Introduction
Ischemic heart disease (IHD) is one of the leading
causes of mortality worldwide (1).
At present, recovering cardiac blood supply is the most effective
treatment against IHD; however, it is also a major cause of
myocardial ischemia/reperfusion injury (MIRI) in clinical therapy
(2). MIRI can result in further
myocardial damage, subsequently worsening the therapeutic outcomes
of reperfusion therapy. Currently, methods for treating MIRI are
insufficiently effective (3,4).
Cardiomyocyte apoptosis is an important characteristic of MIRI that
is observed during early phases of reperfusion. Therefore, further
investigation of the mechanisms underlying reperfusion-induced
cardiomyocyte injury is required to develop treatments for IHD.
Jagged1 belongs to the Delta/Serrate/LAG-2 (DSL)
family of ligands for Notch receptors, which regulate cell
proliferation and differentiation in mammals (5). Following ligand-mediated activation
of the Notch pathway, the Notch intracellular domain (NICD) is
released and binds with the transcription factor CBF-1/suppressor
of hairless/lag to form a transcription complex, and then induces
downstream gene transcription; for example, hairy and enhancer of
split (Hes)-1, Hes-5 and hairy/enhancer-of-split related with YRPW
motif protein 1 (Hey-1) (6,7). The
Notch pathway is an evolutionarily conserved pathway that is widely
involved in cell proliferation, differentiation and apoptosis
(8). Jagged1, a DSL ligand for the
mammalian Notch receptor, activates gene expression and the
suppression of differentiation by binding to Notch and inducing the
proteolytic release of NICD (9).
Activation of the Notch pathway induces downstream proteins
involved in the cell cycle and apoptosis, including NICD, Hes-1,
Hes-5 and Hey-1 (5,9). Previous studies have reported that
the Notch signaling pathway is involved in hypoxia/reoxygenation
(H/R)-induced cardiomyocyte injury (10–12).
Additionally, a previous study indicated that inhibition of
microRNA-449a protects H9C2 cells against H/R-induced damage by
silencing the Notch1 signaling pathway (12).
Curcumin, or
1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, is a
natural phenolic substance present in the rhizome of Curcumae
longae (13,14). Curcumin has received increasing
scientific attention due to its range of reported biological
effects, including anti-inflammatory, antioxidant, anticarcinogenic
and cardioprotective effects (15,16).
Previous studies have reported that by regulating cell
proliferation, apoptosis and antioxidant enzymes, curcumin induces
positive effects on ischemia/reperfusion (I/R) injury in various
organs (17,18). Additionally, a number of studies
have demonstrated that curcumin attenuates I/R injury by regulating
various signaling pathways. In 2017, Liu et al (19) demonstrated that curcumin inhibits
nitric oxide (NO) signaling to protect kidney tubules against renal
I/R injury. Similarly, curcumin also exhibits positive effects on
hepatic I/R injury by suppressing the Toll-like receptor (TLR)4
pathway (20). Furthermore, Kim
et al (21) suggested that
curcumin modulates the TLR2/NF-κB signaling pathway to mitigate
cardiomyocyte I/R-induced injury. Additional studies have reported
that curcumin acts as a G-quadruplex-specific ligand to regulate
telomerase activity, thereby regulating apoptosis (22–24).
However, the protective mechanisms underlying the protective
effects of curcumin against I/R injury are yet to be fully
determined.
Focusing on the regulation of apoptosis, the present
study aimed to determine the underlying mechanisms of curcumin on
H/R-induced cardiomyocyte injury. Additionally, the role of the
Notch signaling pathway in the actions of curcumin on cardiomyocyte
injury were investigated.
Materials and methods
Cell culture
H9C2 cells (ATCC® CRL-1446™; American
Type Culture Collection) were cultured in 6-well plates
(2×104 cells/well) with Dulbecco's modified Eagle's
medium (DMEM; cat. no. D5030; Sigma-Aldrich; Merck KGaA) containing
10% fetal bovine serum (FBS; cat. no. 10099141; Thermo Fisher
Scientific, Inc.); cells were maintained at 37°C in a humidified
incubator containing 5% CO2.
Establishment of the H/R model
According to a previous study (25), H9C2 cells cultured in
phosphate-buffered saline (PBS) alone were exposed to low oxygen
(95% N2 + 5% CO2/O2) for 4 h in a
humidified hypoxia chamber (Stemcell Technologies, Inc.), followed
by reoxygenation (0–12 h) in DMEM supplemented with 0.5% FBS under
normal culture conditions. Cells were harvested to measure cell
viability at 4, 8 and 12 h. Control cells were maintained under
normoxic conditions.
Cell viability assay
The viability of H9C2 cardiomyocytes was evaluated
using a Cell Counting kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc.) according to the manufacturer's protocol.
Briefly, after cells were treated in the aforementioned way, cells
were seeded into 96-well plates (3×105 cells/well) and
incubated at 37°C with 5% CO2 for 24 h. Subsequently,
CCK-8 reagent was added to each well, and cardiomyocytes were
cultured at room temperature for 4 h. Absorbance at 450 nm was
detected using a microplate reader (Cany Precision Instruments Co.,
Ltd.).
Determination of cell injury
H9C2 cells were digested with trypsin and collected
by centrifugation after washing with PBS. Following centrifugation
at 8,000 × g for 10 min at 4°C, the supernatant was collected for
testing. According to the manufacturer's protocols, intracellular
lactate dehydrogenase (LDH), malondialdehyde (MDA) and superoxide
dismutase (SOD) activity levels were detected using LDH (cat. no.
BC0680), MDA (cat. no. BC0020) and SOD (cat. no. BC0170) assay kits
(all Beijing Solarbio Science & Technology Co., Ltd.),
respectively.
