Introduction
Obesity, a common public health concern worldwide,
is associated with the development of chronic metabolic diseases,
including non-alcoholic fatty liver disease (NAFLD), which is
characterized by the excessive accumulation of triglycerides (TAG)
in the liver without inflammation (1). Studies have reported that NAFLD in
patients may progress to non-alcoholic steatohepatitis (NASH),
liver sclerosis and even hepatocellular carcinoma (2,3). Of
these patients, obese individuals are more likely to suffer from
NAFLD/NASH as increases in body weight have been shown to be
positively associated with the accumulation of fat content in the
liver (4). Amelioration of
cellular stress in NAFLD/NASH models revealed an improvement in the
levels of biomarkers related to cellular stress, such as markers of
hypoxia, inflammation and endoplasmic reticulum (ER) stress, has
been reported (5).
Adiponectin has been demonstrated to exert marked
insulin-sensitizing and anti-inflammatory effects (6). Reduced circulating adiponectin levels
(hypoadiponectinemia) have been linked to the etiology of obesity
and obesity-related diseases (7).
Adiponectin can be used to combat various types of liver damage,
including liver disease induced by carbon tetrachloride, fructose,
a high-fat diet, high cholesterol levels and ethanol (8–11).
More importantly, a previous study demonstrated that a decrease in
adiponectin levels is an independent risk factor of developing
NAFLD (12). In recent years,
researchers have found that high molecular weight (HMW) adiponectin
is the predominant active form of adiponectin in the liver, and is
closely associated with obesity and obesity-associated disorders
(13–15). Fibroblast growth factor 21 (FGF21),
a member of the FGF family, is secreted mainly from tissues with
high metabolic activity, such as the liver and adipose tissue
(16). FGF21 treatment has been
reported to attenuate or eliminate the progression to NASH in
animals (17). Furthermore, FGF21
knockdown has been shown to be associated with markedly more severe
hepatic steatosis and inflammation, which can progress to severe
NASH in mouse models of NAFLD (18,19).
Increasing evidence suggests that FGF21 may be developed as a novel
agent candidate for the treatment of NAFLD/NASH (20,21).
Studies have shown that, partially dependent on peroxisome
proliferator-activated receptor-γ activity, FGF21 can stimulate the
transcription and secretion of adiponectin in adipocytes (22,23).
The activation of the FGF21/adiponectin pathway has been reported
to inhibit liver TAG accumulation, thereby reversing hepatic
steatosis and improving NAFLD/NASH (23,24).
Ascorbic acid (AA), also known as vitamin C, is
mainly found in a number of vegetables and fruits. As an excellent
antioxidant, AA has a wide range of benefits, namely
health-promoting and disease-preventing, and therapeutic properties
(25). Studies have shown that AA,
along with other antioxidants, such as vitamin E, can effectively
inhibit oxidative stress, thereby reversing NAFLD (26,27).
Apart from its antioxidative activity, AA can protect cells from
stress via non-antioxidative pathways (28,29);
however, these potential effects of AA alone on NAFLD/NASH and its
mechanisms of action have not yet been well characterized.
Additionally, AA has been reported to promote the secretion of HMW
adiponectin from human adipocytes (30). To the best of our knowledge,
whether AA can increase the expression of HMW adiponectin in
hepatocytes remains unknown.
In spite of accumulating evidence, which typically
describes the beneficial effects of AA on NAFLD/NASH in vivo
or in vitro, further investigation is required to identify
the specific roles of AA in obesity-associated factors-induced cell
stress in hepatocytes. Thus, the aim of the present study was to
determine the effects of AA on obesity-associated cell stress
induced by tumor necrosis factor α (TNFα) in HepG2 cells and
further explore the possible underlying mechanisms.
Materials and methods
Materials
TNFα (cat. no. T6674) and AA (cat. no. A4403) were
purchased from Sigma-Aldrich (Merck KGaA). Antibodies against FGF21
(cat. no. ab171941), hypoxia inducible factor 1α (HIF1α; cat. no.
ab2185) and fibroblast growth factor receptor 2 (FGFR2; cat. no.
ab109372) were obtained from Abcam; phosphorylated 5′AMP-activated
protein kinase (p-AMPK; cat. no. 2531) and AMPK (cat. no. 2532)
antibodies were from Cell Signaling Technology, Inc.; β-actin (cat.
no. sc-47778), the horseradish peroxidase-conjugated secondary
antibody goat-anti-rabbit (cat. no. sc-2004) and goat-anti-mouse
(cat. no. sc-2005) IgG antibodies were obtained from Santa Cruz
Biotechnology, Inc. All other reagents were from Sigma-Aldrich
(Merck KGaA) unless otherwise stated.
Cells and cell culture
HepG2 cells, a human hepatoma-derived cell line,
were obtained from the American Type Culture Collection (HB-8065)
and cultured routinely in a humidified atmosphere containing 5%
CO2 at 37°C with Dulbecco's Modified Eagle's medium
(DMEM) containing 10% fetal bovine serum (FBS), penicillin (100
IU/ml) and streptomycin (30 mg/ml). Cells were seeded into plates
24 h prior to the treatments at ~80% confluence. Cells were
co-cultured with or without AA (0, 100, 200, 500, and 1,000 µM) and
10 ng/ml TNFα for 24 h in a humidified atmosphere containing 5%
CO2 at 37°C. According to the findings from this
experiment, 100 µM AA selected for further experimentation as this
concentration effectively inhibited increases in the expression of
cell stress-related genes induced by TNFα (data not shown). The
cell groups were as follows: Control group, cultured in DMEM; TNFα
group, treated with TNFα (10 ng/ml); and TNFα + AA group, cells
co-cultured with TNFα (10 ng/ml) and AA (100 µM). For each assay,
at least three independent experiments were conducted.
