Introduction
Inflammatory bowel disease (IBD), which includes
Crohn's disease (CD) and ulcerative colitis (UC), is a chronic and
non-resolving intestinal inflammatory disease with poor prognosis
(1,2). A recent study has shown that genetic
and environmental factors, and dysbiosis of the microbiota serve
important roles in the pathogenesis of IBD (3). The intestinal microbiota was
determined to be closely associated with IBD. Our previous studies
revealed that the intestinal population of Roseburia
intestinalis (R. intestinalis) in untreated patients
with CD was markedly lower than in healthy controls (4,5).
R. intestinalis is a butyrate-producing Gram-positive
bacteria belonging to Clostridium subphylum cluster XIVa
(6). Previously, our study
demonstrated that R. intestinalis increased the abundance of
T regulatory (Treg) cells and upregulated the expression of
cytokines thymic stromal lymphopoietin (TSLP) and transforming
growth factor-β (TGF-β) to ameliorate intestinal inflammation
(4); however, whether R.
intestinalis supernatant has a beneficial effect on IBD
requires further investigation.
The microbiota has a direct effect on host immunity;
metabolites secreted by the gut microbiota serve a role in innate
immune responses (7). Important
metabolites include indoles, folate, trimethylamine-N-oxide, and
short-chain fatty acids (SCFAs); among these, SCFAs have been the
subject of much research (7). Gut
microbes ferment dietary fiber to generate SCFAs, specifically
butyrate, which regulates the development and activity of innate
and adaptive immune cells that are crucial for protecting the host
from intestinal injury (8).
Dysbiosis of the microbiota and reduced concentrations of SCFAs are
associated with a significant increase in the number of
pro-inflammatory immune cells in the intestinal mucosa of patients
with IBD (9). Butyrate promotes
the secretion of anti-inflammatory cytokines by colonic macrophages
and dendritic cells by binding to hydroxycarboxylic acid receptor
2, thereby inducing the differentiation of Treg cells and
interleukin (IL)-10-producing T cells to ameliorate
2,4,6-trintirobenzenesulfonic acid (TNBS)-induced intestinal
inflammation (10). A recent study
demonstrated that Faecalibacterium prausnitzii, a commensal
bacterium abundant in the human gut, produces butyrate to maintain
the Th17/Treg balance and suppress colitis (11).
In the present study, the effects of R.
intestinalis supernatant on LPS-induced macrophages were
investigated in vitro, and its role in dextran sulfate
sodium (DSS)- and TNBS-induced colitis was determined in
vivo. In addition, the non-protein components of the
supernatant were analyzed via gas chromatography-mass spectrometry
(GC-MS) analysis. To the best of our knowledge, this is the first
study to examine the effects of R. intestinalis supernatant
on colitis. The results may provide insight into the mechanism by
which the commensal microbiota and its metabolites interact with
the host, and may facilitate the development of novel therapeutic
strategies for treating IBD.
Materials and methods
Animals
Male C57BL/6 mice (6-weeks-old, 17–18 g) were
obtained from the laboratory animals department of Central South
University (Changsha, China) and housed in specific pathogen-free
conditions at 22–26°C, under a 12:12-h light/dark cycle with 40–70%
humidity and ad libitum access to food and water. All animal
experiments were approved by the Ethics Committee of Medical
Research of Central South University and were conducted in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (12).
Bacterial culture
R. intestinalis DSMZ-14610 was grown as
previously described (4). R.
intestinalis supernatant was collected after centrifugation at
2,000 × g and 4°C for 20 min and passed through a 0.22-µm sterile
filter (EMD Millipore) to remove bacteria. Sterile culture medium
was used as placebo. R. intestinalis supernatant and fresh
sterile BD BACTEC™ Lytic/10 Anaerobic/F medium (BD Diagnostics;
Becton-Dickinson and Company) were frozen at −80°C until required.
Medium and supernatant were lyophilized and diluted with PBS by a
factor of two or five prior to administration.
Induction of colitis with DSS
Mice (n=24) were randomly divided into three groups
(n=8 per group): The control, colitis (DSS), and DSS and R.
intestinalis supernatant (DSS + SUP) groups. Between days 0–7,
all mice (except the control group) received 3% DSS (MW,
36,000-50,000; MP Biomedicals). In addition, the control and
colitis groups received 0.2 ml of 5X R. intestinalis culture
medium (BD Diagnostics; Becton-Dickinson and Company) by gavage,
and the DSS + SUP group was treated with 0.2 ml of 5X R.
intestinalis supernatant by gavage once per day following DSS
treatment from day 0 (for 7 days in total). Mice were euthanized
for tissue collection on day 7.
Induction of colitis with TNBS
Mice were assigned to similar groups as
aforementioned (control, TNBS and TNBS + SUP). For the induction of
colitis, mice were pre-sensitized by applying 150 µl of 5% TNBS
(Sigma-Aldrich; Merck KGaA) in a 4:1 v/v ratio with acetone/olive
oil (ratio of 4:1 v/v) mixture onto the shaved dorsal skin mice for
1 day. Control mice were treated with acetone/olive oil mixture
alone (day 1) (4). On days 8 and
10, mice were anesthetized with isoflurane and administered 100 µl
TNBS solution [5% TNBS in 50% ethanol (1:1 v/v ratio)] via the
rectum. Mice were held upside down for 1 min after administration
of TNBS. The control group received 50% ethanol instead of TNBS.
The control and colitis groups received 0.2 ml of 5X R.
intestinalis culture medium (BD Diagnostics; Becton-Dickinson
and Company), and the TNBS + SUP group received R.
intestinalis supernatant via gavage once a day from day 10 (for
5 days in total). Mice were euthanized for tissue collection on day
14 following pre-sensitization.
Disease activity index
The severity of colitis was assessed by measuring
body weight loss, stool characteristics and the occurrence of
hematochezia using the three-component mouse disease activity index
(DAI; n=8; Table SI), as
described previously (5).
Cell isolation and flow cytometry
Murine bone marrow macrophages (BMMs) were cultured
as described (13), with certain
modified as thus described: Briefly, the mouse femur and tibia were
separated and both ends were cut. Then, the bone marrow was flushed
with 1X PBS using a 1-ml syringe. Cells (~2×106/ml) were
cultured in 6-well plates in complete RPMI-1640 containing 10 ng/ml
recombinant macrophage colony stimulating factor (PeproTech, Inc.).