Effects of curcumin pretreatment on
cardiomyocytes subjected to H/R
Curcumin (purity >98%; cat. no. 08511;
Sigma-Aldrich; Merck KGaA) was dissolved in dimethyl sulfoxide.
H9C2 cardiomyocytes were pretreated at 37°C for 2 h in a humidified
incubator containing 5% CO2 with different
concentrations of curcumin (0, 5, 10 and 20 µM) for 24 h, as
previously described (26,27), to determine a non-toxic
concentration. The viability of pretreated H9C2 cells was examined
using a CCK-8 assay; 10 µM was then selected for use in subsequent
experiments.
To determine the effects of curcumin on H/R-induced
cardiomyocyte injury, cells were assigned to the following four
groups: i) H9C2 cells cultured under normoxic conditions (Control);
ii) H9C2 cells cultured with curcumin (10 µM) at 37°C for 2 h under
normoxic conditions (Cur); iii) H9C2 cells subjected to 4 h of
hypoxia followed by 8 h of reoxygenation (H/R); and iv) H9C2 cells
pretreated with 10 µM curcumin for 2 h, then subjected to 4 h of
hypoxia followed by 8 h of reoxygenation (H/R + Cur).
Cardiomyocyte viability was assessed following the
various treatments using CCK-8 assays, and indicators of
cardiomyocyte injury (LDH, MDA and SOD activity) were measured
using the aforementioned assay kits.
Assessment of reactive oxygen species
(ROS) and apoptosis
The levels of intracellular ROS were determined
using a Reactive Oxygen Species Assay kit (cat. no. S0033; Beyotime
Institute of Biotechnology). Trypsinized cells were first collected
by centrifugation in the aforementioned manner, and then washed
with PBS. Cells were cultured with 10 mM dichlorodihydrofluorescein
diacetate at 37°C for 30 min. Subsequently, cells were harvested
and analyzed using a BD FACScalibur flow cytometer (BD Biosciences)
and the data was analyzed using Summit Software V4.3 (Dako; Agilent
Technologies, Inc.).
Apoptosis was evaluated using an Annexin
V-FITC/propidium iodide (PI) staining kit (Thermo Fisher
Scientific, Inc.). Briefly, following incubation for 48 h, the
cells were trypsinized and collected by centrifugation in the
aforementioned manner. Subsequently, the cells were resuspended in
binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM
CaCl2) and cultured with Annexin V (1:20) for 3 min at
room temperature. The cells were then stained with PI (20 µg/ml) in
the dark for 15 min at room temperature. Cell apoptosis was
determined immediately using flow cytometry and the data were
analyzed using BD CellQuest™ Pro Software version 1.2 (BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from H9C2 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and purified with RNase-free DNase (Takara Bio, Inc.). RNA
aliquots (1 µg) from each sample were reversed transcribed into
cDNA using a PrimeScript™ RT reagent kit (Takara Bio, Inc.). The RT
reaction was conducted as follows: 65°C for 5 min, 30°C for 6 min
and 50°C for 60 min. The cDNA was then used for qPCR. Relative mRNA
expression levels were analyzed using an ABI 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with a
SYBR-Green kit (Takara Bio, Inc.). All primer sequences are
presented in Table I. qPCR
amplification was conducted as follows: Denaturation at 94°C for 2
min, followed by 35 cycles of denaturation at 94°C for 30 sec,
annealing at 63°C for 30 sec and 72°C for 1 min, and a final
extension step at 72°C for 7 min. All experiments were conducted in
triplicate, and relative expression levels were determined using
the 2−∆∆Cq method, using GAPDH for normalization
(28).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Primer
sequence |
|---|
| NICD | F:
5′-ATGACTGCCCAGGAAACAAC-3′ |
|
| R:
5′-GTCCAGCCATTGACACACAC-3′ |
| Hes-1 | F:
5′-CAACACGACACCGGACAAAC-3′ |
|
| R:
5′-CGGAGGTGCTTCACTGTCAT-3′ |
| Hes-5 | F:
5′-ACATGGCCTTGGCTGTCTGA-3′ |
|
| R:
5′-TGCACCCACCCATACAAAGG-3′ |
| Hey-1 | F:
5′-GCCGACGAGACCGAATCAAT-3′ |
|
| R:
5′-GGAGACCAGGCGAACACGA-3 |
| GAPDH | F:
5′-GACATGCCGCCTGGAGAAAC-3′ |
|
| R:
5′-AGCCCAGGATGCCCTTTAGT-3 |
Western blot analysis
Total protein was extracted from cells using RIPA
Lysis Buffer (Beyotime Institute of Biotechnology) and centrifuged
at 12,000 × g for 20 min at 4°C. Protein quantification was
performed using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Subsequently, total protein (20 µg)
was subjected to 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore). The membranes were blocked at
37°C for 1.5 h with 5% non-fat milk in TBS-Tween-20 buffer (100 mM
NaCl, 10 mM Tris-HCl, pH 7.4, 0.1% Tween-20) prior to incubation
with primary antibodies at 4°C overnight. Primary antibodies
against NICD (1:100; cat. no. 4147) and GAPDH (1:1,000; cat. no.
2118) were purchased from Cell Signaling Technology, Inc. Membranes
were then incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibodies (cat. no. 7074; 1:2,000; Cell
Signaling Technology, Inc.) at room temperature for 1 h. Protein
bands were visualized using Pierce™ ECL substrate (cat. no. 32106;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol, and the results were normalized to GAPDH expression.
Quantity One software version 4.6.2 (Bio-Rad Laboratories) was used
to quantify western blots.