Reverse transcription-quantitative PCR
(RT-qPCR)
All primers were designed using Primer Express 3.0
software (Applied Biosystems; Thermo Fisher Scientific, Inc.;
Table I). RT reactions were
conducted using an RT kit (cat. no. RR036A; Takara Bio, Inc.) and
SYBR®-Green PCR Super mix (cat. no. A25741; Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The conditions of PCR used Applied
Biosystems ViiA™ 7 Dx qPCR System in this study were as follows:
Initial denaturation: 95°C, 5 min; 40 cycles of denaturation (95°C,
30 sec), annealing (58°C, 30 sec), and elongation (72°C, 60 sec).
The fluorescent signals were detected during the extension phase,
Quantification cycle (Cq) values of the sample were calculated. The
expression of Gapdh was assessed as a housekeeping gene. The
relative expression of the gene of interest was analyzed using the
2−ΔΔCq method (31).
All the experiments were repeated three times.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| β-Actin |
GCAAAGACCTGTACGCCAACA |
TGCATCCTGTCGGCAATG |
| Vegfa |
TTGCCTTGCTGCTCTACCTCCA |
GATGGCAGTAGCTGCGCTGATA |
| Glut1 |
TTGCAGGCTTCTCCAACTGGAC |
CAGAACCAGGAGCACAGTGAAG |
| Mcp1 |
AGAATCACCAGCAGCAAGTGTCC |
TCCTGAACCCACTTCTGCTTGG |
| IL-6 |
AGACAGCCACTCACCTCTTCAG |
TTCTGCCAGTGCCTCTTTGCTG |
| Grp78 |
CTGTCCAGGCTGGTGTGCTCT |
CTTGGTAGGCACCACTGTGTTC |
| Sxbp1 |
CTGCCAGAGATCGAAAGAAGGC |
CTCCTGGTTCTCAACTACAAGGC |
| Fgfr2 |
GTGCCGAATGAAGAACACGACC |
GGCGTGTTGTTATCCTCACCAG |
| Fgf21 |
CTGCAGCTGAAAGCCTTGAAGC |
GTATCCGTCCTCAAGAAGCAGC |
| Hif1α |
GAACGTCGAAAAGAAAAGTCTCG |
CCTTATCAAGATGCGAACTCACA |
Western blot analysis
Cells were harvested and homogenized in lysis buffer
(Pierce; Thermo Fisher Scientific, Inc.) at 4°C. The homogenates
were centrifuged at 12,000 × g at 4°C for 15 min. Protein was
quantified using a Bio-Rad Protein Assay kit (Bio-Rad Laboratories,
Inc.). Protein samples (20 µg) were separated on SDS-PAGE,
transferred to PVDF membranes, and blocked with 5% of BSA in TBS-T
buffer (10 mM Tris HCl, pH 7.5, 150 mM NaCl, 0.15% Tween-20) at
room temperature for 90 min. Following washing with TBST, the
membranes were incubated with primary antibodies at 4°C overnight,
the primary antibodies were as follows: Anti-β-actin (1:1,000; cat.
no. sc-47778; Santa Cruz Biotechnology Inc.); anti-HIF1α (1:200;
cat. no. ab2185; Abcam); anti-FGFR2 (1:1,000; cat. no. ab109372;
Abcam); anti-FGF21 (1:1,000; cat. no. ab171941; Abcam); anti-p-AMPK
(1:1,000; cat. no. 2531; Cell Signaling Technology, Inc.),
anti-AMPK (1:1,000; cat. no. 2532; Cell Signaling Technology,
Inc.), followed by incubation with appropriate secondary antibodies
for 1 h at room temperature, the primary antibodies were as
follows: Goat anti-rabbit IgG antibodies (1:5,000; cat. no.
sc-2004; Santa Cruz Biotechnology, Inc.) and goat anti-mouse IgG
antibodies (1:5,000; cat. no. sc-2005; Santa Cruz Biotechnology,
Inc.). Finally, they were detected using an enhanced
chemiluminescence system (EMD Millipore). The band intensities were
quantified by using ImageJ software version 1.8.0 (National
Institutes of Health, Bethesda, MD, USA). The calculated ratio of
the intensity of the target protein to that of β-actin corresponded
to the expression level of the protein.
ELISA
HepG2 cells were collected under aseptic conditions
and incubated (5×106 cells/well) in 6-well culture
plates at 37°C in humidified 5% CO2. Following treatment
as aforementioned, the conditioned medium from the cells was
collected for determining the secretion of total adiponectin (cat.
no. ab99968, Abcam) and HMW adiponectin (cat. no. CSB-E07270h,
CUSABIO) using ELISA kits. Cells were then washed twice with
ice-cold PBS and lysed in lysis buffer comprising 50 mM Tris (pH
7.4), 150 mM NaCl, 10% (w/v) glycerol, 10 mM EDTA, 1 mM
MgCl2, 20 mM b-glycerophosphosphate, 30 mM NaF, 1%
Triton X-100, 25 mg/ml leupeptin, 25 mg/ml pepstatin and 3 mg/ml
aprotinin. After incubation on ice for 20 min, cell lysates were
centrifuged at 12,000 × g for 30 min at 4°C. A BCA Protein Assay
was used to quantify the total protein of cells. The concentration
of HIFα was measured using a commercially available ELISA kit (cat.
no. ab171577, Abcam) according to the manufacturer's
instructions.
RNA interference
The small interfering RNA (siRNA) sequences
targeting human Fgfr2 were designed using siRNA primer
design software (Guangzhou RiboBio Co., Ltd.; Table II). We mixed the three individual
targeting siRNAs (50 nM of each) at a ratio of 1:1:1 (Fgfr2
siRNA) for transfection. HepG2 cells were seeded on 6-well plates
at 37°C for 24 h until 50–80% confluence was attained. Cells were
subsequently transfected with 50 nM of the Fgfr2 siRNA
mixture or scramble (Scr) control siRNA using riboFECT™ CP
(Guangzhou RiboBio Co., Ltd.).
 | Table II.Fgfr2 siRNA primer used for
transfection. |
Table II.