Cells were used on day 5, at which point ~70% were assessed for
F4/80+CD11b+ by fluorescence activated cell
sorting (FACS) analysis. To prepare peritoneal macrophages, mice
were sacrificed and 5 ml of RPMI-1640 was injected into the
peritoneal cavity. The fluid was collected and centrifuged at 240 ×
g and room temperature for 5 min. To isolate lamina propria cells,
colon tissue was treated with a pre-digestion solution [1X Hank's
Balanced Salt Solution + 5 mM EDTA + 1 mM dithiotreithol + 5% fetal
bovine serum (Biological Industries)] at 37°C for 20 min to remove
epithelial cells. Then, the tissue was treated with digestion
solution [0.3 mg/ml type IV collagenase (Sigma-Aldrich; Merck
KGaA), 3 mg/ml Dispase II (Roche Diagnostics GmbH) and 0.25 mg/ml
DNase I (Sigma-Aldrich; Merck KGaA)] at 37°C for 20 min with gentle
agitation. Cells were collected and purified via a Percoll gradient
(40/80%) prior to FACS analysis. Subsequently, cells were incubated
with anti-mouse antibodies specific for F4/80 (1:50; cat. no.
123113; phycoerythrin-cyanine 7 conjugate; BioLegend, Inc.) and
CD11b (1:50; cat. no. 101205; FITC conjugate; BioLegend, Inc.) at
4°C for 30 min to detect macrophage, or with Leukocyte Activation
Cocktail, BD GolgiPlug™ (BD Biosciences) at 37°C for 5 h to elicit
a primary cytokine response from T cells. The T cells were stained
with anti-mouse Abs specific for IL-17 (1:50; cat. no. 146307;
allophycocyanin conjugate; BioLegend, Inc.) and CD4 markers (1:100;
cat. no. 130308; FITC conjugate; BioLegend, Inc.) at 4°C for 30 min
prior to Th17 analysis. Flow cytometry was performed using a FACS
Arial II flow cytometer (BD Biosciences) and analyzed using FlowJo
7.0 FACS software (FlowJo LLC).
Histology and immunohistochemistry
(IHC)
For H&E detection, proximal and distal colon
tissues were fixed in 4% phosphate-buffered formaldehyde solution
at 4°C for 24 h, embedded in paraffin, cut into 5 µm-thick
sections, and stained with hematoxylin for 30 sec and eosin for 2
min (both at room temperature). The slides were scored for
inflammation by two pathologists (blinded to the treatment
regimens) using previously described criteria (14) (Table
SII). For IHC (n=6), 5 µm-thick formalin-fixed
paraffin-embedded specimens of the distal colon were boiled in
sodium citrate solution (0.01 M, pH 6.0; Wuhan Goodbio Technology
Co., Ltd.) at 100°C for 20 min and then cooled to room temperature.
Sections were incubated at 4°C overnight with an
anti-phosphorylated (p)-STAT3 rabbit mAb (1:200; cat. no. 9145;
Cell Signaling Technology, Inc.) and an anti-IL-6 rabbit polyclonal
Ab (1:200; cat. no. 21865-1-AP; ProteinTech Group, Inc.), followed
by incubation with corresponding secondary biotin-conjugated Abs
(cat. no. KIT-9710, Mai New Biotechnology Development Company) at
37°C for 1 h according to the manufacturer's protocols. The
procedures were performed using a 3′,3′-diaminobenzidene kit (Mai
New Biotechnology Development Company) and scored using a light
microscope (magnification, ×100; DP72; Olympus Corporation) by two
independent pathologists in a blinded manner, with at least 5 high
powered fields counted per sample.
Reverse transcription-quantitative
polymerase chain reaction (qPCR)
Total RNA from colon tissue or cells was isolated by
using TRIzol® (Thermo Fisher Scientific, Inc.), and cDNA
was synthesized using a RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.) according to the manufacturers
protocols. cDNA was amplified by qPCR using SYBR®-Green
Supermix (Vazyme) and a Bio-Rad CFX96 Real-Time PCR Detection
System (Bio-Rad Laboratories, Inc.). The thermocycling conditions
for qPCR were: 95°C for 30 sec, followed by 40 cycles at 95°C for 5
sec and 60°C for 30 sec. The relative expression of target gene
mRNA was calculated using the 2−ΔΔCq method (15) and GAPDH was used as the reference
gene (primer sequences are presented in Table SIII).
Western blotting
Total protein was extracted from colon tissues with
lysis buffer and centrifuged at 13,000 × g and 4°C for 10 min.
Protein concentration was determined by a BCA assay (Thermo Fisher
Scientific, Inc.). Protein samples (30 µg; 1 µg/µl) were boiled for
5 min at 100°C, separated via 10% SDS-PAGE, and transferred onto
polyvinylidene difluoride membranes (Merck KGaA). Membranes were
blocked with 5% bovine serum albumin (Wuhan Goodbio Technology Co.,
Ltd.) at room temperature for 1 h, and then incubated with primary
Abs, including GAPDH mouse mAb (1:2,000; cat. no. 60004-1-Ig;
ProteinTech Group, Inc.), p-STAT3 rabbit mAb (1:1,000; cat. no.
9145; Cell Signaling Technology, Inc.) and STAT3 mouse mAb
(1:1,000; cat. no. 9139; Cell Signaling Technology, Inc.) at 4°C
overnight. Subsequently, membranes were incubated with
corresponding secondary horseradish peroxidase (HRP)-conjugated
anti-mouse Abs (1:5,000; cat. no. SA00001-1; ProteinTech Group,
Inc.) or HRP-conjugated anti-rabbit Abs (1:5,000; cat. no.
SA00001-2; ProteinTech Group, Inc.) for 1 h at 37°C, and then
developed with an enhanced chemiluminescence detection system
(Bio-Rad Laboratories, Inc.).
ELISA
Peripheral blood was collected from mice and
centrifuged at 2,000 × g at room temperature for 15 min to obtain
mouse serum for the following experiments. The concentration of
IL-6 and tumor necrosis factor-α (TNF-α) in mouse serum and the
cell-culture supernatant was quantified using ELISA kits (cat. nos.