Effects of Jagged1 co-treatment with
curcumin on H/R-induced H9C2 cell injury
The recombinant mouse Jagged1 protein was obtained
from Abcam (cat. no. ab109346). H9C2 cells were co-treated with
Jagged1 (50 ng/l) and curcumin (10 µM) in a humidified incubator
with 5% CO2 at 37°C. To investigate the effects of
curcumin on H/R-induced cardiomyocyte injury, cells were assigned
to the following six groups: i) H9C2 cells cultured under normoxic
conditions (Control); ii) H9C2 cells subjected to 4 h of hypoxia
followed by 8 h of reoxygenation (H/R); iii) H9C2 cells cultured
with Jagged1 for 1 h under normoxic conditions (Jagged1); iv) H9C2
cells pretreated with Jagged1 for 1 h and then subjected to 4 h of
hypoxia followed by 8 h of reoxygenation (H/R + Jagged1); v) H9C2
cells pretreated with curcumin for 2 h and then subjected to 4 h of
hypoxia, followed by 8 h of reoxygenation (H/R + Cur); and vi) H9C2
cells co-treated with Jagged1 and curcumin for 3 h and then
subjected to 4 h of hypoxia, followed by 8 h of reoxygenation (H/R
+ Cur + Jagged1).
The viability of H9C2 cells from each group was
evaluated using the CCK-8 assay as aforementioned. Flow cytometry
was used to detect the ROS levels and apoptosis of cardiomyocytes.
Transcriptional and post-transcriptional levels of NICD were
determined via RT-qPCR and western blot analysis, respectively.
Altered mRNA expression of downstream genes in the Notch signaling
pathway was also investigated.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 6.0 software (GraphPad Software, Inc.). All data are
presented as the mean ± standard deviation. Differences were
analyzed using one-way analysis of variance followed by Tukey's
multiple comparison post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
H/R reduces H9C2 cardiomyocyte
viability and contributes to cell injury
As shown in Fig.
1A, H9C2 cell viability was decreased as the duration of
reoxygenation increased. The viability of cardiomyocytes subjected
to 4 h of hypoxia followed by 8 h of reoxygenation was markedly
reduced compared with the control group (P<0.05). The levels of
LDH, an indicator of cardiomyocyte injury, were significantly
increased following 8 h of reoxygenation compared with the control
(Fig. 1B). MDA content was
significantly increased following 4 h of reoxygenation, (Fig. 1C), whereas SOD levels were
significantly decreased following 8 h of reoxygenation, compared
with in the control group (P<0.01). Specific values for these
assays are presented in Table
II.
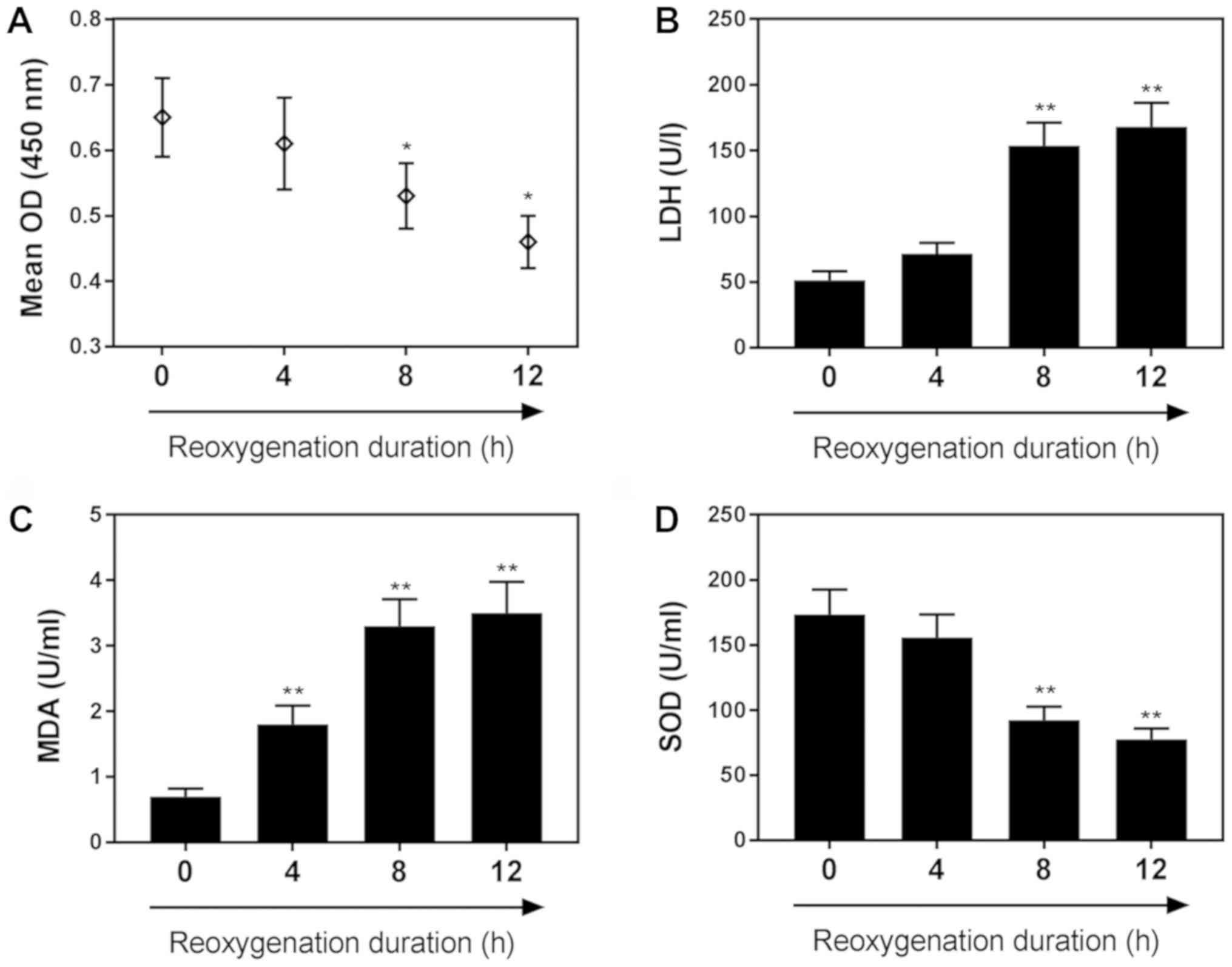 | Figure 1.H/R reduces H9C2 cardiomyocyte
viability and contributes to cell injury. (A) Effects of H/R on
H9C2 cell viability following 0, 4, 8 and 12 h of reoxygenation, as
determined by a Cell Counting Kit-8 assay. Effects of H/R on the
following markers of cardiomyocyte injury: (B) LDH, (C) MDA and (D)
SOD, following 0, 4, 8 and 12 h of reoxygenation. Data are
presented as the mean ± standard deviation (n=3). *P<0.05,
**P<0.01 vs. 0 h. H/R, hypoxia/reoxygenation; LDH, lactate
dehydrogenase; MDA, malondialdehyde; OD, optical density; SOD,
superoxide dismutase. |
 | Table II.Effects of reoxygenation duration on
MDA, LDH and SOD levels. |
Table II.