Fgfr2 siRNA primer used for
transfection.
| siRNA | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| Fgfr2-1 |
GCCCUCCUUCAGUUUAGUUTT |
AACUAAACUGAAGGAGGGCTT |
| Fgfr2-2 |
GGACAAAGAGAUUGAGGUUTT |
AACCUCAAUCUCUUUGUCCTT |
| Fgfr2-3 |
CCAGAGGCAUGGAGUACUUTT |
AAGUACUCCAUGCCUCUGGTT |
Statistical analysis
All experiments were performed in triplicate. The
results are presented as the mean ± standard error of mean.
Significant differences were determined by one-way ANOVA followed
by the Least Significant Difference test using SPSS 18.0 software
(SPSS, Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
AA reduces TNFα -induced cell stress
in HepG2 cells
To establish a cellular model of obesity-related
NAFLD, TNFα, an obesity-associated insult factor, was used to
induce cell stress in HepG2 cells. As presented in Fig. 1, treatment with TNFα (10 ng/ml) for
24 h was determined to successfully promote cellular hypoxia,
inflammation and ER stress, as determined by the significant
increase in HIF1α expression compared with the control. Incubation
with AA (100 µM) for 24 h significantly decreased the protein and
mRNA expression levels of HIF1α in HepG2 cells compared with TNFα
treatment alone (Fig. 1A-C).
Additionally, AA treatment resulted in a significant decrease in
the mRNA levels of glucose transporter 1 (Glut1), an
important target gene of HIF1α (Fig.
1D) (32). In addition, AA
reduced the mRNA levels of the inflammatory factor, monocyte
chemoattractant 1 (Mcp1; Fig.
1E), and the ER stress factor, glucose-regulated protein, 78
kDa (Grp78; Fig. 1F).
However, the mRNA expression levels of vascular endothelial growth
factor A (Vegfa; Fig. 1D),
interleukin (Il)−6 (Fig.
1E) and spliced X-box-binding protein 1 (Sxbp1; Fig. 1F) were markedly unaffected by TNFα
and AA treatment for 24 h.
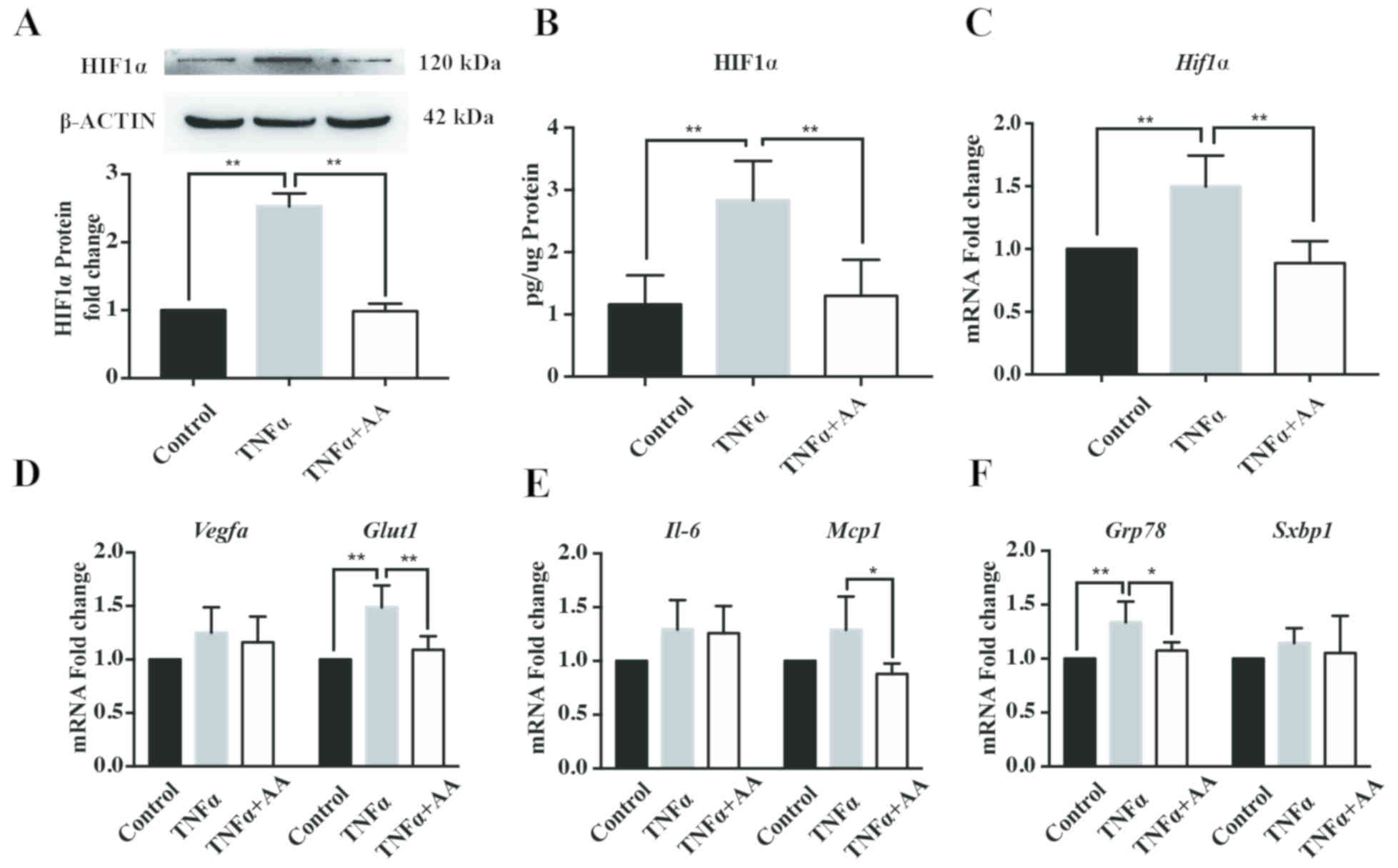 | Figure 1.AA (100 µM) attenuates TNFα-induced
(10 ng/ml) cell stress. (A and B) HIF1α expression in HepG2 cells
was determined by western blotting and ELISA; (C) mRNA expression
levels of mRNA levels in HepG2 cells were assessed cells by
RT-qPCR, respectively. The mRNA expression levels of factors
related to (D) hypoxia, (E) inflammation and (F) endoplasmic
reticulum stress were assessed in HepG2 cells by RT-qPCR. Data are
expressed as fold changes compared with the control group. All data
are presented as the mean ± standard error of the mean. *P<0.05;
**P<0.01. AA, ascorbic acid; Glut1, glucose transporter 1;
Grp78, glucose-regulated protein, 78 kDa; HIF1α, hypoxia inducible
factor 1α; IL-6, interleukin-6; Mcp1, monocyte chemoattractant 1;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; Sxbp1, spliced X-box-binding protein 1; TNFα, tumor
necrosis factor α; Vegfa, vascular endothelial growth factor A. |
AA activates the
FGF21/FGFR2/adiponectin pathway
In addition, we examined the effects of AA treatment
on adiponectin expression, and the secretion of total and HMW
adiponectin. Compared with the control group, the secretion of HMW
adiponectin, but not that of total adiponectin was inhibited by
TNFα treatment. However, TNFα and AA co-treatment resulted in a
significant increase in the secretion of total and HMW adiponectin
compared with TNFα treatment alone (Fig. 2A and B). We also detected the
expression of the upstream molecules involved in the signaling
pathways of adiponectin. The addition of AA significantly increased
the expression of FGFR2 and FGF21 than with TNFα alone; compared
with the control, TNFα treatment notably induced the expression of
FGF21 (Fig. 2C-E). Conversely, the
expression levels of p-AMPK and AMPK were markedly unaffected in
all treatment groups (Fig. 2D and
E), suggesting that the AMPK signaling pathway may not have
been activated by AA.