88-7064-22 and 88-7324-22; eBioscience; Thermo Fisher Scientific,
Inc.) according to the manufacturer's recommendations.
GC-MS analysis of SCFAs
The mouse cecal samples was prepared as described by
Turnbaugh et al (16), and
the supernatant samples was prepared as described by Zhou et
al (11). GC-MS analysis of
concentrated bacterial culture supernatant and mouse cecal samples
was performed using an Agilent 6890N/5975B GC-MS instrument
(Agilent Technologies, Inc.) fitted with an Agilent HP-INNOWAX
column (30 m ×0.25 mm; internal diameter, 0.25 µm). All samples
were collected, frozen and stored under vacuum at −80°C until use.
Samples were assayed using the method of Samuel and Gordon
(17). Prepared samples (1 µl)
were used for detection, 50 µg/ml isocaproic acid was used as an
internal standard and isocaproic acid/diethyl ether (ratio of 1:4
v/v) mixture was used as the solvent. The flow rate was 1
ml/min.
In vitro inflammation model using BMCs
and RAW264.7 cells
The human colon epithelial cell line Caco2 and the
mouse macrophage cell line RAW264.7 were acquired from the Cancer
Research Institute of Central South University. Cells were cultured
at 37°C in 5% CO2. in RPMI-1640 (HyClone; GE Healthcare
Life Sciences) supplemented with 10% fetal bovine serum (Biological
Industries). For the in vitro inflammation model, Caco2
cells, BMCs and RAW264.7 macrophages were induced by exposure to 1
µg/ml lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGA), LPS +
medium, LPS + R. intestinalis supernatant or LPS + sodium
butyrate at 37°C for 12 or 24 h; PBS was used as the control
treatment. Then, macrophages were co-treated with R.
intestinalis supernatant (2X) at 37°C for 12 or 24 h.
Statistical analysis
Data were expressed as the mean ± standard deviation
of at least three independent experiments. P<0.05 was considered
to indicate a statistically significant difference. Statistical
significance was analyzed by one-way ANOVA with a
Student-Newman-Keuls post-hoc test (SPSS 18.0; SPSS, Inc.).
Results
R. intestinalis supernatant suppresses
proinflammatory cytokine expression via LPS-induced macrophages in
vitro
It has been shown that R. intestinalis
bacteria have significant anti-inflammatory effects in the context
of TNBS-induced colitis (4);
however, analysis tends to be conducted using direct culture
systems or with bacteria that were in contact with intestinal
epithelial cells (IECs). To the best of our knowledge, no studies
have investigated the effects of components secreted by R.
intestinalis on the culture supernatant. Therefore, in
vitro analyses were conducted to examine the possible
anti-inflammatory effects of R. intestinalis supernatant on
LPS-induced macrophages and Caco2 colonic cells. First, the effects
of R. intestinalis supernatant (2X raw supernatant) on
LPS-induced RAW264.7 macrophages were determined. The results
revealed that the mRNA expression of IL-6, STAT3 and TNF-α by
RAW264.7 cells were markedly increased upon exposure to LPS
compared with blank treatment (Fig.
1A-C); however, the expression of IL-6 and STAT3, but not
TNF-α, significantly decreased in cells treated with LPS + R.
intestinalis supernatant compared with LPS alone and LPS +
medium (Fig. 1A-C). ELISA of IL-6
and TNF-α concentrations yielded similar results (Fig. 1D and E). The effects of R.
intestinalis supernatant on LPS-induced Caco2 colon cells were
also examined; no significant alterations in IL-6 concentration
were observed (Fig. S1A).
Additionally, similarly with the results obtained for RAW264.7
