Introduction
Osteonecrosis of the femoral head (ONFH) is a
debilitating skeletal disorder that occurs in young individuals. Up
to 20,000 patients are diagnosed with ONFH in the United States
annually (1,2). In China, it is estimated that the
total number of patients with ONFH is 5–7.5 million with
10,000-20,000 new cases each year (3). However, the pathogenesis of ONFH
remains unclear, and effective preventive treatment strategies are
urgently required. Once diagnosed, ~80% of patients typically
progress to femoral head collapse within 1–5 years if it is not
treated in a timely manner, and total hip arthroplasty (THA) is
often required (4). However,
limitations in the field of material science mean that, artificial
hip joints are still associated with a limited service life and
serious complications (5).
Therefore, it is vital to identify methods to prevent the collapse
of the femoral head and delay the occurrence of THA in the early
stages, as well as develop effective treatment methods for ONFH
(6). Core decompression combined
with bone mesenchymal stem cell (BMSC)-based therapy was recently
revealed to be a promising method to treat the early stages of ONFH
(7–12).
BMSCs are multipotent stem cells that have the
ability to self-renew and differentiate into osteoblasts,
chondrocytes and adipocytes to reproduce skeletal tissues. In
addition, BMSCs are associated with various technical advantages,
including easy collection, rapid expansion and low immunogenicity
(13,14). Thus, BMSCs have become an optimal
seed cell choice in bone tissue engineering. Tissue engineering
technology primarily uses exogenous/endogenous seed cells,
biomaterial scaffolds with good biocompatibility and degradability,
and biologically active molecules for reconstruction of defective
tissues or organs (15). A number
of studies suggested that BMSC-based tissue engineering has
specific effects on cartilage repair and bone regeneration
(16–19). For instance, a dually optimized
silk-fibroin-gelatin scaffold combined with endogenic BMSC could
repair cartilage injury in vitro and in vivo
(16). Another study demonstrated
that gene-transfected MSCs seeded on β-tricalcium phosphate (β-TCP)
ceramic scaffolds promoted bone regeneration, suggesting this may
be a potential strategy for the treatment of ONFH (17). Unfortunately, the low usage
efficiency of exogenous BMSCs due to their poor adhesion on
biomaterial scaffolds is a complication in BMSC-based tissue
engineering (18,20,21).
Bioactive molecules, particularly polypeptides, have been used to
modify biomaterial scaffolds (19,22–24).
Compared with linear peptides which these studies have focused on
(19,22–24),
cyclic peptides have several favorable properties including high
affinity, selectivity and stability to protein targets (25). Phage display technology has been
widely used to screen small peptides with high affinity and
specificity for targets of interest, including small molecules,
cells, tissues and materials, through biopanning procedures
(26–28).
Previous studies have proven the success of using
BMSCs combined with various biomaterial scaffolds in bone
regeneration (29,30). The β-TCP scaffold is a bone tissue
engineering material that has been widely used in the clinic for
decades. The β-TCP scaffolds have the advantages of
biocompatibility and biodegradability, and favorable osteogenesis
and angiogenesis properties (31–33).
Several studies have demonstrated that using β-TCP scaffolds
combined with BMSCs could promote bone regeneration in vitro
and in vivo (18,34–36).
The porous structure of β-TCP provides a scaffold for the adhesion
and growth of BMSCs while the connection between pores facilitates
the blood supply of the scaffolds. However, the adhesion of BMSCs
on β-TCP scaffolds is considered unsatisfactory, reducing the
effectiveness of this promising method (18,21,37).
Recently, studies have demonstrated that the implantation of β-TCP
bioceramic scaffolds can treat early stage ONFH effectively
(31,38,39).
However, up to date, no research has focused on the use of cyclic
peptides to improve the adhesion of BMSCs on β-TCP scaffolds in the
tissue engineering treatments for ONFH.
In the present study, β-TCP scaffolds were covered
with bioactive cyclic peptides in order to improve the biofunction
of β-TCP scaffolds in tissue engineering in vitro. A
specific affinity cyclic peptide for rat BMSCs was screened from
the cyclic peptide phage display library (Ph.D.™-C7C) using phage
display technology. The affinity of the cyclic peptide for BMSCs
was detected by fluorescence staining and flow cytometry.
Furthermore, the cyclic peptides were also placed over the surface
of the β-TCP scaffolds in order to investigate their effect on the
adhesion, expansion and proliferation of BMSCs.
Materials and methods
BMSC culture
Sprague-Dawley rat BMSCs were purchased from Cyagen
Biosciences, Inc. (cat. no. RASMX-01001). Cells were cultured in
low glucose Dulbecco's modified Eagle's medium (DMEM; cat. no.
01-051-1A; Biological Industries, Ltd.) with 10% fetal bovine serum
(cat. no. 04-400-1A; Biological Industries, Ltd.) and 1%
penicillin-streptomycin at 37°C in a 5% CO2 incubator.
The trilineage-induced differentiation capacity of rat BMSCs was
confirmed following a previously described method (22). Briefly, passage 2 BMSCs were
induced with osteogenic, adipogenic and chondrogenic
differentiation medium obtained from Cyagen Biosciences, Inc. (cat.
nos. RASMX-90021, 90031 and 90041) according to the manufacturer's
instructions. After two weeks of culture, BMSCs were fixed with 4%
neutral formaldehyde for 30 min. The cells for osteogenesis were
stained with Alizarin red staining solution for 5 min and the cells
for adipogenesis were stained with Oil Red O staining solution for
30 min, both at room temperature. The cells for chondrogenesis were
fixed with 4% neutral formaldehyde for 30 min after three weeks of
culture and were stained with Alcian Blue staining solution for 30
min at room temperature. Rat BMSCs of passages 3–6 were used in
subsequent experiments.
Biopanning of cyclic peptides by phage
display
Based on the different binding affinity abilities of
the cyclic peptide in a loop-constrained heptapeptide (Ph.D.™-C7C)
phage display library and the target cell, the peptide that
specifically bound to the BMSCs was selected through biopanning of
the phage display technology. The biopanning procedure was
performed following a previous described method with modifications
(23,40).
