.
Int J Pharm Phytopharmacol Res. 6:20–26. 2016. View Article : Google Scholar
|
|
12
|
Sallam AA, Hitotsuyanagi Y, Mansour ES,
Ahmed AF, Gedara S, Fukaya H and Takeya K: Cucurbitacins from
Bryonia cretica. Phytochem Lett. 3:117–121. 2010. View Article : Google Scholar
|
|
13
|
Nakashima S, Matsuda H, Kurume A, Oda Y,
Nakamura S, Yamashita M and Yoshikawa M: Cucurbitacin E as a new
inhibitor of cofilin phosphorylation in human leukemia U937 cells.
Bioorg Med Chem Lett. 20:2994–2997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuda H, Nakashima S, Abdel-Halim OB,
Morikawa T and Yoshikawa M: Cucurbitane-type triterpenes with
anti-proliferative effects on U937 cells from an egyptian natural
medicine, Bryonia cretica: Structures of new triterpene glycosides,
bryoniaosides A and B. Chem Pharm Bull (Tokyo). 58:747–751. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chekroun E, Bechiri A, Azzi R, Adida H,
Benariba N and Djaziri R: Antidiabetic activity of two aqueous
extracts of two cucurbitaceae: Citrullus colocynthis and
Bryonia dioica. Phytothérapie. 15:57–66. 2017. View Article : Google Scholar
|
|
16
|
Dhouioui M, Boulila A, Jemli M, Schiets F,
Casabianca H and Zina MS: Fatty acids composition and antibacterial
activity of Aristolochia longa L. and Bryonia dioïca
Jacq. Growing wild in tunisia. J Oleo Sci. 65:655–661. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chekroun E, Benariba N, Adida H, Bechiri
A, Azzi R and Djaziri R: Antioxidant activity and phytochemical
screening of two Cucurbitaceae: Citrullus colocynthis fruits
and Bryonia dioica roots. Asian Pac J Trop Dis. 5:632–637.
2015. View Article : Google Scholar
|
|
18
|
Benarba B, Meddah B and Aoues A:
Bryonia dioica aqueous extract induces apoptosis through
mitochondrial intrinsic pathway in BL41 Burkitt's lymphoma cells. J
Ethnopharmacol. 141:510–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Díaz-Rodríguez E, Sanz E and Pandiella A:
Antitumoral effect of Ocoxin, a natural compound-containing
nutritional supplement, in small cell lung cancer. Int J Oncol.
53:113–123. 2018.PubMed/NCBI
|
|
20
|
Díaz-Rodríguez E, El-Mallah AM, Sanz E and
Pandiella A: Antitumoral effect of Ocoxin in hepatocellular
carcinoma. Oncol Lett. 14:1950–1958. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sathish S, Janakiraman N and Johnson M:
Phytochemical analysis of vitex altissima L. using UV–VIS, FTIR and
GC-MS. Int J Pharm Sci Drug Res. 4:56–62. 2012.
|
|
22
|
Rodríguez-Gonzalo E, García-Gómez D and
Carabias-Martínez R: Development and validation of a method for the
detection and confirmation of biomarkers of exposure in human urine
by means of restricted access material-liquid chromatography-tandem
mass spectrometry. J Chromatogr A. 1217:40–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roy S, Banerjee B and Vedasiromoni JR:
Cytotoxic and apoptogenic effect of Swietenia mahagoni (L.) Jacq.
leaf extract in human leukemic cell lines U937, K562 and HL-60.
Environ Toxicol Pharm. 37:234–247. 2014. View Article : Google Scholar
|
|
24
|
Buranrat B, Mairuae N and Kanchanarach W:
Cytotoxic and antimigratory effects of Cratoxy formosum extract
against HepG2 liver cancer cells. Biomed Rep. 6:441–448. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benarba B, Meddah B and Tir-Touil A:
Response of bone resorption markers to Aristolochia longa
intaked by Algerian breast cancer postmenopausal women. Adv
Pharmacol Sci. 2014:1–4. 2014. View Article : Google Scholar
|
|
26
|
Sun X, Ma X, Li Q, Yang Y, Xu X, Sun J, Yu
M, Cao K, Yang L, Yang G, et al: Anticancer effects of fisetin on
mammary carcinoma cells via regulation of the PI3K/Akt/mTOR
pathway: In vitro and in vivo studies. Int J Mol Med.