Effects of reoxygenation duration on
MDA, LDH and SOD levels.
| Reoxygenation
duration | 0 h | 4 h | 8 h | 12 h |
|---|
| LDH (U/l) | 50.1±4.7 | 73.8±5.6 |
150.5±48.5a |
164.6±41.6a |
| MDA (U/ml) | 0.8±0.1 |
1.8±0.3a |
3.1±0.5a |
3.4±0.5a |
| SOD (U/ml) | 174.3±39.6 | 149.5±38.4 |
82.1±19.6a |
64.5±13.2a |
Curcumin attenuates H/R-induced H9C2
cell apoptosis
Curcumin treatment did not affect H9C2 cell
viability under normoxic conditions (Fig. 2). Therefore, 10 µM curcumin was
selected for subsequent experiments. As presented in Fig. 3A, curcumin significantly increased
the viability of H/R-treated cardiomyocytes compared with H/R
treatment alone, whereas it induced no significant effects on the
viability of cells cultured under normoxic conditions.
Cardiomyocyte injury was induced by H/R treatment, as indicated by
the significantly upregulated release of LDH and MDA, and the
significantly decreased release of SOD, compared with in the
control group. Following curcumin pretreatment, these effects were
significantly attenuated compared with in the H/R group (Fig. 3B-D). Additionally, ROS levels were
significantly increased in the H/R group compared with in the
control group (P<0.01; Fig.
3E). Curcumin significantly reduced the H/R-induced increase in
ROS levels (P<0.05). As determined using flow cytometry, the
percentage of apoptotic H/R-treated H9C2 cells was 15.13%, which
was significantly increased compared with the control group (4.59%;
P<0.01); conversely, curcumin pretreatment significantly
decreased the rate of apoptosis in H/R-treated cells to 7.7%
(P<0.05; Fig. 3F).
 | Figure 3.Curcumin attenuates H/R-induced
injury and apoptosis in H9C2 cells. (A) Curcumin reversed the
inhibitory effects of H/R on H9C2 cell viability. Curcumin
pretreatment attenuated the effects of H/R on the levels of (B)
LDH, (C) MDA and (D) SOD. Effects of curcumin on H/R-induced
increases in (E) ROS levels and (F) apoptosis. Data are presented
as the mean ± standard deviation (n=3). *P<0.05, **P<0.01 vs.
Con; ^P<0.05, ^^P<0.01 vs. H/R;
#P<0.05, ##P<0.01 vs. Cur. Con, control
(normoxic culture conditions); Cur, curcumin; H/R,
hypoxia/reoxygenation; LDH, lactate dehydrogenase; MDA,
malondialdehyde; OD, optical density; PI, propidium iodide; ROS,
reactive oxygen species; SOD, superoxide dismutase. |
Curcumin pretreatment inhibits the
Notch signaling pathway
The levels of NICD, Hes-1, Hes-5 and Hey-1 were
measured in H9C2 cells following H/R-induced injury to investigate
the effects of curcumin on H/R-induced cardiomyocytes. As presented
in Fig. 4A and B, the
transcriptional and post-transcriptional levels of NICD were
significantly increased following H/R treatment compared with in
the control group (P<0.01). Conversely, the expression levels of
NICD in the Cur + H/R group were significantly decreased compared
with in the H/R group. Additionally, as presented in Fig. 4C, the mRNA expression levels of
Hes-1, Hes-5 and Hey-1 following H/R were significantly upregulated
compared with in the control group, whereas curcumin pretreatment
significantly attenuated this H/R-induced upregulation of genes
downstream of the Notch pathway.
 | Figure 4.Curcumin pretreatment inhibits the
Notch signaling pathway. (A) mRNA and (B) protein expression levels
of NICD as determined via RT-qPCR and western blotting,
respectively. (C) Expression of downstream genes (Hes-1, Hes-5 and
Hey-1) of the Notch pathway as determined via RT-qPCR analysis.