The cyclic peptide phage display library used in the
biopanning experiment was constructed using a Ph.D.™-C7C Phage
Display Peptide Library kit (cat. no. E8120S; New England BioLabs,
Inc.). The Ph.D.™-C7C library has complexity on the order of
109 independent clones. A pair of bilateral cysteine
residues of the randomized 7 amino acids were oxidized to form a
disulfide linkage, presenting the peptides to the cells as loops.
BMSCs were cultured in 6 mm petri dishes and were washed twice with
PBS (cat. no. 02-024-1A; Biological Industries, Ltd.) before
blocking with DMEM supplemented with 5 mg/ml bovine serum albumin
(BSA; cat. no. B2064-10G; Sigma-Aldrich; Merck KGaA) at 37°C in an
atmosphere containing 5% CO2 for 1 h. Cells were then
washed six times with Tris-buffered saline with Tween 20 (TBST; 50
mM Tris-HCl pH 7.5, 150 mM NaCl +0.1% Tween-20) 6 times. Following
this, the BMSCs were incubated with the Ph.D.™-C7C phage display
library [1×1011 phage forming units (PFU)] for 1 h to
allow the peptides to bind to the cells. Subsequently, the unbound
phage clones were discarded and the poorly bound peptides were
removed by washing BMSCs 10 times with TBST. The bound phages were
eluted with 1 ml of 0.2 M glycine-HCl (pH 2.2; Sigma-Aldrich; Merck
KGaA) combined with 1 mg/ml of BSA for 10 min. The elutions were
neutralized with 150 µl of 1 M Tris-HCl (pH 9.1). The eluted phage
clones that had bound to the BMSCs were then amplified in
Escherichia coli ER2738 (provided in the Ph.D.™-C7C kit;
cat. no. E8120S; New England BioLabs, Inc.). Subsequently,
titration and purification of the bound phage clones were performed
according to the manufacturer's protocol. In total, three rounds of
biopanning were conducted to screen the BMSC-specific affinity
cyclic peptides.
Peptide sequencing and cyclic peptide
synthesis
Following two or three rounds of biopanning, the
phage clones were selected randomly for sequencing. Phage DNA were
sequenced by Genewiz, Inc., with Sanger sequencing. The primer used
for sequencing was 5′-HOCCCTCATAGTTAGCGTAACG-3′ (the −96
gIII sequencing primer was provided in the Ph.D.™-C7C kit).
Subsequently, the amino acid sequences of the presented cyclic
peptides were analyzed using Chromas 2.6.6 software (Technelysium
Pty Ltd.).
Through the biopanning experiment, a specific cyclic
peptide in the BMSC-affinity clones was identified in the present
study, which was termed C7. A scrambled version of the peptide
containing the same seven amino acids between the two ends of
cysteine in C7 was used as the negative control and termed S7. A
fibronectin-derived peptide containing three amino acids (arginine,
glycine and aspartic acid), which was confirmed to have a high
affinity for BMSCs, was used as the positive control and was termed
RGD (21). All of the peptides
were chemically synthesized using solid-phase peptide synthesis
(Scilight Biotechnology, LLC), according to our previous study
(41). The molecular weight of the
synthesized C7 was determined using matrix-assisted laser
desorption/ionization-time of flight mass spectrometry (JBI
Scientific, LLC.) with positive mode, according to our previous
study (42). The calibration
matrix was α-cyano-4-hydroxycinnamic acid and the instrument
settings were as follows: 20,000 V accelerating voltage, 95% grid
voltage, 200 nsec extraction delay time and αν acquisition mass
range between 600 and 2,000 kDa. An extra aminohexanoic acid was
connected at the amino (N) terminus of all peptides to facilitate
fluorescein-5- isothiocyanate (FITC; Scilight Biotechnology, LLC)
labeling. Following FITC labeling, the peptides were stored at
−20°C. The FITC-labeled peptides were then dissolved in PBS at the
concentration of 1 mg/ml prior to usage.
Cyclic peptide affinity assay
The cyclic peptide affinity assay was performed in
accordance with previous studies with minor modifications (22,40).
BMSCs were cultured on 6-well tissue culture plates
until 70–80% confluence was reached. Following this, BMSCs were
cultured with 10 µM FITC-labeled peptides for 1 h at 37°C to allow
binding of the peptides to the cells and internalization. The cells
were washed with PBS three times to remove non-bound peptides.
Cells were then digested from the culture plate and centrifuged at
250 × g for 5 min at room temperature. Cell pellets were
resuspended in PBS and subjected to flow cytometry (BD LSR
Fortessa™; Becton, Dickinson and Company). The BMSC affinity
properties of the cyclic peptide and two control peptides were
analyzed quantitively by flow cytometry at a wavelength of 488 nm,
using FlowJo v10 (Tree Star, Inc). Furthermore, the BMSCs were
cultured on 3.5 mm culture dishes (cat. no. D35-10-0-N; Cellvis)
specifically for confocal laser microscopy until 70–80% confluence
was reached. Cells were incubated with 10 µM FITC-labeled peptides
for 1 h at 37°C and washed three times with PBS. Following this,
the cells were incubated with phalloidin (Phalloidin-iFluor 594
reagent; cat. no. ab176757; Abcam) for 45 min to display the
cytoskeleton. The nuclei were counterstained with DAPI (cat. no.
C0065; Beijing Solarbio Science & Technology Co., Ltd.) and the
cells were examined under a confocal laser microscope (Zeiss LSM
800; Carl Zeiss Meditec AG). The cyclic peptide affinity assay was
repeated at least three times.
Bonding of cyclic peptides and β-TCP
scaffolds
Sterile β-TCP scaffolds were purchased from Shanghai
Bio-lu Biomaterials Co., Ltd. Each scaffold was cylindrically
shaped, with a diameter of 10 mm and a thickness of 3 mm. The
porous β-TCP scaffolds had micropores (diameter, 500–600 µm;
interconnection diameter, 120 µm). The C7, S7 and RGD peptides were
bound to the scaffolds through absorption and a freeze-drying
process according to a previously described method with minor
modifications (42,43). The peptides were dissolved in
sterile PBS to a concentration of 0.1 mg/ml. Following this, the
β-TCP scaffolds were placed in the peptide solution and gently
shaken to ensure that the peptides were fully adsorbed onto all
exposed surfaces of the β-TCP scaffolds. The β-TCP scaffolds and
peptide solution were incubated at 4°C for 24 h. Subsequently, the
scaffolds were washed gently with PBS to remove the non-adsorbed
peptides, and dried in a freeze dryer (Alpha 1–2 LDplus; Martin
Christ Gefriertrocknungsanlagen GmbH) for 1 h. After bonding with
cyclic peptide, the scaffolds were stored in Eppendorf tubes at 4°C
without light or moisture prior to the experiments. The
peptide-bound β-TCP scaffolds were observed under a confocal laser
microscope.