cells, R. intestinalis supernatant led to a significant
reduction in the protein expression of IL-6 by LPS-treated BMMs
compared with the LPS and LPS + medium groups (Fig. 1F). These results indicated that
R. intestinalis supernatant inhibited the inflammatory
response via LPS-induced macrophages in vitro.
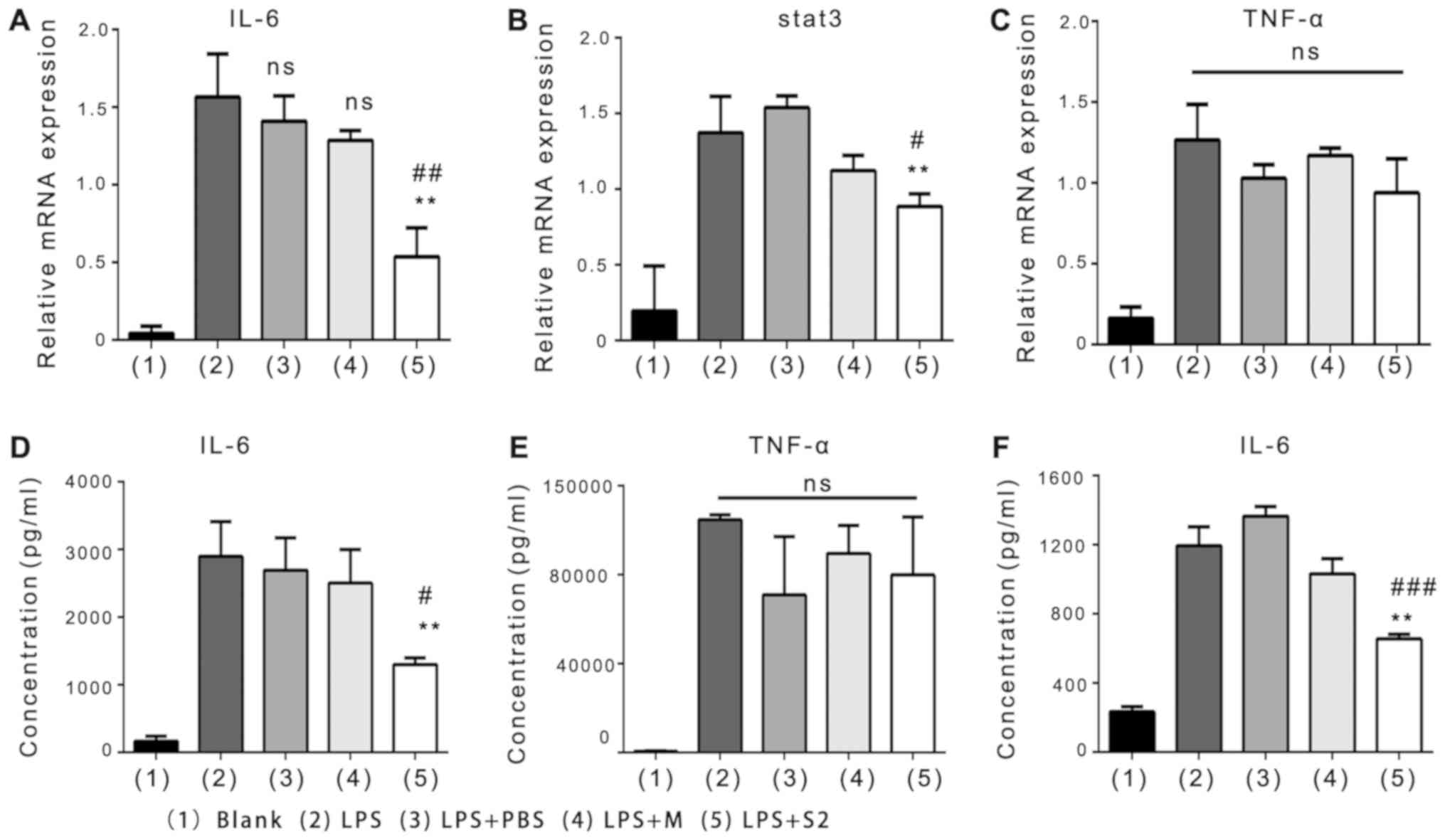 | Figure 1.Effects of R. intestinalis
supernatant on macrophages in vitro. Reverse
transcription-quantitative polymerase chain reaction analysis of
(A) IL-6, (B) STAT3 and (C) TNF-α in RAW264.7 cells induced for 24
h with LPS and then treated with PBS, M or S2. (D and E) ELISA of
IL-6 and TNF-α levels in the supernatant of RAW264.7 cells exposed
to LPS for 24 h. (F) ELISA of IL-6 in the supernatant of bone
marrow macrophage cells (isolated from normal C57BL/6 mice) exposed
to LPS for 12 h. Data are representative of three independent
experiments. Error bars represent mean ± standard error of the
mean. **P<0.01 vs. LPS; #P<0.05,
##P<0.01 and ###P<0.001 vs. LPS + M;
ns, non-significant; IL, interleukin; LPS, lipopolysaccharide; M,
medium; S2, 2X R. intestinalis supernatant; STAT3, signal
transducer and activator 3; TNF-α, tumor necrosis factor-α. |
R. intestinalis supernatant suppresses
colitis in murine models of DSS- and TNBS-induced IBD
The effects of R. intestinalis supernatant on
mouse models of DSS- and TNBS-induced colitis were investigated
(Fig. 2A and B), both of which are
well-established experimental models of colitis used to observe the
therapeutic effects of drugs (18).
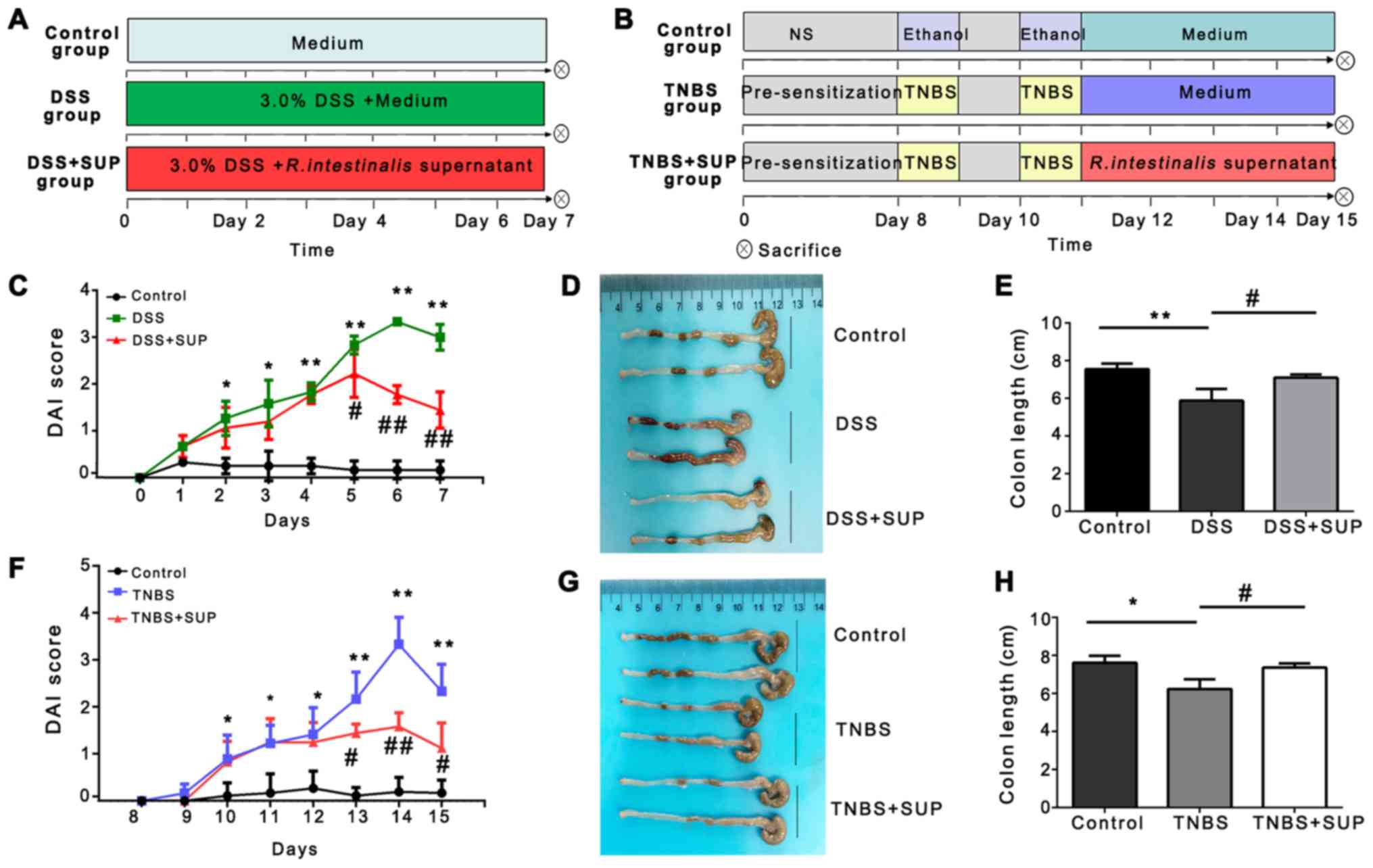 | Figure 2.R. intestinalis supernatant
improves the clinical signs and reduces colon shortening in mice
with DSS- and TNBS-induced colitis. Flow diagram showing the
generation of the (A) DSS-induced and (B) TNBS-induced colitis
model in mice, and treatment with R. intestinalis
supernatant or control medium. (C) DAI score of control, DSS, and
DSS + SUP mice (n=8). (D and E) Representative images of colons and
histograms of the colon length for each group. (F) DAI score of
control, TNBS and TNBS + SUP mice (n=8). (G and H) Representative
images of colons, and histograms of the colon length for the
different groups. Data are representative of three independent
experiments. Error bars represent mean ± standard error of the
mean. *P<0.05, **P<0.01 vs. control. #P<0.05,
##P<0.01 vs. DSS or TNBS; ns, non-significant; DAI,
disease activity index; DSS, dextran sulfate sodium; TNBS,
2,4,6-trintirobenzenesulfonic acid; SUP, supernatant treatment. |
From day 5 post-treatment with supernatant, the DAI
score of the DSS + SUP group of mice was significantly lower, and
the colon length was longer than that of the DSS group (Fig. 2C-E). In addition, the DAI score of
mice in the TNBS + SUP group improved significantly faster, and the
colon length was notably longer than that of the TNBS group
(Fig. 2F-H).
Then, the severity of colonic inflammation and
ulcers was analyzed by H&E staining of tissue sections. The
results revealed that the distal or mid colon of non-colitic
controls exhibited normal crypts and no infiltration by
polymorphonuclear cells; however, polymorphonuclear cell
infiltration, crypt damage, mucosal injury, edema and other
inflammatory features were observed in mice treated with DSS or
TNBS (Fig. 3A and B). Intestinal
inflammation was clear throughout the mid and distal colon;
however, treatment with R. intestinalis supernatant
alleviated these clinical signs, resulting in a significant
reduction in the histological inflammation score compared with the