42:811–820. 2018.PubMed/NCBI
|
|
27
|
Piao XM, Gao F, Zhu JX, Wang LJ, Zhao X,
Li X, Sheng MM and Zhang Y: Cucurbitacin B inhibits tumor
angiogenesis by triggering the mitochondrial signaling pathway in
endothelial cells. Int J Mol Med. 42:1018–1025. 2018.PubMed/NCBI
|
|
28
|
Sahpazidou D, Geromichalos GD, Stagos D,
Apostolou A, Haroutounian SA, Tsatsakis AM, Tzanakakis GN, Hayes AW
and Kouretas D: Anticarcinogenic activity of polyphenolic extracts
from grape stems against breast, colon, renal and thyroid cancer
cells. Toxicol Lett. 230:218–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Lima AP, Pereira Fde C, Vilanova-Costa
CA, Soares JR, Pereira LC, Porto HK, Pavanin LA, Dos Santos WB and
Silveira-Lacerda Ede P: Induction of cell cycle arrest and
apoptosis by ruthenium complex
cis-(dichloro)tetramineruthenium(III) chloride in human lung
carcinoma cells A549. Biol Trace Elem Res. 147:8–15. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koo BC, Kim DH, Kim IR, Kim GC, Kwak HH
and Park BS: A natural product, chios gum mastic, induces the death
of HL-60 cells via apoptosis and cell cycle arrest. Int J Oral
Biol. 36:13–21. 2011.
|
|
31
|
Hostanska K, Nisslein T, Freudenstein J,
Reichling J and Saller R: Cimicifuga racemosa extract
inhibits proliferation of estrogen receptor-positive and negative
human breast carcinoma cell lines by induction of apoptosis. Breast
Cancer Res Treat. 84:151–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chakravarti B, Maurya R, Siddiqui JA, Bid
HK, Rajendran SM, Yadav PP and Konwar R: In vitro anti-breast
cancer activity of ethanolic extract of Wrightia tomentosa: Role of
pro-apoptotic effects of oleanolic acid and urosolic acid. J
Ethnopharmacol. 142:72–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferreira AK, de-Sá-Júnior PL, Pasqualoto
KF, de Azevedo RA, Câmara DA, Costa AS, Figueiredo CR, Matsuo AL,
Massaoka MH, Auada AV, et al: Cytotoxic effects of dillapiole on
MDA-MB-231 cells involve the induction of apoptosis through the
mitochondrial pathway by inducing an oxidative stress while
altering the cytoskeleton network. Biochimie. 99:195–207. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye B, Li J, Li Z, Yang J, Niu T and Wang
S: Anti-tumor activity and relative mechanism of ethanolic extract
of Marsdenia tenacissima (Asclepiadaceae) against human hematologic
neoplasm in vitro and in vivo. J Ethnopharmacol. 153:258–267. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee DH, Park KI, Park HS, Kang SR,
Nagappan A, Kim JA, Kim EH, Lee WS, Hah YS, Chung HJ, et al:
Flavonoids isolated from Korea citrus aurantium L. induce G2/M
phase arrest and apoptosis in human gastric cancer AGS cells. J
Evid Based Complementary Altern Med. 2012:1–11. 2012. View Article : Google Scholar
|
|
36
|
Zhang Q, Zhao XH and Wang ZJ: Flavones and
flavonols exert cytotoxic effects on a human oesophageal
adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing
apoptosis. Food Chem Toxicol. 46:2042–2053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gómez-Alonso S, Collins VJ, Vauzour D,
Rodríguez-Mateos A, Corona G and Spencer JPE: Inhibition of colon
adenocarcinoma cell proliferation by flavonols is linked to a G2/M
cell cycle block and reduction in cyclin D1 expression. Food Chem.
130:493–500. 2012. View Article : Google Scholar
|
|
38
|
Youssef Moustafa AM, Khodair AI and Saleh
MA: Isolation, structural elucidation of flavonoid constituents
from Leptadenia pyrotechnica and evaluation of their toxicity and
antitumor activity. Pharm Biol. 47:539–552. 2009. View Article : Google Scholar
|
|
39
|
Sisa M, Bonnet SL, Ferreira D and Van der
Westhuizen JH: Photochemistry of flavonoids. Molecules.
15:5196–5245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsimogiannis D, Samiotaki M, Panayotou G
and Oreopoulou V: Characterization of flavonoid subgroups and
hydroxy substitution by HPLC-MS/MS. Molecules. 12:593–606. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee HN, Shin SA, Choo GS, Kim HJ, Park YS,
Kim BS, Kim SK, Cho SD, Nam JS, Choi CS, et al: Anti-inflammatory
effect of quercetin and galangin in LPS-stimulated RAW264.7
macrophages and DNCB-induced atopic dermatitis animal models. Int J
Mol Med. 41:888–898. 2018.PubMed/NCBI
|
|
42
|
Romagnolo DF and Selmin OI: Flavonoids and
cancer prevention: A review of the evidence. J Nutr Gerontol
Geriatr. 31:206–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Barros L, Dueñas M, Ferreira ICFR,
Carvalho AM and Santos-Buelga C: Use of HPLC-DAD-ESI/MS to profile
phenolic compounds in edible wild greens from Portugal. Food Chem.