BMSC behavior on the cyclic peptide-bound
β-TCP scaffolds
Cell adhesion
The BMSC suspensions (100 µl; 3×105
cells) were seeded on the C7-bound β-TCP scaffolds in 24-well
culture plates. BMSCs were also seeded, at the same concentration
as the C7-bound scaffolds, on the S7-bound and pure β-TCP scaffolds
as the negative control and on RGD-bound scaffolds as the positive
control. Following incubation for 12 and 24 h, the effluent
non-adherent cells of four types of scaffolds were separately
collected. Meanwhile, the scaffolds were washed with PBS three
times to collect the inner non-adherent BMSCs. The total effluent
cells were counted using a hemocytometer. The effluent cells of
different scaffolds referred to BMSCs which were not adherent to
the scaffolds. The number of effluent cells of each scaffold
revealed the extent of BMSC adhesion and the recruitment capacity
of BMSCs (16). The experiment was
repeated at least three times.
Cell expansion
BMSCs were seeded on the C7-bound β-TCP scaffolds at
a density of 2×104 cells/ml. The S7-bound scaffolds and
the pure β-TCP scaffolds were used as the negative control while
the RGD-bound scaffolds were used as the positive control.
Following 24 h of culture, the scaffolds were stained with
phalloidin for 45 min to reveal the cytoskeleton of the cells and
examined under a confocal laser microscope. The experiment was
repeated at least three times.
Cell proliferation
To determine BMSC viability and proliferation on the
different scaffolds, a Cell Counting Kit-8 (CCK-8; cat. no. CK04;
Dojindo Molecular Technologies, Inc.) assay was performed according
to the manufacturer's protocol. The C7-bound scaffolds, pure β-TCP
scaffolds (negative control) and the RGD-bound scaffolds (positive
control) were used. BMSCs (1×104 cells) were seeded onto
the three types of peptide-bound scaffolds in 24-well culture
plates. Following 1, 3, 5 and 7 days of culture, 30 µl of CCK-8
solution was added to fresh DMEM and incubated at 37°C for 2 h.
Following this, the optical density (OD) value was measured using a
96-well reader plate at 450 nm. The experiment was repeated at
least three times.
Statistical analysis
All data were expressed as the mean ± standard
deviation, and were analyzed using one-way or two-way analysis of
variance followed by Tukey's test for comparison of multiple
groups. GraphPad Prism 7.0 software (GraphPad Software, Inc.) was
used for data analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of the high-affinity
rat BMSC cyclic peptide
Rat BMSCs were characterized using the
trilineage-induced differentiation experiment (Fig. S1). The recovery efficiency after
each round of biopanning was counted as the output titer divided by
the input titer of the phage clones. The input titer of the C7C
phages was 1.0×1011 PFU for each round of biopanning. As
presented in Table I, the optimal
recovery efficiency was obtained in the third round of biopanning,
which was 310-fold higher than that of the first round. The C7C
phage clones in the last two rounds of biopanning were selected for
sequencing. A total of 12 affinity phage clones were selected
randomly in each round of sequencing. The BMSC-specific affinity
cyclic peptide, CTTNPFSLC (termed C7), was subsequently identified
using Sanger sequencing and Chromas analysis software (Table II and Fig. 1A). Furthermore, the C7 phage clones
appeared five and six times (50%) among all of the assayed clones,
in the second and third rounds of sequencing, respectively
(Table II). In addition, other
cyclic peptides (Table II) were
also identified as candidates for BMSC-affinity peptide through the
biopanning process. These results suggested high BMSC affinity of
C7, which was further explored in the subsequent experiments.
 | Table I.Recovery efficiency of
biopanning. |
Table I.
Recovery efficiency of
biopanning.
|
| Input titer
(PFU) | Output titer
(PFU) | Recovery
efficiency | Fold increase |
|---|
| Round 1 |
1.0×1011 |
5.8×104 |
5.8×10−7 | 1 |
| Round 2 |
1.0×1011 |
3.9×106 |
3.9×10−5 | 67 |
| Round 3 |
1.0×1011 |
1.8×107 |
1.8×10−4 | 310 |
 | Table II.Peptide sequences. |
Table II.
Peptide sequences.
| Round 1 | Round 2 | Round 3 |
|---|
| No | CTTNPFSLC
(5/12) | CTTNPFSLC
(6/12) |
| sequencing | CRLSMETVC
(3/12) | CQAPHKPWC
(2/12) |
|
| CQAPHKPWC
(1/12) | CRLSMETVC
(1/12) |
|
| CHNSKSTTC
(1/12) | CPSSMRGTC
(1/12) |
|
| CPSSMRGTC
(1/12) | CDLLESERC
(1/12) |
|
| CILKKNVSC
(1/12) | CKMWNGSGC
(1/12) |
Affinity of C7 for BMSCs
The affinity of the C7 cyclic peptide for BMSCs was
further assayed. The C7 cyclic peptide was synthesized and the
molecular weight of C7 was examined to be 1,006.95 kDa by mass
spectrometry (Fig. 1B). A peptide
with the same seven amino acids between the two ends of cysteine as
C7 in a scrabbled order (CTFNLPTSC) was used as the negative
control (S7). A peptide containing three amino acids (arginine,
glycine and aspartic acid) was used as the positive control (RGD).