 | Figure 2.AA activates the
FGF21/FGFR2/adiponectin pathway in HepG2 cells. ELISA of (A) total
adiponectin and (B) HMW adiponectin secretion in all groups. (C)
Reverse transcription-quantitative polymerase chain reaction
analysis of Fgf21 and Fgfr2 gene expression in HepG2
cells. (D) Representative images of FGFR2, FGF21, p-AMPK and AMPK
protein levels from 3 independent experiments and (E) fold changes
of protein levels relative to the control group are shown. Control
group, cultured in DMEM medium; TNFα group, treated with TNFα (10
ng/ml); and TNFα + AA group, co-cultured with TNFα (10 ng/ml) and
AA (100 µM). All data are normalized to the band density of
β-actin. *P<0.05; **P<0.01. AA, ascorbic acid; AMPK,
5′AMP-activated protein kinase; Fgf21, fibroblast growth factor 21;
Fgfr2, fibroblast growth factor receptor 2; HMW, high molecular
weight; p, phosphorylated; TNFα, tumor necrosis factor α. |
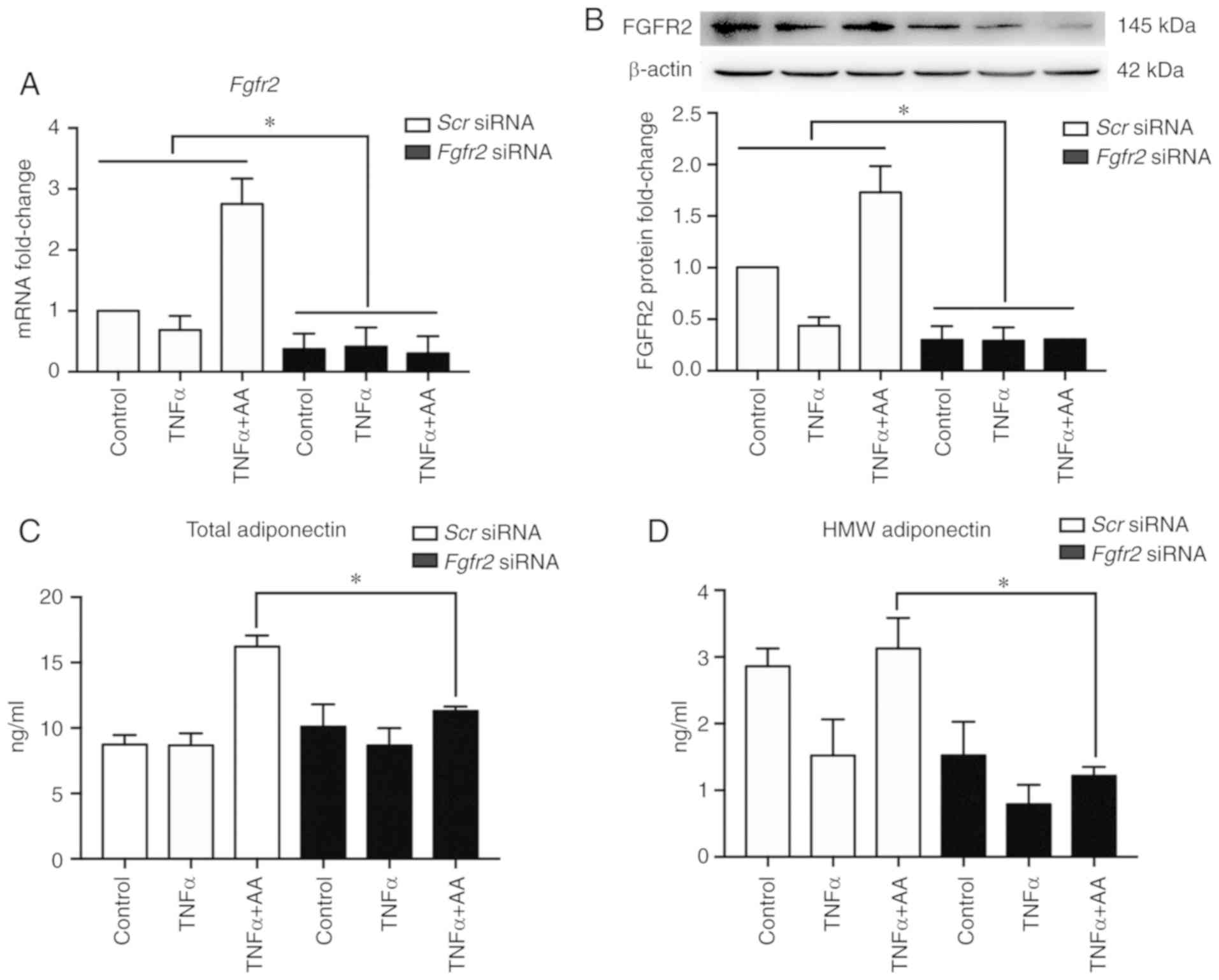
Fgfr2 knockdown attenuates the effects
of AA on total and HMW adiponectin
In order to confirm the role of FGF21/FGFR2 in
beneficial effects of AA in this study, FGFR2 expression was
silenced via RNA interference. Compared with Scr siRNA, the
transfection of siRNA targeting Fgfr2 successfully inhibited
the expression of FGFR2 (P<0.001; Fig. 3A and B). Compared with Scr
siRNA, Fgfr2 knockdown significantly suppressed the increase
in the secretion of both total adiponectin and HMW adiponectin
induced by AA (Fig. 3C and D).