GAPDH was used as an internal control. Data are presented as the
mean ± standard deviation (n=3). *P<0.05, **P<0.01 vs. Con;
^P<0.05, ^^P<0.01 vs. H/R;
#P<0.05, ##P<0.01 vs. Cur. Con, control
(normoxic culture conditions); Cur, curcumin; H/R,
hypoxia/reoxygenation; Hes, hairy and enhancer of split; Hey-1,
hairy/enhancer-of-split related with YRPW motif protein 1; NICD,
Notch intracellular domain; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Jagged1 suppresses the protective
effects of curcumin against H/R-induced H9C2 cell death
Notch signaling was activated using Jagged1 (50
ng/l), and Jagged1 affected the protective effects of curcumin on
H/R-induced H9C2 cardiomyocytes. As presented in Fig. 5A, activation of the Notch signaling
pathway by Jagged1 partially reversed the protective effects of
curcumin on the viability of H/R-damaged cells. Furthermore, it was
observed that ROS levels were also significantly increased in the
H/R + Cur + Jagged1 group compared with in the H/R + Cur group
(P<0.01). Additionally, the apoptotic rate of cells was
significantly increased from 7.78% in the H/R + Cur group to 14.21%
in the H/R + Cur + Jagged1 group (Fig.
5E).
 | Figure 5.Jagged1 attenuates the protective
effects of curcumin against H/R-induced cardiomyocyte cell death.
(A) Jagged1 reversed the inhibitory effects of curcumin against
H/R-induced reductions in H9C2 cell viability. (B and D) Jagged1
treatment attenuated the downregulation of ROS levels induced by
curcumin. (C and E) Jagged1 alleviated the anti-apoptotic effects
of curcumin. Data are presented as the mean ± standard deviation
(n=3). *P<0.05, **P<0.01 vs. Con; ^P<0.05,
^^P<0.01 vs. H/R; #P<0.05,
##P<0.01 vs. Jagged1; +P<0.05,
++P<0.01 vs. H/R + Jagged1;
&P<0.05, &&P<0.01 vs. H/R +
Cur. Con, control (normoxic culture conditions); Cur, curcumin;
H/R, hypoxia/reoxygenation; OD, optical density; PI, propidium
iodide; ROS, reactive oxygen species. |
The increased expression of NICD and downstream
genes of the Notch pathway in the Jagged1 group indicated that
Jagged1 alone activated Notch signaling (Fig. 6). In addition, NICD, Hes-1, Hes-5
and Hey-1 levels were significantly upregulated in the H/R + Cur +
Jagged1 group compared with in the H/R + Cur group (Fig. 6), suggesting that curcumin
protected cardiomyocytes from H/R-induced apoptosis by modulating
the Notch signaling pathway. A summary of the proposed mechanisms
underlying the protective effects of curcumin on H/R injury
reported during the present study is presented in Fig. 7.
 | Figure 6.Jagged1 promotes the expression of
Notch pathway-associated genes. Jagged1 upregulated the (A) mRNA
and (B) protein expression levels of NICD. (C) Expression of
downstream genes (Hes-1, Hes-5 and Hey-1) in the Notch pathway was
upregulated by Jagged1. GAPDH was used as an internal control. Data
are presented as the mean ± standard deviation (n=3). *P<0.05,
**P<0.01 vs. Con; ^P<0.05, ^^P<0.01
vs. H/R; #P<0.05, ##P<0.01 vs. Jagged1;
++P<0.01 vs. H/R + Jagged1;
&&P<0.01 vs. H/R + Cur. Con, control
(normoxic culture conditions); Cur, curcumin; H/R,
hypoxia/reoxygenation; Hes, hairy and enhancer of split; Hey-1,
hairy/enhancer-of-split related with YRPW motif protein 1; NICD,
Notch intracellular domain. |
 | Figure 7.Schematic of the proposed mechanisms
underlying the effects of curcumin and Jagged1 reported in the
present study. H/R treatment induced cardiomyocyte injury via the
elevation of oxidative stress (including ROS production, reduced
SOD activity, enhanced MDA content and increased LDH activity),
increased apoptosis and decreased cell viability. Curcumin
attenuated H/R injury, whereas Jagged1 exacerbated H/R-induced
injury and reversed the effects of curcumin. Cur, curcumin; H/R,
hypoxia/reoxygenation; Hes, hairy and enhancer of split; Hey-1,
hairy/enhancer-of-split related with YRPW motif protein 1; LDH,
lactate dehydrogenase; MDA, malondialdehyde; NICD, Notch
intracellular domain; ROS, reactive oxygen species; SOD, superoxide
dismutase. |
Discussion
Reperfusion treatment substantially alleviates
myocardial ischemia; however, it can lead to irreversible
cardiomyocyte apoptosis and even cardiac remodeling, and as a
result, MIRI is a leading cause of IHD (29). The prevention of cardiomyocyte
apoptosis is hypothesized to be one of the key approaches to
preventing MIRI (30). A number of
studies have reported that curcumin effectively reduces H/R-induced
H9C2 cell injury by modulating various pathways (31–33);
however, the mechanisms underlying the protective effects of
curcumin on cardiomyocytes remain unclear, and the contribution of
the Notch pathway to cardiomyocyte injury induced by H/R is yet to
be fully elucidated. In the present study, an important role for
the Notch signaling pathway in the protective effects of curcumin
against H/R-induced H9C2 cell death was observed. Curcumin
significantly decreased cardiomyocyte apoptosis following H/R,
potentially by downregulating the Notch pathway.
Firstly, the viability, and the levels of LDH, MDA
and SOD were evaluated to determine the effects of curcumin on a
cardiomyocyte model of H/R. As biomarkers of lipid peroxidation and
oxidative stress, LDH, MDA and SOD levels are closely associated
with the degree of cell injury (34). In the present study, LDH and MDA
contents progressively increased as the duration of reoxygenation
was increased, suggesting that the injury of H9C2 cells, and
oxidative stress and lipid peroxidation, were aggravated as the
period of reoxygenation was extended. Previous studies have
suggested that curcumin protects cardiomyocytes against MIRI by
inhibiting the leakage of LDH, enhancing SOD activity and reducing
MDA production (33,35). These results were consistent with
the present study, in which curcumin modulated the levels of LDH,
MDA and SOD to improve H/R-induced oxidative stress and increase
antioxidative activity in H9C2 cells. In addition, MIRI promotes
the production of ROS, elevated levels of which are sufficiently
toxic to damage all cellular components (36); however, the present findings
revealed that curcumin treatment significantly decreased
H/R-induced ROS production, reducing the toxic effects of ROS on
cardiomyocytes. It has been reported that MIRI induces
cardiomyocyte apoptosis via a number of apoptotic pathways,
including the Janus kinase 2/STAT3 and TLR4/NF-κB pathways
(10,20,37).