Following incubation with FITC-C7, FITC-S7 and FITC-RGD for 1 h,
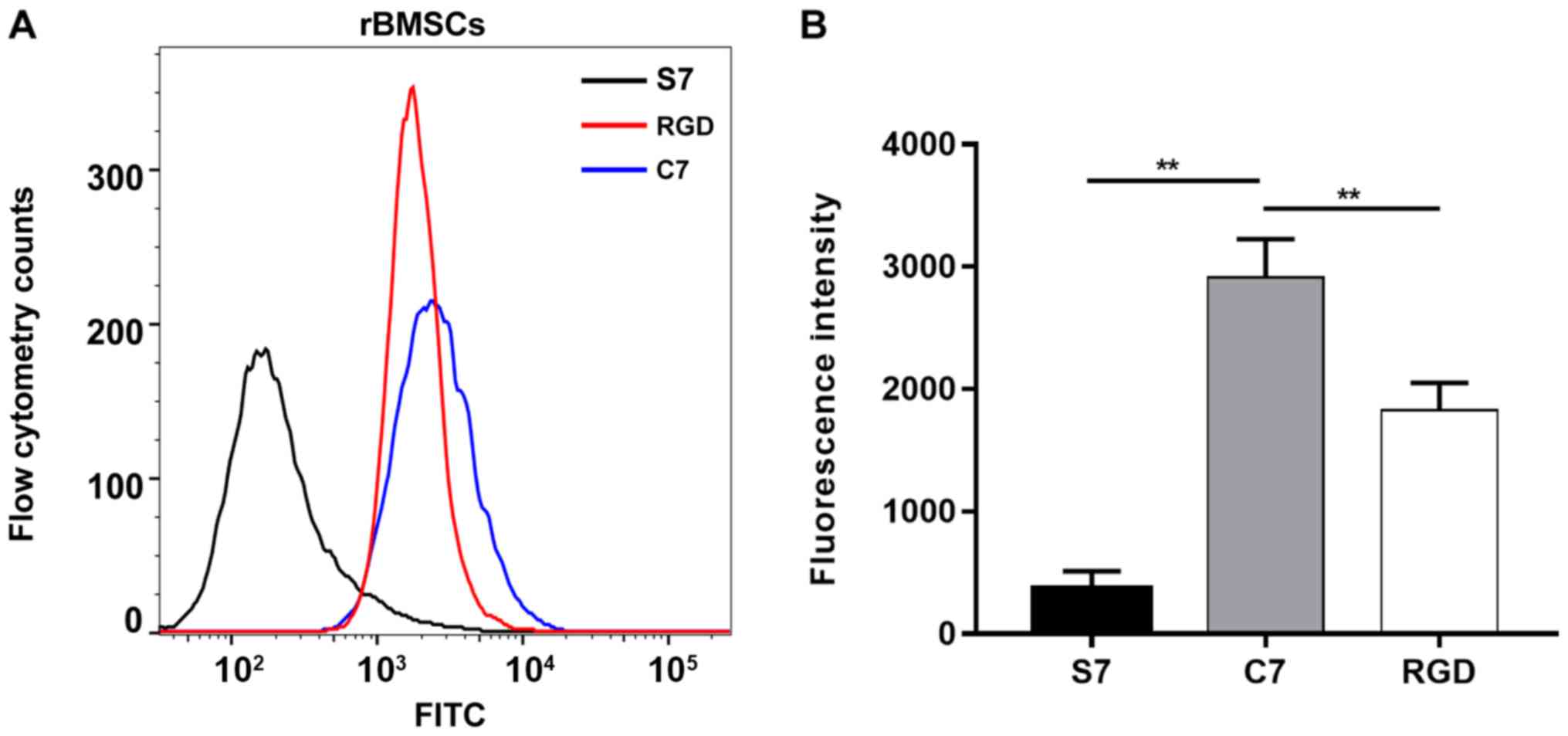
the BMSCs were examined by flow cytometry. The average fluorescence
intensity was 2,913.0±311.0 for the rat BMSCs incubated with
FITC-C7, 1,928.0±222.5 for the rat BMSCs incubated with FITC-RGD
and 369.0±141.1 for the rat BMSCs incubated with FITC-S7 (Fig. 2A). The average fluorescence
intensity of the BMSCs incubated with FITC-C7 was significantly
increased compared with the FITC-S7 and FITC-RGD groups (n=3;
P<0.01; Fig. 2B). In addition,
strong fluorescent signals were observed in the FITC-C7 and
FITC-RGD groups under confocal laser microscopy, whereas faint
signals were observed in FITC-S7 treated cells (Fig. 3). These results suggested that C7
had a high affinity for the BMSCs, which was superior when compared
with the positive control RGD.