DSS and TNBS groups (Fig. 3C and
D). Thus, R. intestinalis supernatant was suggested to
reduce the DAI score, prevent colon shortening and alleviate
colonic inflammation in both IBD models.
 | Figure 3.R. intestinalis supernatant
inhibits invasion of the colon by inflammatory cells.
Representative H&E stained images of the (A) distal and (B) mid
colon. Upper and lower images are at ×40 and 200 magnification
(scale bars, 500 and 50 µm). (C and D) Pathological score for the
different groups (n=6). Data are representative of three
independent experiments. Error bars represent mean ± standard error
of the mean. **P<0.01, ***P<0.001 vs. control;
#P<0.05, ##P<0.01,
###P<0.001 vs. DSS or TNBS; ns, non-significant; DSS,
dextran sulfate sodium; TNBS, 2,4,6-trintirobenzenesulfonic acid;
SUP, supernatant treatment. |
R. intestinalis supernatant alters the
activity of inflammatory macrophages and the production of
proinflammatory cytokines
To explore alterations in immune cell activity, the
abundance of F4/80+CD11b+ macrophages, which
are recruited to sites of inflammation and are regarded as
activated inflammatory macrophages, were investigated; these cells
produce nitric oxide (NO) and other inflammatory mediators, such as
IL-6 and TNF-α (19,20). Such macrophages contribute, at
least in part, to the pathogenesis of IBD (21,22).
A significant increase in the abundance of
F4/80+CD11b+ macrophages was detected in the
DSS and TNBS groups compared with the control. A significant
reduction was observed in the number of colonic macrophages in the
TNBS + SUP group compared with the model, but not in the DSS + SUP
group (Fig. 4A and C). In
addition, the number of peritoneal macrophages in DSS and TNBS mice
treated with R. intestinalis supernatant significantly
decreased compared with the respective models (Fig. 4B and D).
 | Figure 4.Fewer
F4/80+CD11b+ macrophages are detected in
DSS/TNBS-induced colitis mice treated with R. intestinalis
supernatant. Representative flow cytometry plots of (A) colonic
F4/80+CD11b+ macrophages and (B) peritoneal
F4/80+CD11b+ macrophages (n=6). (C and D)
Statistical analysis of the number of
F4/80+CD11b+ cells in the colon (left) and
peritoneal cavity (right) (n=6). ELISA of (E) IL-6 and (F) TNF-α
levels in mouse serum (n=6). Representative immunohistochemistry
images of (G) IL-6 and (H) p-STAT3 expression in colon tissues
(scale bar, 20 µm; magnification, ×200; n=6). (I) Western blot
analysis of STAT3 and p-STAT3 in colon tissues. Data are
representative of three independent experiments. Error bars
represent mean ± standard error of the mean. *P<0.05,
***P<0.001 vs. control; #P<0.05,
##P<0.01 vs. DSS or TNBS; ns, non-significant; DSS,
dextran sulfate sodium; IL, interleukin; p, phosphorylated; PE,
phycoerythrin; STAT3, signal transducer and activator 3; SUP,
supernatant treatment; TNBS, 2,4,6-trintirobenzenesulfonic acid;
TNF-α, tumor necrosis factor-α. |
DSS and TNBS treatment increased the serum
concentrations of IL-6 and TNF-α in both models; however, a
significant decrease in the serum levels of IL-6 was observed
following treatment with R. intestinalis supernatant
(Fig. 4E and F). Previous studies
have suggested that IL-6 and STAT3 are potential therapeutic
targets in IBD (23). In line with
reductions in IL-6 expression in the colon, downregulation of STAT3
was also observed. The results of IHC revealed that expression of
IL-6 and p-STAT3 (the active form of STAT3) in the DSS and TNBS
groups markedly increased, whereas that in the DSS/TNBS + SUP
groups decreased (Fig. 4G and H).