In the present study, curcumin pretreatment reduced the H/R-induced
apoptosis of H9C2 cells. Collectively, the present findings
demonstrated the positive effects of curcumin on H9C2 cardiomyocyte
apoptosis induced by H/R.
Furthermore, to investigate the potential role of
the Notch pathway in MIRI, the expression levels of NICD and
certain downstream genes were determined. Previous studies have
demonstrated that the Notch signaling pathway serves a positive
role in inhibiting MIRI, protecting cardiomyocytes against H/R
damage (38–40). Additionally, certain medicines
exert inhibitory effects on MIRI via the Notch signaling pathway;
for example, relaxin protects myocardial cells against H/R injury
by upregulating Notch1 signaling (41). Conversely, opposing findings were
reported in the present study, as it was observed that the
expression levels of NICD, Hes-1, Hes-5 and Hey-1 were
significantly increased in response to H/R treatment. Following
curcumin treatment, the expression levels of Notch pathway genes
were downregulated, as was H/R-induced cardiomyocyte apoptosis.
Therefore, it was hypothesized that Notch pathway activation
promoted H/R-induced cardiomyocyte injury. To test this hypothesis,
Jagged1 protein, a ligand that interacts with four receptors in the
Notch pathway, was used to reactivate the Notch1 receptor and NICD
release following curcumin treatment. Notably, it was observed that
ROS levels and the apoptotic rate were significantly increased
following Jagged1 treatment, and that the mRNA expression levels of
Hes-1, Hes-5 and Hey-1 were also significantly unregulated. These
results were consistent with the hypothesis that the Notch pathway
not only contributed to H/R-induced H9C2 cardiomyocyte apoptosis,
but also exacerbated H/R-induced injury. It has been reported that
Jagged1 is involved in cell injury (42–44).
Therefore, the role of Jagged1 in H/R-induced H9C2 cardiomyocyte
injury, and the involvement of Notch signaling in the effects of
curcumin were investigated. The results revealed that curcumin
possessed the ability to suppress H/R-induced H9C2 cell apoptosis,
and that Jagged1 attenuated the inhibitory effects of curcumin by
activating the Notch pathway. Therefore, curcumin and Jagged1
induced opposing effects on apoptotic processes in H9C2 cells.
Previous studies have also indicated that the ligand Jagged2
exhibits anti-apoptotic effects (45,46).
In addition, it was previously reported that inhibition of the
Delta1/Notch1 pathway affects apoptosis (47). Pelullo et al (48) revealed that Jagged1 potentially
contributes to the occurrence of acute lymphoblastic leukemia via
Notch3. The exact mechanisms underlying the interaction/competition
between the two molecules were not investigated in the present
study. In addition, various signaling pathways, including the NO
(49), TLR4 (50) and TLR/NF-κB signaling pathways
(51) have also been reported to
be involved in the regulation of IR injury; however, the
involvement of these pathways in the effects of curcumin or Jagged1
were not investigated in the present study.
In conclusion, the present study revealed that H/R
injury activated the Notch signaling pathway, and induced H9C2
cardiomyocyte injury and apoptosis; however, in vivo
experiments are required to validate these findings. Additionally,
curcumin not only inhibited the association between H/R injury and
Notch signaling by inhibiting NICD expression, but also reduced the
H/R-induced apoptosis of H9C2 cells. Furthermore, Jagged1
recombinant protein treatment further suggested that the Notch
pathway contributed to H/R damage in cardiomyocytes. These findings
provide improved understanding of the mechanisms via which curcumin
affects H/R-induced cardiomyocyte apoptosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ and MY made substantial contributions to the
conception and design of the study. HH, ML, SL, ZC, CS, ZK, AL and
QW performed the acquisition and analysis of data, and interpreted
the data. PZ and MY drafted the manuscript. All authors approved of
the final version of the manuscript to be published. All authors
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soriano JB, Rojas-Rueda D, Alonso J, Anto
JM, Cardona PJ, Fernandez E, Garcia-Basteiro AL, Benavides FG,
Glenn SD, Krish V, et al: The burden of disease in Spain: Results
from the global burden of disease 2016. Med Clin (Barc).
151:171–190. 2018.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bell MT, Puskas F, Agoston VA, Cleveland
JC Jr, Freeman KA, Gamboni F, Herson PS, Meng X, Smith PD, Weyant
MJ, et al: Toll-like receptor 4-dependent microglial activation
mediates spinal cord ischemia-reperfusion injury. Circulation. 128
(Suppl 1):S152–S156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang D, Chen TY and Liu FJ: Che-1
attenuates hypoxia/reoxygenation-induced cardiomyocyte apoptosis by
upregulation of Nrf2 signaling. Eur Rev Med Pharmacol Sci.