Fgfr2 knockdown abolishes the effects
of AA on cell stress
We finally assessed the effects of Fgfr2
knockdown on cellular stress in HepG2 cells. In the Scr
siRNA-transfected group, AA treatment appeared to reverse the
increase in all cell stress markers at the mRNA and protein levels
due to TNFα, including HIF1α (Fig. 4A
and B) and its target genes, Glut and Vegfa
(Fig. 4C and D), and the gene
expression of inflammatory-related factors (Mcp1 and
Il-6; Fig. 4E and F) and ER
stress-related factors (Grp78 and Sxbp1; Fig. 4G and H); significant differences
were observed between the Scr-siRNA and Fgfr2-siRNA
groups following co-treatment. Furthermore, the role of AA in
improving NAFLD/NASH proposed in the present study is summarized as
a schematic diagram in Fig. 4I. AA
was suggested to suppress obesity-associated insult via the
inhibition of TNFα-induced cellular hypoxia, inflammation and ER
stress. These benefits of AA are likely to be mediated by the
activation of the FGF21/FGFR2/adiponectin signaling pathway in
hepatocytes.
 | Figure 4.Fgfr2 knockdown suppresses the
inhibitory effects of AA on cell stress. Hif1α (A) mRNA and
(B) protein expression levels were assessed in HepG2 cells by
RT-qPCR and ELISA, respectively. The gene expression of (C)
Glut1 and (D) Vegfa, (E) Mcp1 and (F)
Il-6, (G) Grp78 and (H) Sxbp1 were determined
by RT-qPCR. Data are expressed as fold changes compared with
Scr siRNA. *P<0.05; **P<0.01. (I) Schematic model of
the effects of AA on hepatocyte stress. AA treatment was proposed
to be beneficial for relieving cell stress (hypoxia, inflammation
and ER stress) in hepatocytes via the regulation of the
FGF21/FGFR2/adiponectin pathway, which may improve NAFLD/NASH. AA,
ascorbic acid; ER, endoplasmic reticulum; FGF21, fibroblast growth
factor 21; Fgfr2, fibroblast growth factor receptor 2; Glut1,
glucose transporter 1; Grp78, glucose-regulated protein, 78 kDa;
HIF1α, hypoxia inducible factor 1α; IL-6, interleukin-6; Mcp1,
monocyte chemoattractant 1; NAFLD, non-alcoholic fatty liver
disease; NASH, non-alcoholic steatohepatitis; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Scr,
scramble; siRNA, small interfering RNA; Sxbp1, spliced
X-box-binding protein 1; TNFα, tumor necrosis factor α; Vegfa,
vascular endothelial growth factor A. |
Discussion
Long-term exposure to obesity-associated insults
induced by TNFα and saturated fatty acids causes lipid accumulation
in the liver, which leads to fatty liver disease, known as NAFLD,
which may progress to NASH (33).
In the present study, TNFα-treated HepG2 cells were employed to
examine the effects of AA on hepatocyte stress in obesity-related
NAFLD in vitro. Our results revealed that AA: i)
Significantly ameliorated TNFα-induced cell stresses, including
hypoxia, inflammation and ER stress; ii) significantly promoted the
secretion of HMW adiponectin from TNFα-treated cells; and iii)
activated the FGF21/FGFR2/adiponectin signaling pathway to exert
these beneficial effects as mentioned above.
Our data demonstrated that AA co-treatment
effectively attenuated TNFα-induced cell stress. The expression of
TNFα, a known pro-inflammatory factor, is significantly elevated in
patients with NAFLD/NASH, and is involved in liver lipid metabolism
and inflammation (33,34). Thus, TNFα-induced HepG2 cells were
employed in the present study to establish an in vitro model
in order to mimic the unhealthy state of hepatocytes in the
obesity-related NAFLD/NASH population. Our results revealed that
TNFα successfully induced hepatocyte stress in the form of
increased hypoxia, inflammation and ER stress.
The human body cannot synthesize vitamin C; a lower
consumption of vitamin C has been associated with a decreased
plasma level of AA, which may increase the risk of developing
metabolic diseases, including NAFLD/NASH (27). In addition, hepatocytes from
patients with NAFLD/NASH suffer from various types of stress, such
as hypoxia, inflammation and ER stress (35–37).
Therefore, we aimed to investigate the effects of AA on NAFLD/NASH
beyond its traditional characteristic as an excellent antioxidant.