Western blotting also demonstrated that the expression of STAT3 and
p-STAT3 notably decreased in the DSS/TNBS + SUP groups compared
with the respective model groups (Fig.
4I). Collectively, these results indicated that R.
intestinalis supernatant inhibited inflammatory macrophages and
the IL-6/STAT3 signaling pathway.
R. intestinalis supernatant inhibits
Th17 cell differentiation
In addition to inflammatory macrophages,
infiltration of the colon by CD4+ T cells contributes to
the pathogenesis of IBD (24). CD
and ulcerative colitis (UC) are associated with distinct
populations of Th cells: High Th1 cell numbers are observed in the
injured intestinal lamina propria of patients with CD, whereas Th2
cells are observed in UC (24). In
addition, Th17 cells have been associated with CD and UC (25). A significant increase in Th17 cell
numbers in the DSS and TNBS groups was reported compared with the
control; however, a significant reduction was observed in model
groups treated with R. intestinalis (Fig. 5A and B). On the contrary, no
significant changes in the overall numbers of Th1 and Th2 cells
were observed between the model and R. intestinalis-treated
groups (Fig. 5C and D). IL-6 is a
pleiotropic cytokine that functions as a central regulator of the
adaptive immune response in IBD; it can also serve as a promoter of
Th17 cell differentiation (11,26).
Inhibition of IL-6 expression may be the cause of depleted Th17
cell numbers. Thus, R. intestinalis supernatant was proposed
to inhibit the differentiation of CD4+ T cells,
particularly Th17 cells.
 | Figure 5.Fewer Th17 cells are detected in mice
with colitis exposed to R. intestinalis supernatant. (A)
Representative flow cytometry plots and (B) statistical analysis of
the percentage of CD4+IL-17+ cells (Th17
cells) within the splenic cell population (n=6). The percentage of
(C) CD4+IFN-γ+ T cells (Th1) and (D)
CD4+IL-4+ T cells (Th2) in the spleen was
analyzed by flow cytometry (n=6). Data are representative of three
independent experiments. Error bars represent the mean ± standard
error of the mean. *P<0.05, **P<0.01 vs. control.
##P<0.01 vs. DSS or TNBS; ns, non-significant; APC,
allophycocyanin; DSS, dextran sulfate sodium; FITC, fluorescein
isothiocyanate; IFN-γ, interferon-γ; IL, interleukin; SUP,
supernatant treatment; TNBS, 2,4,6-trintirobenzenesulfonic
acid. |
R. intestinalis produces SCFAs,
including butyrate
Finally, GC-MS analysis was conducted to examine
whether non-protein components in the bacterial supernatant serve a
role in regulating anti-inflammatory responses. Propionic acid,
isobutyric acid, butyric acid, isovaleric acid, valeric acid and
hexanoic acid were detected, and the presence of SCFAs in the R.
intestinalis supernatant was confirmed (data not shown). In
addition, SCFAs in the cecal contents of mice were determined. A
notable increase in the levels of SCFAs in the DSS/TNBS + SUP
groups was observed compared with in the DSS and TNBS groups,
although the differences were not statistically significant
(Fig. 6A and B). The results also
revealed that propionic acid and butyric acid were the most
frequently detected SCFAs in the samples, which may be associated
with the anti-inflammatory effects of R. intestinalis in the
colitis models employed in the present study. Sodium butyrate and
R. intestinalis supernatant had similar effects with respect
to inhibiting IL-6 expression by macrophages in vitro
(Fig. S2).
Discussion
Our previous study examined the anti-inflammatory
properties of R. intestinalis bacteria; reductions in IL-17
levels were reported in an animal model of colitis treated with
R. intestinalis and in a cellular model of inflammation
(LPS-treated NCM460 colon cells co-cultured with R.
intestinalis) (5).
Additionally, R. intestinalis was reported to exert
protective effects in a TNBS-induced colitis model by promoting
Treg differentiation in the colon and the secretion of
anti-inflammatory mediators, including IL-10, TSLP, and TGF-β
(4). In addition, the bacteria
have similar anti-inflammatory effects on LPS-induced Caco2 colon
cells (4). The findings of these
aforementioned studies suggest that R. intestinalis could be
a candidate probiotic for the treatment of IBD; however, the
safety, efficacy and dose of bacteria for clinical practice remains
uncertain and further investigation is required (4,5). On
the contrary, examining the effects of R. intestinalis
supernatant may improve understanding of the interaction between
bacteria and host, and may aid the development of safer treatment
strategies for future clinical use.
The effects of R. intestinalis supernatant
were determined in the present study. The results indicated that
the supernatant inhibited the expression of IL-6 induced by LPS in
RAW264.7 macrophages and BMMs in vitro. A recent study
showed that the metabolites generated by the microbiota protect
IECs, and serve a role in the interaction between IECs and
macrophages (27). Conversely, it
was reported that R. intestinalis supernatant had no
significant effects on IL-6 expression by Caco2 colon cells in
vitro. Thus, it was proposed that R. intestinalis
supernatant exerts its function via immune cells and thus its
function was investigated in vivo.
In addition, the effects of the bacterial
supernatant were investigated in murine models of DSS- and
TNBS-induced colitis in the present study. Mice with DSS-induced
colitis are considered to be a model of human UC, whereas mice with
TNBS-induced colitis represent a model of human CD (18). These models are used widely to
undertake research on other commensal microbiota, such as
Faecalibacterium prausnitzii (11). Furthermore, compounds such as
recombinant human milk fat globule epidermal growth factor VIII
have been investigated in DSS- and TNBS-induced models to determine
their therapeutic potential in IBD (28). The present study revealed that
R. intestinalis supernatant ameliorated DSS- and
TNBS-induced colitis. These similar findings obtained from both
models suggest that R. intestinalis supernatant may exert
anti-inflammatory effects against IBD.
Macrophages in the colonic mucosa serve vital roles
in innate immune responses. The cells possess numerous functions,
including phagocytosis to remove pathogenic microorganisms and
apoptotic cells to maintain homeostasis, the secretion of various
cytokines involved in immune regulation, the presentation of
antigens to T cells and T cell activation (29,30).