C7 cyclic peptide binding to the β-TCP
scaffolds
The C7, S7 and RGD peptides were bound to the β-TCP
scaffolds for 24 h using natural absorption and the freeze-drying
process. The peptide-bound β-TCP scaffolds were observed under a
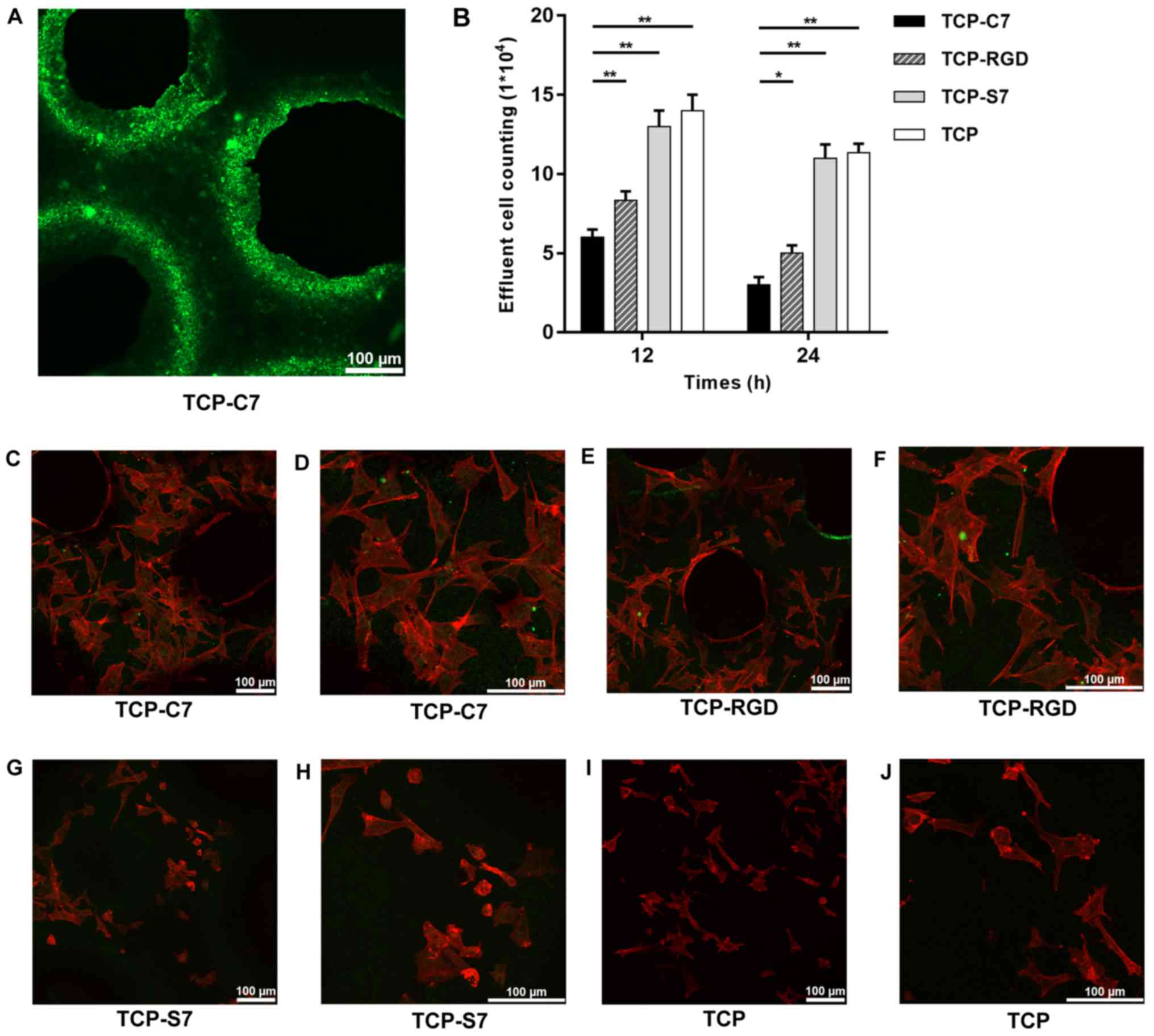
confocal laser microscope. As indicated in Fig. 4A, C7-bound β-TCP scaffolds
exhibited homogeneous green fluorescence, indicating that the
peptides were bound homogeneously on the surface of the β-TCP
scaffolds. This result indicated the stable construction of
C7-bound β-TCP scaffolds.
Impact of the C7 cyclic peptide on the
adhesion, expansion and proliferation of BMSCs on β-TCP
scaffolds
Following 12 and 24 h incubation on the four types
of scaffolds, the effluent amount of BMSCs on the C7-bound
scaffolds was lower compared with the S7-bound, the RGD-bound and
the pure β-TCP scaffolds (Fig.
4B), indicating that the C7 peptide improved the cell adhesion
and the recruitment capacity of BMSCs on the β-TCP scaffolds.
To investigate the expansion of the BMSCs, cells
were seeded on C7-, S7-, RGD-bound and pure β-TCP scaffolds.
Following 24 h of culture in vitro, the cells on the C7- and
RGD-bound scaffolds were fully attached to the scaffolds and
exhibited superior expansion morphologies when compared with the
surface of the S7-bound and the pure β-TCP scaffolds (Fig. 4C-J). These results suggested that
the C7 peptide promoted the expansion of BMSCs on the β-TCP
scaffolds.
Next, the impact of C7 on cell proliferation was
assayed. There was a significant difference in the numbers of
viable BMSCs following 1, 3, 5 and 7 days of incubation among the
C7- and RGD-bound, and pure β-TCP scaffolds. The OD value of BMSCs
on C7-bound scaffolds was significantly increased compared with the
pure TCP scaffolds and the RGD-bound scaffolds following 1, 3, 5
and 7 days of incubation (n=3; P<0.05; Fig. 5). Thus, it could be concluded that
the C7-bound scaffolds significantly improved the proliferation of
BMSCs following a period of incubation. In addition, the OD value
of BMSCs seeded on C7-bound scaffolds increased with the passage of
time, demonstrating that the C7-bound scaffolds had good
biocompatibility and no cytotoxicity towards BMSCs.
Discussion
In the present study, a BMSC-specific affinity
cyclic peptide (C7), which contained 7 amino acids and was
disulfide cyclized, was screened using phage display technology
with a Ph.D.™-C7C phage display library. The biopanning process
revealed that C7 had a specific affinity for BMSCs. Following the
identification of C7, flow cytometry and fluorescent staining
assays were conducted in the present study, which confirmed the
high affinity of C7 for BMSCs in vitro. In addition, β-TCP
scaffold surfaces were uniformly covered with C7. Subsequent
experiments demonstrated improved adhesion, expansion and
proliferation on C7-bound β-TCP scaffolds when compared with pure
β-TCP scaffolds. In summary, the present study identified a
BMSC-affinity cyclic peptide (C7) which could promote adhesion,
expansion and proliferation of BMSCs on β-TCP scaffolds in
vitro.
Recently, research into the bio-functionalization of
bone tissue engineering materials has become a primary focus in the
field of bone tissue engineering. The application of bioactive
substances can modify bone tissue engineering materials to improve
their histocompatibility and biological activity. Thus, tissue
engineering materials are developed from inactive materials to
active materials. A common method is to modify bioactive
substances, including short peptides, proteins, growth factors and
other bioactive molecules, on to the surface of the materials by
means of absorption or covalent coupling (44,45).
Among those bioactive molecules, the biomimetic peptides are the
most widely studied in the modification of bio-functionalized
scaffolds due to their high purity, great bioactivity and
convenient synthesis (46).
Notably, our previous studies attempted to modify synthesized
scaffolds with the RGD peptide and linear peptides screened by
phage display technology in order to promote cell adhesion and
osteogenesis (24,47). The RGD peptide is an essential cell
adhesion peptide derived from fibronectin in the extracellular
matrix (48). Furthermore, the RGD
peptide acts as a binding ligand to the extracellular matrix
protein of the targeted cells, and binds to the integrin subunit of
the cell membrane. A number of biomaterials have been modified with
RGD for medical studies and applications (49,50).