Our results reported that AA reversed the aforementioned stresses
triggered by TNFα. Similar findings have also been reported in
human intervention studies. For example, overweight adults
supplemented with AA (1,000 mg/day) for 2 months exhibited
significantly reduced plasma c-reactive protein levels (38). Of note, we reported AA to reduce
the levels of HIF1α protein. Generally, the total level of HIF1α
does not usually change under hypoxia (39); although the mechanism associated
with the expression of HIF1α under these conditions was not
explored in our study, further investigation is required. AA can
inhibit the stability and transcriptional activity of HIF1α
protein. As mentioned in a previous study (32), HIF-1 is downregulated by
iron-containing 2-oxoglutarate-dependent enzymes that require AA as
a cofactor; HIF-1-dependent gene expression is effectively
suppressed by AA and is inhibited even under conditions that allow
HIF-1α protein stabilization. Additionally, Vissers et al
(40) have found that AA is the
main regulator of the hypoxic response in normal cells, and the
optimal level of AA has an important impact on HIF-1 regulation
(41). Taken together, these
results demonstrate that AA alone may be beneficial for the
amelioration of obesity-induced NAFLD/NASH by attenuating a wide
range of hepatocyte stresses.
This study also measured the secretion of total
adiponectin and HMW adiponectin levels, as obesity-associated
NAFLD/NASH has been associated with decreased levels of circulating
adiponectin and HMW adiponectin (42). We found that TNFα reduced the
secretion of HMW adiponectin, but exerted no notable effects on
total adiponectin levels. Treatment with AA not only increased
total adiponectin secretion, but also attenuated reductions in HMW
adiponectin secretion induced by TNFα. Our results were consistent
with those of a recent study, which indicated that the beneficial
effects of AA are closely associated with improvements in
adiponectin profiles rather than changes in total adiponectin
levels (43). Therefore, the
findings of the present study may suggest that AA may exert its
beneficial effects on hepatocyte stress by increasing the levels of
adiponectin levels, particularly HMW adiponectin.
Over the past few years, the specific roles of
FGF21/FGFR2 in modulating obesity and obesity-associated metabolic
diseases, such as NAFLD/NASH and type 2 diabetes have undergone
extensive study (44–46). Although FGF21 expression has been
shown to be notably elevated in obese individuals, long-term
treatment with FGF21 was determined to favorably improve the
outcomes linked to obesity and NAFLD (47,48).
Adiponectin is a downstream effector of FGF21/FGFR2 (49,50).
A recent study also suggested that the FGF21/adiponectin/IL-17A
pathway is crucial in alleviating hepatic steatosis and
inflammation in a mouse model of NASH (24). Nevertheless, to the best of our
knowledge, no studies have investigated the effects of AA on the
FGF21/FGFR2 signaling pathway, although white pitaya juice, which
contains AA, was proposed to attenuate hepatic steatosis in obese
mice (51). The results of our
study demonstrated that AA significantly increased the mRNA and
protein expression levels of FGF21 and FGFR2, and adiponectin
secretion. Furthermore, FGFR2 knockdown almost abolished the
protective effects of AA in terms of blocking the reduction of
HIF1α and the downregulation of cellular stress-related genes.
Unexpectedly, TNFα also induced FGF21 expression; further
investigations are warranted to determine the mechanisms underlying
this effect. An overlap in energy metabolism has been reported
between the AMPK and FGF21 signaling pathways (52–54).
However, in our specific model, AA did not activate the AMPK
pathway; this was inconsistent with the findings of another study
which reported that strawberry extract attenuated lipid
accumulation in HepG2 cells via AMPK activation (55). This inconsistency may be explained
by the different agents used; strawberry extract contains other
bioactive substances apart from AA that may activate the AMPK
pathway. Collectively, these results illustrated that the
FGF21/FGFR2/adiponectin signaling pathway could serve a crucial
role in attenuating obesity-related insult-associated hepatocyte
stress by AA.
In conclusion, the present study demonstrated that
AA effectively attenuated hepatocyte stress induced by
obesity-related insults through activation of the
FGF21/FGFR2/adiponectin pathway, which may suggest a novel
mechanism of AA in alleviating NAFLD/NASH. Further investigation is
required to verify the role of AA in NAFLD/NASH in future
studies.
Our study has certain limitations in that HepG2
cells were employed to establish an in vitro model of NAFLD.
Under certain culture conditions, HepG2 cells display robust
morphological and functional differentiation. Therefore, this cell
line can be important for the study of human liver diseases, yet
this cell line represent as a model of cancer rather than normal
human liver. Thus, the results of the present study require further
verification in vivo.
Acknowledgements
Not applicable.
Funding
This study was supported by the Fundamental Research
Funds for the Central Universities (grant. no. xjj2017186);
National Natural Science Foundation of China (grant. no. 81874263);
and China Postdoctoral Science Foundation (grant. no.
2017T100759).
Availability of data and materials
All data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XQG, XL and XQL made substantial contributions to
the design of this study. XQG, ZYH, KJW and YFZ performed the
experiments. ZYH, YXW and XML conducted the RT-qPCR and western
blot analysis experiments. YFY, JJ and XQG interpreted the results
and wrote the draft of the manuscript. XQL and XL critically
revised the manuscript for important intellectual content. All
authors read and approved the final manuscript and agreed to the
publication of the final manuscript.
Ethics approval and consent to
participate
Not applicable
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of nonalcoholic fatty
liver disease-Meta-analytic assessment of prevalence, incidence,
and outcomes. Hepatology. 64:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Castera L, Vilgrain V and Angulo P:
Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol.
10:666–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmed A, Wong RJ and Harrison SA:
Nonalcoholic fatty liver disease review: Diagnosis, treatment, and
outcomes. Clin Gastroenterol Hepatol. 13:2062–2070. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fabbrini E, Sullivan S and Klein S:
Obesity and nonalcoholic fatty liver disease: Biochemical,
metabolic, and clinical implications. Hepatology. 51:679–689. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Handa P, Vemulakonda AL, Maliken BD,
Morgan-Stevenson V, Nelson JE, Dhillon BK, Hennessey KA, Gupta R,
Yeh MM and Kowdley KV: Differences in hepatic expression of iron,
inflammation and stress-related genes in patients with nonalcoholic
steatohepatitis. Ann Hepatol. 16:77–85. 2017. View Article : Google Scholar
|
|
6
|
Trujillo ME and Scherer PE:
Adiponectin-journey from an adipocyte secretory protein to
biomarker of the metabolic syndrome. J Intern Med. 257:167–175.