In IBD, macrophages accumulate in the injured mucosa and secrete
IL-1β, IL-6 and TNF-α to induce inflammatory responses and maintain
inflammatory conditions (21). In
the present study, a significant reduction in the abundance of
colonic macrophages in TNBS + SUP group was observed, but not in
the DSS + SUP group. These differences between the two groups may
be due to the differing susceptibilities to the effects of R.
intestinalis between the DSS- and TNBS-induced colitis models.
A previous study reported that transgenic mouse expressing
TNF-α-induced protein 3 in IECs were protected from DSS-induced
colitis but not TNBS-induced colitis, also indicating the different
susceptibilities between DSS- and TNBS-induced colitis models
(31). In addition, dynamic
alterations in the number of colon macrophages in the DSS-induced
colitis model may underlie these observations (32). Further investigation using flow
cytometry assays of colon macrophages at several continuous time
points may provide further insight. However, the present study
reported an increase in the number of peritoneal inflammatory
macrophages in mice with DSS- and TNBS-induced colitis. Increased
IL-6 production was detected in LPS-induced macrophages in
vitro but was ameliorated by R. intestinalis
supernatant. These results suggest that R. intestinalis
supernatant functions by regulating the activity of macrophages.
Furthermore, accumulating evidence suggests that circulating or
local levels of IL-6 affect IBD, and that IL-6 promotes the
differentiation of CD4+ T cells into Th17 cells
(26,33). IL-6 is upregulated via activation
of the STAT3 signaling pathway under inflammatory conditions
(34). The results of the present
study revealed upregulated IL-6 and STAT3 expression, and increased
numbers of Th17 cells in DSS- and TNBS-induced colitis models;
opposing effects were observed following treatment with R.
intestinalis supernatant.
The components of R. intestinalis, such as
the flagellin protein, are essential for suppressing colitis via
binding to unique receptors such as Toll-like receptor 5 (TLR5)
(35). To determine whether
non-protein components exhibits any effects on BMMs, R.
intestinalis supernatant was irradiated with UV, heated to
>100°C and treated with trypsin. The supernatant was then used
to treat BMMs isolated from TLR5−/− mice. The results
suggested that R. intestinalis supernatant downregulated the
levels of IL-6 (Fig. S1B),
supporting that R. intestinalis supernatant contains active
non-protein components.
Additionally, GC-MS was conducted in the present
study to identify substances in the R. intestinalis
supernatant; SCFAs, including butyrate were detected. Butyrate
exerts an anti-inflammatory effect on immune cells involved in
inflammation; it suppresses Th9 and Th2-mediated immune responses
to attenuate inflammation in the lungs (36). Furthermore, microbiota-derived
butyrate inhibited the apoptosis of IECs, decreased the production
of inflammatory cytokines, including interferon-γ and IL-1β,
reduced intestinal inflammation and maintained intestinal
homeostasis (37). Several studies
have proposed that the molecular mechanism underlying the
anti-inflammatory effects of butyrate may involve downregulating
the expression of proinflammatory factors, such as NO, IL-6, and
IL-12, and regulating the activity of macrophages in the lamina
propria (14,27,38).
On the contrary, butyrate has no effect on the secretion of TNF-α
and C-X-C motif chemokine 12 by macrophages (14). In addition, R. intestinalis
supernatant did not significantly affect the expression of
LPS-induced TNF-α, opposing the effects of Faecalibacterium
prausnitzii supernatant (39).
Finally, LPS-induced RAW264.7 macrophages were
treated with the same concentration of sodium butyrate detected in
the supernatant. The similar results obtained for the sodium
butyrate and the R. intestinalis supernatant treatment
groups suggest that butyrate present in R. intestinalis
supernatant exerts anti-inflammatory effects (Fig. S2). However, further investigation
using sodium butyrate as a control, and combined with a butyrate
receptor inhibitor are required for further confirmation.
Collectively, the results of the present study
indicated that R. intestinalis supernatant regulated immune
responses and ameliorated DSS- and TNBS-induced colitis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81670504 and
81472287) and Natural Science Foundation of Hunan (grant no.
2017JJ3468).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL, BT and MX performed the experiments. CZ, LT and
YQ collected the data SW, ZY, XWu and XM analyzed the data, and WL
prepared the manuscript. ZS, MD and XL revised the manuscript and
contributed to study design. XWa designed the study and supervised
the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethical
Committee of Medical Research of Central South University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torres J, Mehandru S, Colombel JF and
Peyrin-Biroulet L: Crohn's disease. Lancet. 389:1741–1755. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ungaro R, Mehandru S, Allen PB,
Peyrin-Biroulet L and Colombel JF: Ulcerative colitis. Lancet.
389:1756–1770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Souza HSP, Fiocchi C and Iliopoulos D:
The IBD interactome: An integrated view of aetiology, pathogenesis
and therapy. Nat Rev Gastroenterol Hepatol. 14:739–749. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen Z, Zhu C, Quan Y, Yang J, Yuan W,
Yang Z, Wu S, Luo W, Tan B and Wang X: Insights into Roseburia
intestinalis which alleviates experimental colitis pathology by
inducing anti-inflammatory responses. J Gastroenterol Hepatol.
33:1751–1760. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu C, Song K, Shen Z, Quan Y, Tan B, Luo
W, Wu S, Tang K, Yang Z and Wang X: Roseburia intestinalis inhibits
Interleukin17 Excretion and promotes regulatory T cells
differentiation in colitis. Mol Med Rep. 17:7567–7574.