However, this peptide lacks BMSC specificity due to the existence
of fibronectin receptors in all cells. Recently, the utility of
phage display technology has become a promising technique to search
for peptides that have high BMSC affinity and specificity (22,23).
According to several studies, cyclic peptides are associated with
properties that make them attractive potential agents for research
applications, drug development and therapeutics (25,51,52).
Compared with linear peptides, cyclic peptides have higher affinity
and selectivity for targets of interests, and they are more stable
due to their constrained-conformation. In addition, cyclic peptides
have low inherent toxicity, can be efficiently synthesized and
easily labeled (25). Salmasi
et al (53) reported that
the number of seed cells, and cell adhesion and expansion on
biomaterial scaffolds determined the ability of bone tissue
regeneration in bone tissue engineering.
Thus, in order to improve the recruitment and the
efficiency of BMSCs on biomaterial scaffolds in bone tissue
engineering, the present study identified a BMSC-specific affinity
cyclic peptide, C7, using phage display technology. Using three
rounds of biopanning, the recovery efficiency increased round by
round. Notably, the recovery efficiency of the third round
increased by 310-fold when compared with the initial round,
indicating that C7 could have a specific affinity for BMSCs. The
results of flow cytometry demonstrated that C7 exhibited improved
BMSC affinity compared with the control S7 and RGD peptides. In
addition, observation under a confocal laser microscope supported
the high affinity of C7 for BMSCs. These results were similar to
the affinity properties of the linear E7 peptide, which has also
been identified using phage display technology (21). However, it was hypothesized that C7
may have the advantage of increased affinity due to the
loop-constrained conformation with a disulfide linkage in the amino
acid sequence. Following modification of the β-TCP scaffolds with
C7 via absorption to their surface, results indicated that C7-bound
β-TCP scaffolds exhibited superior adhesion and expansion
morphologies under the confocal laser microscope when compared with
pure and S7-bound β-TCP scaffolds, indicating that the C7-bound
β-TCP scaffolds improved the biocompatibility of β-TCP scaffolds
and promoted the adhesion and expansion of BMSCs. The results of
effluent cell counting also supported that C7 could increase BMSC
adhesion to the β-TCP scaffolds when compared with the other three
types of scaffolds, including RGD-bound scaffolds. Thus, it could
be inferred that C7 may have a superior influence on BMSC adhesion
compared with the RGD peptide in vitro. The present study
also examined the proliferation ability of BMSCs on the different
scaffolds and the results were consistent with the results of cell
adhesion and expansion. The OD value at 1, 3, 5 and 7 days
demonstrated that C7-bound β-TCP scaffolds promoted the
proliferation of BMSCs, compared with RGD-bound scaffolds and pure
β-TCP scaffolds. The present results demonstrated that the cyclic
peptide C7 had a specific affinity for BMSCs and improved cell
adhesion, expansion and proliferation on β-TCP scaffolds. The
present study suggested that C7 could be used to modify different
biomaterials to construct bioactive scaffolds in BMSC-based tissue
engineering. Future studies will need to investigate further the
species specificity of C7 prior to its use in human-derived
mesenchymal stem cells in clinical research in patients.
The present study has several limitations. Although
C7 was isolated using phage display technology, the specificity of
C7 to other subtypes of cells has not been investigated. The exact
mechanism of the affinity interaction between the specific cyclic
peptide and BMSCs remains unclear due to the complexities of the
bioreceptors on the BMSC surface. Further studies should identify
the target receptors of C7 on BMSCs and clarify how C7 activates
the intracellular signaling pathway of BMSCs, thereby elucidating
how the biological activities of BMSCs are regulated. In addition,
further validation tests for the affinity of C7 for BMSCs in bone
tissue engineering are needed. The present study lacks results from
apoptosis assays to provide additional explanation of BMSCs
viability on β-TCP. Furthermore, combined with core decompression,
further investigation of C7-bound β-TCP scaffolds on the treatment
efficacy of ONFH in vivo is required in the future,
specifically regarding the chemotaxis of recruiting
exogenous/endogenous BMSCs and the impact on bone regeneration.
In conclusion, the present study identified a
specific affinity cyclic peptide, C7, for rat BMSCs through
biopanning using phage display technology. The loop-constrained
heptapeptide was confirmed to be specific and have high BMSC
affinity in vitro. In addition, in the present study,
C7-bound β-TCP scaffolds were constructed, which exhibited improved
BMSC adhesion, expansion and proliferation abilities when compared
with pure β-TCP scaffolds in vitro. These results suggested
that C7 could be used to enhance the recruitment of BMSCs on
biomaterial scaffolds, which could provide a novel method to
improve BMSC-based bone tissue engineering therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81702152) and the
Natural Science Foundation of Shandong Province, China (grant nos.
ZR2018MH007 and ZR2017BH015).
Availability of data and materials
The datasets generated and analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
TS, ZM and SS designed the experiments. TS performed
the experiments. TS, CP, and GW analyzed the data. TS wrote the
manuscript. TS revised the manuscript. All authors reviewed and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mont MA and Hungerford DS: Non-traumatic
avascular necrosis of the femoral head. J Bone Joint Surg Am.
77:459–474. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sultan AA, Mohamed N, Samuel LT, Chughtai
M, Sodhi N, Krebs VE, Stearns KL, Molloy RM and Mont MA:
Classification systems of hip osteonecrosis: An updated review. Int
Orthop. 43:1089–1095. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui L, Zhuang Q, Lin J, Jin J, Zhang K,
Cao L, Lin J, Yan S, Guo W, He W, et al: Multicentric epidemiologic
study on six thousand three hundred and ninety five cases of
femoral head osteonecrosis in China. Int Orthop. 40:267–276. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mont MA, Cherian JJ, Sierra RJ, Jones LC
and Lieberman JR: Nontraumatic osteonecrosis of the femoral head:
Where do we stand today? A ten-year update. J Bone Joint Surg Am.
97:1604–1627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu XW, Zi Y, Xiang LB and Wang Y: Total
hip arthroplasty: areview of advances, advantages and limitations.