2010. View Article : Google Scholar
|
|
7
|
Kawano J and Arora R: The role of
adiponectin in obesity, diabetes, and cardiovascular disease. J
Cardiometab Syndr. 4:44–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hebbard L and George J: Animal models of
nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol.
8:35–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mandal P, Park PH, McMullen MR, Pratt BT
and Nagy LE: The anti-inflammatory effects of adiponectin are
mediated via a heme oxygenase-1-dependent pathway in rat Kupffer
cells. Hepatology. 51:1420–1429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Polyzos SA, Kountouras J, Zavos C and
Tsiaousi E: Role of adiponectin in the pathogenesis and treatment
of nonalcoholic fatty liver disease. Diabetes Obes Metab.
12:365–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Finelli C and Tarantino G: What is the
role of adiponectin in obesity related non-alcoholic fatty liver
disease? World J Gastroenterol. 19:802–812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phillips LK, Peake JM, Zhang X, Hickman
IJ, Briskey DR, Huang BE, Simpson P, Li SH, Whitehead JP, Martin JH
and Prins JB: Postprandial total and HMW adiponectin following a
high-fat meal in lean, obese and diabetic men. Eur J Clin Nutr.
67:377–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Tong W, Zhao X, Zhang H, Tang Y and
Deng X: Chinese herb extract improves liver steatosis by promoting
the expression of high molecular weight adiponectin in NAFLD rats.
Mol Med Rep. 16:5580–5586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Sun LR, Li MZ, et al: The changes
of serum high molecular weight adiponectin levels in T2DM with
nonalcoholic fatty liver disease. Medical Innovation of China.
9:5–7. 2013.
|
|
16
|
Gimeno RE and Moller DE: FGF21-based
pharmacotherapy-potential utility for metabolic disorders. Trends
Endocrinol Metab. 25:303–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JH, Kang YE, Chang JY, Park KC, Kim
HW, Kim JT, Kim HJ, Yi HS, Shong M, Chung HK and Kim KS: An
engineered FGF21 variant, LY2405319, can prevent non-alcoholic
steatohepatitis by enhancing hepatic mitochondrial function. Am J
Transl Res. 8:4750–4763. 2016.PubMed/NCBI
|
|
18
|
Fisher FM, Chui PC, Nasser IA, Popov Y,
Cunniff JC, Lundasen T, Kharitonenkov A, Schuppan D, Flier JS and
Maratos-Flier E: Fibroblast growth factor 21 limits lipotoxicity by
promoting hepatic fatty acid activation in mice on methionine and
choline-deficient diets. Gastroenterology. 147:1073–1083.e6. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fisher FM and Maratos-Flier E:
Understanding the physiology of FGF21. Annu Rev Physiol.
78:223–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inagaki T: Research perspectives on the
regulation and physiological functions of FGF21 and its association
with NAFLD. Front Endocrinol (Lausanne). 6:1472015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Musso G, Cassader M and Gambino R:
Non-alcoholic steatohepatitis: Emerging molecular targets and
therapeutic strategies. Nat Rev Drug Discov. 15:249–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vernia S, Cavanagh-Kyros J, Garcia-Haro L,
Sabio G, Barrett T, Jung DY, Kim JK, Xu J, Shulha HP, Garber M, et
al: The PPARα-FGF21 hormone axis contributes to metabolic
regulation by the hepatic JNK signaling pathway. Cell Metab.
20:512–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piccinin E and Moschetta A:
Hepatic-specific PPARα-FGF21 action in NAFLD. Gut. 65:1075–1076.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao L, Yin J, Gao W, Wang Q, Yao W and Gao
X: A long-acting FGF21 alleviates hepatic steatosis and
inflammation in NASH mice partly through an FGF21- adiponectin-
IL17A pathway. Br J Pharmacol. 175:3379–3393. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duerbeck NB, Dowling DD and Duerbeck JM:
Vitamin C: Promises not kept. Obstet Gynecol Surv. 71:187–193.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oliveira CP, Gayotto LC, Tatai C, Della
Nina BI, Lima ES, Abdalla DS, Lopasso FP, Laurindo FR and Carrilho
FJ: Vitamin C and vitamin E in prevention of nonalcoholic fatty
liver disease (NAFLD) in choline deficient diet fed rats. Nutr J.
2:92003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hadzi-Petrushev N, Dimovska K, Jankulovski
N, Mitrov D and Mladenov M: Supplementation with Alpha-Tocopherol
and ascorbic acid to nonalcoholic fatty liver disease's statin
therapy in men. Adv Pharmacol Sci. 2018:46730612018.PubMed/NCBI
|
|
28
|
Fischer AP and Miles SL: Ascorbic acid,
but not dehydroascorbic acid increases intracellular vitamin C
content to decrease Hypoxia Inducible Factor-1 alpha activity and
reduce malignant potential in human melanoma. Biomed Pharmacother.
86:502–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pires AS, Marques CR, Encarnação JC,
Abrantes AM, Mamede AC, Laranjo M, Gonçalves AC, Sarmento-Ribeiro
AB and Botelho MF: Ascorbic acid and colon cancer: An oxidative
stimulus to cell death depending on cell profile. Eur J Cell Biol.
95:208–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rose FJ, Webster J, Barry JB, Phillips LK,
Richards AA and Whitehead JP: Synergistic effects of ascorbic acid
and thiazolidinedione on secretion of high molecular weight
adiponectin from human adipocytes. Diabetes Obes Metab.