2018.PubMed/NCBI
|
|
6
|
Duncan SH, Hold GL, Barcenilla A, Stewart
CS and Flint HJ: Roseburia intestinalis sp. nov., a novel
saccharolytic, butyrate-producing bacterium from human faeces. Int
J Syst Evol Microbiol. 52:1615–1620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cani PD: Human gut microbiome: Hopes,
threats and promises. Gut. 67:1716–1725. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goncalves P, Araujo JR and Di Santo JP: A
Cross-talk between Microbiota-derived short-chain fatty acids and
the host mucosal immune system regulates intestinal homeostasis and
inflammatory bowel disease. Inflamm Bowel Dis. 24:558–572. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arpaia N, Campbell C, Fan X, Dikiy S, van
der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ and
Rudensky AY: Metabolites produced by commensal bacteria promote
peripheral regulatory T-cell generation. Nature. 504:451–455. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh N, Gurav A, Sivaprakasam S, Brady E,
Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et
al: Activation of Gpr109a, receptor for niacin and the commensal
metabolite butyrate, suppresses colonic inflammation and
carcinogenesis. Immunity. 40:128–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou L, Zhang M, Wang Y, Dorfman RG, Liu
H, Yu T, Chen X, Tang D, Xu L, Yin Y, et al: Faecalibacterium
prausnitzii produces butyrate to maintain Th17/Treg balance and to
ameliorate colorectal colitis by inhibiting histone deacetylase 1.
Inflamm Bowel Dis. May 23–2018.(Epub ahead of print). doi:
10.1093/ibd/izy182. View Article : Google Scholar :
|
|
12
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory A, .
The National Academies Collection: Reports funded by National
Institutes of Health. Guide for the Care and Use of Laboratory
Animals. 8th. National Academies Press (US); Washington, DC: 2011,
PubMed/NCBI
|
|
13
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang PV, Hao L, Offermanns S and
Medzhitov R: The microbial metabolite butyrate regulates intestinal
macrophage function via histone deacetylase inhibition. Proc Natl
Acad Sci USA. 111:2247–2252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini
V, Mardis ER and Gordon JI: An obesity-associated gut microbiome
with increased capacity for energy harvest. Nature. 444:1027–1031.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samuel BS and Gordon JI: A humanized
gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc
Natl Acad Sci USA. 103:10011–10016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wirtz S, Popp V, Kindermann M, Gerlach K,
Weigmann B, Fichtner-Feigl S and Neurath MF: Chemically induced
mouse models of acute and chronic intestinal inflammation. Nat
Protoc. 12:1295–1309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dale DC, Boxer L and Liles WC: The
phagocytes: Neutrophils and monocytes. Blood. 112:935–945. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gren ST and Grip O: Role of monocytes and
intestinal macrophages in crohn's disease and ulcerative colitis.
Inflamm Bowel Dis. 22:1992–1998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou B, Xia X, Wang P, Chen S, Yu C, Huang
R, Zhang R, Wang Y, Lu L, Yuan F, et al: Induction and amelioration
of methotrexate-induced gastrointestinal toxicity are related to
immune response and gut microbiota. EBioMedicine. 33:122–133. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitsuyama K, Matsumoto S, Masuda J,
Yamasakii H, Kuwaki K, Takedatsu H and Sata M: Therapeutic
strategies for targeting the IL-6/STAT3 cytokine signaling pathway
in inflammatory bowel disease. Anticancer Res. 27:3749–3756.
2007.PubMed/NCBI
|
|
24
|
Imam T, Park S, Kaplan MH and Olson MR:
Effector T helper cell subsets in inflammatory bowel diseases.
Front Immunol. 9:12122018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen ML and Sundrud MS: Cytokine networks
and T-Cell subsets in inflammatory bowel diseases. Inflamm Bowel
Dis. 22:1157–1167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagashima H, Ishii N and So T: Regulation
of interleukin-6 receptor signaling by TNF receptor-associated
factor 2 and 5 during differentiation of inflammatory CD4(+) T
cells. Front Immunol. 9:19862018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen G, Ran X, Li B, Li Y, He D, Huang B,
Fu S, Liu J and Wang W: Sodium butyrate inhibits inflammation and
maintains epithelium barrier integrity in a TNBS-induced
inflammatory bowel disease mice model. EBioMedicine. 30:317–325.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Brenner M, Yang WL and Wang P:
Recombinant human MFG-E8 ameliorates colon damage in DSS- and
TNBS-induced colitis in mice. Lab Invest. 95:480–490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rhee L, Murphy SF, Kolodziej LE, Grimm WA,
Weber CR, Lodolce JP, Chang JE, Bartulis SJ, Messer JS, Schneider
JR, et al: Expression of TNFAIP3 in intestinal epithelial cells
protects from DSS-but not TNBS-induced colitis. Am J Physiol
Gastrointest Liver Physiol. 303:G220–G227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang W, XY L, Zheng D, Zhang D, Huang S,
Zhang X, Ai F, Wang X, Ma J, Xiong W, et al: Dynamic changes of
peritoneal macrophages and subpopulations during ulcerative colitis
to metastasis of colorectal carcinoma in a mouse model. Inflamm
Res. 62:669–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bettelli E, Carrier Y, Gao W, Korn T,
Strom TB, Oukka M, Weiner HL and Kuchroo VK: Reciprocal
developmental pathways for the generation of pathogenic effector
TH17 and regulatory T cells. Nature. 441:235–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quan Y, Song K, Zhang Y, Zhu C, Shen Z, Wu
S, Luo W, Tan B, Yang Z and Wang X: Roseburia intestinalis-derived
flagellin is a negative regulator of intestinal inflammation.
Biochem Biophys Res Commun. 501:791–799. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vieira RS, Castoldi A, Basso PJ, Hiyane
MI, Câmara NOS and Almeida RR: Butyrate attenuates lung
inflammation by negatively modulating Th9 cells. Front Immunol.
10:672019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhong X, Zhang Z, Wang S, Cao L, Zhou L,
Sun A, Zhong Z and Nabben M: Microbial-Driven butyrate regulates
Jejunal homeostasis in piglets during the weaning stage. Front
Microbiol. 9:33352019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chakravortty D, Koide N, Kato Y, Sugiyama
T, Mu MM, Yoshida T and Yokochi T: The inhibitory action of
butyrate on lipopolysaccharide-induced nitric oxide production in
RAW 264.7 murine macrophage cells. J Endotoxin Res. 6:243–247.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martin R, Chain F, Miquel S, Lu J,
Gratadoux JJ, Sokol H, Verdu EF, Bercik P, Bermudez-Humaran LG and
Langella P: The commensal bacterium Faecalibacterium prausnitzii is
protective in DNBS-induced chronic moderate and severe colitis
models. Inflamm Bowel Dis. 20:417–430. 2014. View Article : Google Scholar : PubMed/NCBI
|




