Int J Clin Exp Med. 8:27–36. 2015.PubMed/NCBI
|
|
6
|
Millikan PD, Karas V and Wellman SS:
Treatment of osteonecrosis of the femoral head with vascularized
bone grafting. Curr Rev Musculoskelet Med. 8:252–259. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Persiani P, De Cristo C, Graci J, Noia G,
Gurzi M and Villani C: Stage-related results in treatment of hip
osteonecrosis with core-decompression and autologous mesenchymal
stem cells. Acta Orthop Belg. 81:406–412. 2015.PubMed/NCBI
|
|
8
|
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang
L, Liu B and Yu X: Treatment of early stage osteonecrosis of the
femoral head with autologous implantation of bone marrow-derived
and cultured mesenchymal stem cells. Bone. 50:325–330. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao Q, Jin H, Liao F, Xiao L, Chen D and
Tong P: The efficacy of targeted intraarterial delivery of
concentrated autologous bone marrow containing mononuclear cells in
the treatment of osteonecrosis of the femoral head: A five year
follow-up study. Bone. 57:509–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Papakostidis C, Tosounidis TH, Jones E and
Giannoudis PV: The role of ‘cell therapy’ in osteonecrosis of the
femoral head. A systematic review of the literature and
meta-analysis of 7 studies. Acta Orthop. 87:72–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piuzzi NS, Chahla J, Schrock JB, LaPrade
RF, Pascual-Garrido C, Mont MA and Muschler GF: Evidence for the
Use of Cell-Based Therapy for the Treatment of Osteonecrosis of the
Femoral Head: A Systematic Review of the Literature. J
Arthroplasty. 32:1698–1708. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piuzzi NS, Chahla J, Jiandong H, Chughtai
M, LaPrade RF, Mont MA, Muschler GF and Pascual-Garrido C: Analysis
of cell therapies used in clinical trials for the treatment of
osteonecrosis of the femoral head: A systematic review of the
literature. J Arthroplasty. 32:2612–2618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie XH, Wang XL, He YX, Liu Z, Sheng H,
Zhang G and Qin L: Promotion of bone repair by implantation of
cryopreserved bone marrow-derived mononuclear cells in a rabbit
model of steroid-associated osteonecrosis. Arthritis Rheum.
64:1562–1571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harada N, Watanabe Y, Sato K, Abe S,
Yamanaka K, Sakai Y, Kaneko T and Matsushita T: Bone regeneration
in a massive rat femur defect through endochondral ossification
achieved with chondrogenically differentiated MSCs in a degradable
scaffold. Biomaterials. 35:7800–7810. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghanaati S, Booms P, Orlowska A, Kubesch
A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C and
Choukroun J: Advanced platelet-rich fibrin: A new concept for
cell-based tissue engineering by means of inflammatory cells. J
Oral Implantol. 40:679–689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi W, Sun M, Hu X, Ren B, Cheng J, Li C,
Duan X, Fu X, Zhang J, Chen H and Ao Y: Structurally and
functionally optimized silk-fibroin-gelatin scaffold using 3d
printing to repair cartilage injury in vitro and in vivo. Adv
Mater. 29:2017. View Article : Google Scholar :
|
|
17
|
Guo X, Zheng Q, Kulbatski I, Yuan Q, Yang
S, Shao Z, Wang H, Xiao B, Pan Z and Tang S: Bone regeneration with
active angiogenesis by basic fibroblast growth factor gene
transfected mesenchymal stem cells seeded on porous beta-TCP
ceramic scaffolds. Biomed Mater. 1:93–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mebarki M, Coquelin L, Layrolle P,
Battaglia S, Tossou M, Hernigou P, Rouard H and Chevallier N:
Enhanced human bone marrow mesenchymal stromal cell adhesion on
scaffolds promotes cell survival and bone formation. Acta Biomater.
59:94–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Zhang X, Hu X, Shao Z, Zhu J, Dai
L, Man Z, Yuan L, Chen H, Zhou C, et al: A functional biphasic
biomaterial homing mesenchymal stem cells for in vivo cartilage
regeneration. Biomaterials. 35:9608–9619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diez-Escudero A, Espanol M, Bonany M, Lu
X, Persson C and Ginebra MP: Heparinization of beta tricalcium
phosphate: Osteo-immunomodulatory effects. Adv Healthc Mater.
7:2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kasten P, Luginbuhl R, van Griensven M,
Barkhausen T, Krettek C, Bohner M and Bosch U: Comparison of human
bone marrow stromal cells seeded on calcium-deficient
hydroxyapatite, beta-tricalcium phosphate and demineralized bone
matrix. Biomaterials. 24:2593–2603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shao Z, Zhang X, Pi Y, Wang X, Jia Z, Zhu
J, Dai L, Chen W, Yin L, Chen H, et al: Polycaprolactone
electrospun mesh conjugated with an MSC affinity peptide for MSC
homing in vivo. Biomaterials. 33:3375–3387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramaraju H, Miller SJ and Kohn DH:
Dual-functioning peptides discovered by phage display increase the
magnitude and specificity of BMSC attachment to mineralized
biomaterials. Biomaterials. 134:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Man Z, Sha D, Sun S, Li T, Li B, Yang G,
Zhang L, Wu C, Jiang P, Han X, et al: In vitro bioactivity study of
RGD-coated titanium alloy prothesis for revision total hip
arthroplasty. Biomed Res Int. 2016:86279782016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deyle K, Kong XD and Heinis C: Phage
selection of cyclic peptides for application in research and drug
development. Acc Chem Res. 50:1866–1874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan Y, Tian T, Liu W, Zhu Z and C JY:
Advance in phage display technology for bioanalysis. Biotechnol J.
11:732–745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gray BP and Brown KC: Combinatorial
peptide libraries: Mining for cell-binding peptides. Chem Rev.
114:1020–1081. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, He B, Liu K, Ning L, Luo D, Xu K,
Zhu W, Wu Z, Huang J and Xu X: A novel peptide specifically binding
to VEGF receptor suppresses angiogenesis in vitro and in vivo.