22:1084–1093. 2018.PubMed/NCBI
|
|
5
|
Bash J, Zong WX, Banga S, Rivera A,
Ballard DW, Ron Y and Gelinas C: Rel/NF-kappaB can trigger the
Notch signaling pathway by inducing the expression of Jagged1, a
ligand for Notch receptors. EMBO J. 18:2803–2811. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geisler F and Strazzabosco M: Emerging
roles of Notch signaling in liver disease. Hepatology. 61:382–392.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo J, Wang P, Wang R, Wang J, Liu M,
Xiong S, Li Y and Cheng B: The Notch pathway promotes the cancer
stem cell characteristics of CD90(+) cells in hepatocellular
carcinoma. Oncotarget. 7:9525–9537. 2016.PubMed/NCBI
|
|
9
|
Zavadil J, Cermak L, Soto-Nieves N and
Bottinger EP: Integration of TGF-beta/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO J.
23:1155–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai W, Yang X, Han S, Guo H, Zhao Z, Wang
H, Guan H, Jia Y, Gao J, Yang T, et al: Notch1 pathway protects
against burn-induced myocardial injury by repressing reactive
oxygen species production through JAK2/STAT3 signaling. Oxid Med
Cell Longev. 2016:56389432016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng J, Wu Q, Lv R, Huang L, Xu B, Wang
X, Chen A and He F: MicroRNA-449a inhibition protects H9C2 cells
against hypoxia/reoxygenation-induced injury by targeting the
Notch-1 signaling pathway. Cell Physiol Biochem. 46:2587–2600.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esatbeyoglu T, Huebbe P, Ernst IM, Chin D,
Wagner AE and Rimbach G: Curcumin--from molecule to biological
function. Angew Chem Int Ed Engl. 51:5308–5332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeh CH, Chen TP, Wu YC, Lin YM and Jing
Lin P: Inhibition of NFkappaB activation with curcumin attenuates
plasma inflammatory cytokines surge and cardiomyocytic apoptosis
following cardiac ischemia/reperfusion. J Surg Res. 125:109–116.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park BH, Lim JE, Jeon HG, Seo SI, Lee HM,
Choi HY, Jeon SS and Jeong BC: Curcumin potentiates antitumor
activity of cisplatin in bladder cancer cell lines via ROS-mediated
activation of ERK1/2. Oncotarget. 7:63870–63886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kunnumakkara AB, Bordoloi D, Padmavathi G,
Monisha J, Roy NK, Prasad S and Aggarwal BB: Curcumin, the golden
nutraceutical: Multitargeting for multiple chronic diseases. Br J
Pharmacol. 174:1325–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen SQ, Zhang Y, Xiang JJ and Xiong CL:
Protective effect of curcumin against liver warm
ischemia/reperfusion injury in rat model is associated with
regulation of heat shock protein and antioxidant enzymes. World J
Gastroenterol. 13:1953–1961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yucel AF, Kanter M, Pergel A, Erboga M and
Guzel A: The role of curcumin on intestinal oxidative stress, cell
proliferation and apoptosis after ischemia/reperfusion injury in
rats. J Mol Histol. 42:579–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu F, Ni W, Zhang J, Wang G, Li F and Ren
W: Administration of curcumin protects kidney tubules against renal
ischemia-reperfusion injury (RIRI) by modulating nitric oxide (NO)
signaling pathway. Cell Physiol Biochem. 44:401–411. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Li N, Lin D and Zang Y: Curcumin
protects against hepatic ischemia/reperfusion induced injury
through inhibiting TLR4/NF-κB pathway. Oncotarget. 8:65414–65420.
2017.PubMed/NCBI
|
|
21
|
Kim YS, Kwon JS, Cho YK, Jeong MH, Cho JG,
Park JC, Kang JC and Ahn Y: Curcumin reduces the cardiac
ischemia-reperfusion injury: involvement of the toll-like receptor
2 in cardiomyocytes. J Nutr Biochem. 23:1514–1523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jha N, Mishra S, Mamidi A, Mishra A, Jha S
and Surolia A: Targeting Human Telomeric G- Quadruplexes DNA with
curcumin and its synthesized analogues under molecular crowding
condition. RSC Advances. 6:7474–7487. 2016. View Article : Google Scholar
|
|
23
|
Lin S, Gao W, Tian Z, Yang C, Lu L, Mergny
JL, Leung CH and Ma DL: Luminescence switch-on detection of protein
tyrosine kinase-7 using a G-quadruplex-selective probe. Chem Sci.
6:4284–4290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang M, Mao Z, Kang TS, Wong CY, Mergny
JL, Leung CH and Ma DL: Conjugating a groove-binding motif to an
Ir(iii) complex for the enhancement of G-quadruplex probe behavior.
Chem Sci. 7:2516–2523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park M, Youn B, Zheng XL, Wu D, Xu A and
Sweeney G: Globular Adiponectin, acting via adipoR1/APPL1, protects
H9c2 cells from hypoxia/reoxygenation-induced apoptosis. Plos One.
6:e191432011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Magalhães LG, Machado CB, Morais ER,
Moreira EB, Soares CS, Da SS, Da SFA and Rodrigues V: In vitro
schistosomicidal activity of curcumin against Schistosoma mansoni
adult worms. Parasitology Research. 104:1197–1201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu Q, Cai Y, Huang C, Shi Q and Yang H:
Curcumin increases rat mesenchymal stem cell osteoblast
differentiation but inhibits adipocyte differentiation.
Pharmacognosy Magazine. 8:202–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto T and Sadoshima J: Protection of
the heart against ischemia/reperfusion by silent information
regulator 1. Trends Cardiovasc Med. 21:27–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Jing X, Dong A, Bai B and Wang H:
Overexpression of TIMP3 protects against cardiac
ischemia/reperfusion injury by inhibiting myocardial apoptosis
through ROS/Mapks pathway. Cell Physiol Biochem. 44:1011–1023.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu K, Chen H, You QS, Ye Q, Wang F, Wang
S, Zhang SL, Yu KJ and Lu Q: Curcumin attenuates myocardial
ischemia-reperfusion injury. Oncotarget. 8:112051–112059. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang R, Zhang JY, Zhang M, Zhai MG, Di SY,
Han QH, Jia YP, Sun M and Liang HL: Curcumin attenuates IR-induced
myocardial injury by activating SIRT3. Eur Rev Med Pharmacol Sci.