127:169–173. 2011. View Article : Google Scholar
|
|
44
|
Marfe G, Tafani M, Indelicato M,
Sinibaldi-Salimei P, Reali V, Pucci B, Fini M and Russo MA:
Kaempferol induces apoptosis in two different cell lines via Akt
inactivation, Bax and SIRT3 activation, and mitochondrial
dysfunction. J Cell Biochem. 106:643–650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Leung HW, Lin CJ, Hour MJ, Yang WH, Wang
MY and Lee HZ: Kaempferol induces apoptosis in human lung non-small
carcinoma cells accompanied by an induction of antioxidant enzymes.
Food Chem Toxicol. 45:2005–2013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brusselmans K, Vrolix R, Verhoeven G and
Swinnen JV: Induction of cancer cell apoptosis by flavonoids is
associated with their ability to inhibit fatty acid synthase
activity. J Biol Chem. 280:5636–5645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kang JW, Kim JH, Song K, Kim SH, Yoon JH
and Kim KS: Kaempferol and quercetin, components of Ginkgo biloba
extract (EGb 761), induce caspase-3-dependent apoptosis in oral
cavity cancer cells. Phytother Res. 24 (Suppl 1):S77–S82. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Devi KP, Rajavel T, Habtemariam S, Nabavi
SF and Nabavi SM: Molecular mechanisms underlying anticancer
effects of myricetin. Life Sci. 142:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim ME, Ha TK, Yoon JH and Lee JS:
Myricetin induces cell death of human colon cancer cells via
BAX/BCL2-dependent pathway. Anticancer Res. 34:701–706.
2014.PubMed/NCBI
|
|
50
|
Xu Y, Xie Q, Wu S, Yi D, Yu Y, Liu S, Li S
and Li Z: Myricetin induces apoptosis via endoplasmic reticulum
stress and DNA double-strand breaks in human ovarian cancer cells.
Mol Med Rep. 13:2094–2100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zheng AW, Chen YQ, Zhao LQ and Feng JG:
Myricetin induces apoptosis and enhances chemosensitivity in
ovarian cancer cells. Oncol Lett. 13:4974–4978. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Morales P and Haza AI: Selective apoptotic
effects of piceatannol and myricetin in human cancer cells. J Appl
Toxicol. 32:986–993. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang S, Wang L, Liu H, Zhao G and Ming L:
Enhancement of recombinant myricetin on the radiosensitivity of
lung cancer A549 and H1299 cells. Diagn Pathol. 9:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
de Oliveira Júnior RG, Christiane Adrielly
AF, da Silva Almeida JRG, Grougnet R, Thiéry V and Picot L:
Sensitization of tumor cells to chemotherapy by natural products: A
systematic review of preclinical data and molecular mechanisms.
Fitoterapia. 129:383–400. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lu J, Papp LV, Fang J, Rodriguez-Nieto S,
Zhivotovsky B and Holmgren A: Inhibition of mammalian thioredoxin
reductase by some flavonoids: Implications for myricetin and
quercetin anticancer activity. Cancer Res. 66:4410–4418. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Maggioni D, Nicolini G, Rigolio R, Biffi
L, Pignataro L, Gaini R and Gravello W: Myricetin and naringenin
inhibit human squamous cell carcinoma proliferation and migration
in vitro. Nutr Cancer. 66:1257–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Deepa M, Sureshkumar T, Satheeshkumar PK
and Priya S: Antioxidant rich Morus alba leaf extract induces
apoptosis in human colon and breast cancer cells by the
downregulation of nitric oxide produced by inducible nitric oxide
synthase. Nutr Cancer. 65:305–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jayakumar JK, Nirmala P, Praveen Kumar BA
and Kumar AP: Evaluation of protective effect of myricetin, a
bioflavonoid in dimethyl benzanthracene-induced breast cancer in
female Wistar rats. South Asian J Cancer. 3:107–111. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Semwal DK, Semwal RB, Combrinck S and
Viljoen A: Myricetin: A dietary molecule with diverse biological
activities. Nutrients. 8:902016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Martínez-Pérez C, Ward C, Turnbull AK,
Mullen P, Cook G, Meehan J, Jarman EJ, Thomson PI, Campbell CJ,
McPhail D, et al: Antitumour activity of the novel flavonoid
Oncamex in preclinical breast cancer models. Br J Cancer.
114:905–916. 2016. View Article : Google Scholar : PubMed/NCBI
|