12:1084–1089. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuiper C, Dachs GU, Currie MJ and Vissers
MC: Intracellular ascorbate enhances hypoxia-inducible factor
(HIF)-hydroxylase activity and preferentially suppresses the HIF-1
transcriptional response. Free Radic Biol Med. 69:308–317. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanuri G, Spruss A, Wagnerberger S,
Bischoff SC and Bergheim I: Role of tumor necrosis factor α (TNFα)
in the onset of fructose-induced nonalcoholic fatty liver disease
in mice. J Nutr Biochem. 22:527–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ajmal MR, Yaccha M, Malik MA, Rabbani MU,
Ahmad I, Isalm N and Abdali N: Prevalence of nonalcoholic fatty
liver disease (NAFLD) in patients of cardiovascular diseases and
its association with hs-CRP and TNF-α. Indian Heart J. 66:574–579.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mann JP, Raponi M and Nobili V: Clinical
implications of understanding the association between oxidative
stress and pediatric NAFLD. Expert Rev Gastroenterol Hepatol.
11:371–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahesh K, Kumar PP, Ali MS, et al:
Endoplasmic reticulum (ER) stress in non-alcoholic fatty liver
disease (NAFLD). J Clin Exp Hepatol. 2:S38–S39. 2012.(In Chinese).
View Article : Google Scholar
|
|
37
|
Ao N, Yang J, Wang X and Du J:
Glucagon-like peptide-1 preserves non-alcoholic fatty liver disease
through inhibition of the endoplasmic reticulum stress-associated
pathway. Hepatol Res. 46:343–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Block G, Jensen CD, Dalvi TB, Norkus EP,
Hudes M, Crawford PB, Holland N, Fung EB, Schumacher L and Harmatz
P: Vitamin C treatment reduces elevated C-reactive protein. Free
Radic Biol Med. 46:70–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schumacker PT: Hypoxia-inducible factor-1
(HIF-1). Crit Care Med. 33:423–425. 2005. View Article : Google Scholar
|
|
40
|
Vissers MC, Gunningham SP, Morrison MJ,
Dachs GU and Currie MJ: Modulation of hypoxia-inducible factor-1
alpha in cultured primary cells by intracellular ascorbate. Free
Radic Biol Med. 42:765–772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miyata T, Wada Y, Cai Z, Iida Y, Horie K,
Yasuda Y, Maeda K, Kurokawa K and van Ypersele de Strihou C:
Implication of an increased oxidative stress in the formation of
advanced glycation end products in patients with end-stage renal
failure. Kidney Int. 51:1170–1181. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li G, Yin J, Fu J, Li L, Grant SFA, Li C,
Li M, Mi J, Li M and Gao S: FGF21 deficiency is associated with
childhood obesity, insulin resistance and hypoadiponectinaemia: The
BCAMS study. Diabetes Metab. 43:253–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McMorrow AM, Connaughton RM, Magalhães TR,
McGillicuddy FC, Hughes MF, Cheishvili D, Morine MJ, Ennis S, Healy
ML, Roche EF, et al: Personalized cardio-metabolic responses to an
anti-inflammatory nutrition intervention in obese adolescents: A
randomized controlled crossover trial. Mol Nutr Food Res.
62:e17010082018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen
M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, et al:
Fibroblast growth factor 21 reverses hepatic steatosis, increases
energy expenditure, and improves insulin sensitivity in
diet-induced obese mice. Diabetes. 58:250–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Singhal G, Kumar G, Chan S, Fisher FM, Ma
Y, Vardeh HG, Nasser IA, Flier JS and Maratos-Flier E: Deficiency
of fibroblast growth factor 21 (FGF21) promotes hepatocellular
carcinoma (HCC) in mice on a long term obesogenic diet. Mol Metab.
13:56–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Samms RJ, Lewis JE, Norton L, Stephens FB,
Gaffney CJ, Butterfield T, Smith DP, Cheng CC, Perfield JW II,
Adams AC, et al: FGF21 is an insulin-dependent postprandial hormone
in adult humans. J Clin Endocrinol Metab. 102:3806–3813. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J and Li Y: Fibroblast growth factor
21, the endocrine FGF pathway and novel treatments for metabolic
syndrome. Drug Discovery Today. 19:579–589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Talukdar S, Zhou Y, Li D, Rossulek M, Dong
J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM, et al: A
long-acting FGF21 molecule, PF-05231023, decreases body weight and
improves lipid profile in non-human primates and type 2 diabetic
subjects. Cell Metab. 23:427–440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Holland WL, Adams AC, Brozinick JT, Bui
HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng
CC, et al: An FGF21-adiponectin-ceramide axis controls energy
expenditure and insulin action in mice. Cell Metab. 17:790–797.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin Z, Tian H, Lam KS, Lin S, Hoo RC,
Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A and Li X: Adiponectin
mediates the metabolic effects of FGF21 on glucose homeostasis and
insulin sensitivity in mice. Cell Metab. 17:779–789. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Song H, Zheng Z, Wu J, Lai J, Chu Q and
Zheng X: White pitaya (Hylocereus undatus) juice attenuates insulin
resistance and hepatic steatosis in diet-induced obese mice. PLoS
One. 11:e01496702016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Berglund ED, Kang L, Lee-Young RS,
Hasenour CM, Lustig DG, Lynes SE, Donahue EP, Swift LL, Charron MJ
and Wasserman DH: Glucagon and lipid interactions in the regulation
of hepatic AMPK signaling and expression of PPARalpha and FGF21
transcripts in vivo. Am J Physiol Endocrinol Metab. 299:E607–E614.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu X, Wang Y, Hou L, Xiong Y and Zhao S:
Fibroblast growth factor 21 (FGF21) promotes formation of aerobic
myofibers via the FGF21-SIRT1-AMPK-PGC1α pathway. J Cell Physiol.
232:1893–1906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Salminen A, Kauppinen A and Kaarniranta K:
FGF21 activates AMPK signaling: Impact on metabolic regulation and
the aging process. J Mol Med (Berl). 95:123–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Forbes-Hernández TY, Giampieri F,
Gasparrini M, Afrin S, Mazzoni L, Cordero MD, Mezzetti B, Quiles JL
and Battino M: Lipid accumulation in HepG2 cells is attenuated by
strawberry extract through AMPK activation. Nutrients. 9:E6212017.
View Article : Google Scholar : PubMed/NCBI
|