Signal Transduct Target Ther. 2:170102017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agarwal R and Garcia AJ: Biomaterial
strategies for engineering implants for enhanced osseointegration
and bone repair. Adv Drug Deliv Rev. 94:53–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Battiwalla M and Barrett AJ: Bone marrow
mesenchymal stromal cells to treat complications following
allogeneic stem cell transplantation. Tissue Eng Part B Rev.
20:211–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu Y, Lu X, Li M, Chen X, Liu Y, Feng X,
Yu J, Zhang C, Niu D, Wang S, et al: Minimally invasive treatment
for osteonecrosis of the femoral head with angioconductive
bioceramic rod. Int Orthop. 42:1567–1573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lobo SE, Glickman R, da Silva WN, Arinzeh
TL and Kerkis I: Response of stem cells from different origins to
biphasic calcium phosphate bioceramics. Cell Tissue Res.
361:477–495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stulajterova R, Medvecky L, Giretova M and
Sopcak T: Structural and phase characterization of bioceramics
prepared from tetracalcium phosphate-monetite cement and in vitro
osteoblast response. J Mater Sci Mater Med. 26:1832015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin J, Shao J, Juan L, Yu W, Song X, Liu
P, Weng W, Xu J and Mehl C: Enhancing bone regeneration by
combining mesenchymal stem cell sheets with β-TCP/COL-I scaffolds.
J Biomed Mater Res B Appl Biomater. 106:2037–2045. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li B, Liu Z, Yang J, Yi Z, Xiao W, Liu X,
Yang X, Xu W and Liao X: Preparation of bioactive β-tricalcium
phosphate microspheres as bone graft substitute materials. Mater
Sci Eng C Mater Biol App. 70:1200–1205. 2017. View Article : Google Scholar
|
|
36
|
Masaoka T, Yoshii T, Yuasa M, Yamada T,
Taniyama T, Torigoe I, Shinomiya K, Okawa A, Morita S and Sotome S:
Bone defect regeneration by a combination of a β-tricalcium
phosphate scaffold and bone marrow stromal cells in a non-human
primate model. Open Biomed Eng J. 10:2–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oe K, Miwa M, Nagamune K, Sakai Y, Lee SY,
Niikura T, Iwakura T, Hasegawa T, Shibanuma N, Hata Y, et al:
Nondestructive evaluation of cell numbers in bone marrow stromal
cell/beta-tricalcium phosphate composites using ultrasound. Tissue
sEng Part C Methods. 16:347–353. 2010. View Article : Google Scholar
|
|
38
|
Li B, Hu R, Sun L, Luo R, Zhao J and Tian
X: A CARE-compliant article: Biomechanics of treating early-stage
femoral-head osteonecrosis by using a beta-tricalcium phosphate
bioceramic rod system: A 3-dimensional finite-element analysis.
Medicine (Baltimore). 97:e108082018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kawai T, Shanjani Y, Fazeli S, Behn AW,
Okuzu Y, Goodman SB and Yang YP: Customized, degradable,
functionally graded scaffold for potential treatment of early stage
osteonecrosis of the femoral head. J Orthop Res. 36:1002–1011.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pi Y, Zhang X, Shi J, Zhu J, Chen W, Zhang
C, Gao W, Zhou C and Ao Y: Targeted delivery of non-viral vectors
to cartilage in vivo using a chondrocyte-homing peptide identified
by phage display. Biomaterials. 32:6324–6332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang G, Man Z, Zhang N, Xin H, Li Y, Sun T
and Sun S: Biopanning of mouse bone marrow mesenchymal stem cell
affinity for cyclic peptides. Mol Med Rep. 19:407–413.
2019.PubMed/NCBI
|
|
42
|
Wang G, Man Z, Xin H, Li Y, Wu C and Sun
S: Enhanced adhesion and proliferation of bone marrow mesenchymal
stem cells on betatricalcium phosphate modified by an affinity
peptide. Mol Med Rep. 19:375–381. 2019.PubMed/NCBI
|
|
43
|
Bhatnagar RS, Qian JJ, Wedrychowska A,
Sadeghi M, Wu YM and Smith N: Design of biomimetic habitats for
tissue engineering with P-15, a synthetic peptide analogue of
collagen. Tissue Eng. 5:53–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lutolf MP and Hubbell JA: Synthetic
biomaterials as instructive extracellular microenvironments for
morphogenesis in tissue engineering. Nat Biotechnol. 23:47–55.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weber D, Kotzsch A, Nickel J, Harth S,
Seher A, Mueller U, Sebald W and Mueller TD: A silent H-bond can be
mutationally activated for high-affinity interaction of BMP-2 and
activin type IIB receptor. BMC Struct Biol. 7:62007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Collier JH and Segura T: Evolving the use
of peptides as components of biomaterials. Biomaterials.
32:4198–4204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Man Z, Li T, Zhang L, Yuan L, Wu C, Li P,
Sun S and Li W: E7 peptide-functionalized Ti6Al4V alloy for BMSC
enrichment in bone tissue engineering. Am J Transl Res.
10:2480–2490. 2018.PubMed/NCBI
|
|
48
|
Ruoslahti E and Pierschbacher MD:
Arg-Gly-Asp: A versatile cell recognition signal. Cell. 44:517–518.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hersel U, Dahmen C and Kessler H: RGD
modified polymers: Biomaterials for stimulated cell adhesion and
beyond. Biomaterials. 24:4385–4415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang H, Lin CY and Hollister SJ: The
interaction between bone marrow stromal cells and RGD-modified
three-dimensional porous polycaprolactone scaffolds. Biomaterials.
30:4063–4069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Valeur E, Gueret SM, Adihou H,
Gopalakrishnan R, Lemurell M, Waldmann H, Grossmann TN and
Plowright AT: New modalities for challenging targets in drug
discovery. Angew Chem Int Ed Engl. 56:10294–10323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zorzi A, Deyle K and Heinis C: Cyclic
peptide therapeutics: Past, present and future. Curr Opin Chem
Biol. 38:24–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Salmasi S, Nayyer L, Seifalian AM and
Blunn GW: Nanohydroxyapatite effect on the degradation,
osteoconduction and mechanical properties of polymeric bone tissue
engineered scaffolds. Open Orthop J. 10:900–919. 2016. View Article : Google Scholar : PubMed/NCBI
|