22:1150–1160. 2018.PubMed/NCBI
|
|
33
|
Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan
J, Wang N, Deng C, Zhang S, Li Y, et al: SIRT1 activation by
curcumin pretreatment attenuates mitochondrial oxidative damage
induced by myocardial ischemia reperfusion injury. Free Radic Biol
Med. 65:667–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Wei S, Tang JM, Guo LY, Zheng F,
Yang JY, Kong X, Huang YZ, Chen SY and Wang JN: PEP-1-CAT protects
hypoxia/reoxygenation-induced cardiomyocyte apoptosis through
multiple sigaling pathways. J Transl Med. 11:1132013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duan W, Yang Y, Yan J, Yu S, Liu J, Zhou
J, Zhang J, Jin Z and Yi D: The effects of curcumin post-treatment
against myocardial ischemia and reperfusion by activation of the
JAK2/STAT3 signaling pathway. Basic Res Cardiol. 107:2632012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bagheri F, Khori V, Alizadeh AM,
Khalighfard S, Khodayari S and Khodayari H: Reactive oxygen
species-mediated cardiac-reperfusion injury: Mechanisms and
therapies. Life Sci. 165:43–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeong CW, Yoo KY, Lee SH, Jeong HJ, Lee CS
and Kim SJ: Curcumin protects against regional myocardial
ischemia/reperfusion injury through activation of RISK/GSK-3β and
inhibition of p38 MAPK and JNK. J Cardiovasc Pharmacol Ther.
17:387–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou XL, Wan L and Liu JC: Activated
Notch1 reduces myocardial ischemia reperfusion injury in vitro
during ischemic postconditioning by crosstalk with the RISK
signaling pathway. Chin Med J (Engl). 126:4545–4551.
2013.PubMed/NCBI
|
|
39
|
Zhang S, Zhang R, Wu F and Li X:
MicroRNA-208a regulates H9c2 cells simulated ischemia-reperfusion
myocardial injury via targeting CHD9 through Notch/NF-kappa B
signal pathways. Int Heart J. 59:580–588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jing R, Zhou Z, Kuang F, Huang L and Li C:
microRNA-99a reduces lipopolysaccharide-induced oxidative injury by
activating Notch pathway in H9c2 cells. Int Heart J. 58:422–427.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boccalini G, Sassoli C, Formigli L, Bani D
and Nistri S: Relaxin protects cardiac muscle cells from
hypoxia/reoxygenation injury: Involvement of the Notch-1 pathway.
FASEB J. 29:239–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang K, Ding R, Ha Y, Jia Y, Liao X, Wang
S, Li R, Shen Z, Xiong H, Guo J and Jie W: Hypoxia-stressed
cardiomyocytes promote early cardiac differentiation of cardiac
stem cells through HIF-1α/Jagged1/Notch1 signaling. Acta Pharm Sin
B. 8:795–804. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kamei N, Kwon SM, Ishikawa M, Ii M,
Nakanishi K, Yamada K, Hozumi K, Kawamoto A, Ochi M and Asahara T:
Endothelial progenitor cells promote astrogliosis following spinal
cord injury through Jagged1-dependent Notch signaling. J
Neurotrauma. 29:1758–1769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu X, Zou Y, Zhou Q, Huang L, Gong H, Sun
A, Tateno K, Katsube K, Radtke F, Ge J, et al: Role of Jagged1 in
arterial lesions after vascular injury. Arterioscler Thromb Vasc
Biol. 31:2000–2006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu Y, Su H, Li X, Guo G, Cheng L, Qin R,
Qing G and Liu H: The NOTCH ligand JAGGED2 promotes pancreatic
cancer metastasis independent of NOTCH signaling activation. Mol
Cancer Ther. 14:289–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Francis JC, Radtke F and Logan MP: Notch1
signals through Jagged2 to regulate apoptosis in the apical
ectodermal ridge of the developing limb bud. Dev Dyn.
234:1006–1015. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang H, Ye Y, Bai Z and Wang S: The COX-2
selective inhibitor-independent COX-2 effect on colon carcinoma
cells is associated with the Delta1/Notch1 pathway. Dig Dis Sci.
53:2195–2203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pelullo M, Quaranta R, Talora C, Checquolo
S, Cialfi S, Felli MP, te Kronnie G, Borga C, Besharat ZM, Palermo
R, et al: Notch3/Jagged1 circuitry reinforces notch signaling and
sustains T-ALL. Neoplasia. 16:1007–1017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weerateerangkul P, Chattipakorn S and
Chattipakorn N: Roles of the nitric oxide signaling pathway in
cardiac ischemic preconditioning against myocardial
ischemia-reperfusion injury. Med Sci Monit. 17:RA44–RA52. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu L, Ye T, Tang Q, Wang Y, Wu X, Li H
and Jiang Y: Exercise preconditioning regulates the toll-like
receptor 4/nuclear Factor-κB signaling pathway and reduces cerebral
ischemia/reperfusion inflammatory injury: A study in rats. J Stroke
Cerebrovasc Diseases. 25:2770–2779. 2016. View Article : Google Scholar
|
|
51
|
Zheng Y, Bu J, Yu L, Chen J and Liu H:
Nobiletin improves propofol-induced neuroprotection via regulating
Akt/mTOR and TLR 4/NF-κB signaling in ischemic brain injury in
rats. Biomed Pharmacother. 91:494–503. 2017. View Article : Google Scholar : PubMed/NCBI
|





















