Introduction
Platelets are derived from mature megakaryocytes,
and are biconvex in shape, when they have not been activated, have
no nuclei and have a diameter of 2–4 µm. Platelets have a short
lifespan, typically 1–2 weeks. In addition to hemostasis, platelets
serve an important role in pathophysiological processes, including
angiogenesis, atherosclerosis, tissue regeneration and immune
regulation (1,2).
Platelet-derived microparticles (PMPs) are submicron
particles with a diameter of 0.1–1 µm that are secreted by
activated or apoptotic platelets (3). Particles of <0.1 µm in diameter
are called exosomes, while particles >1 µm in diameter are
termed apoptotic bodies (4,5).
Microparticles have been demonstrated to act as carriers of signals
for communication between cells (1). However, because of their small size
and the diverse structures and phenotypes of PMPs, there are
considerable challenges in handling and characterizing these
particles (6).
In the present study, the traditional method of
extracting PMPs was improved upon, and differences in the
expression of molecular markers on the surface of PMPs were
investigated. Transmission electron microscopy (TEM) was used to
identify platelets and their microparticles. Platelets were readily
activated using a simple protocol, and a large quantity of
microparticles of varying compositions, sizes and structures were
derived from the activated platelets. A high degree of purity of
PMPs was attained through gradient centrifugation, and additionally
it was demonstrated that the standard practice of using flow
cytometry to identify PMPs may underestimate the actual number of
microparticles.
Materials and methods
Sample acquisition
Platelet-rich plasma (PRP) was obtained from healthy
volunteers from the blood station of Changhai Hospital affiliated
to The Second Military Medical University between May and December
2015. Volunteers provided informed consent for the collection of
blood samples and the protocol used in the present was approved by
The Ethics Committee of Changhai Hospital. Blood (200 ml) was
collected in the morning at the blood station prior to breakfast
from each volunteer. There were 32 volunteers, 23 males and 9
females. The age of volunteers was 20–40 years old. The
free-flowing technique using a 16G needle was employed for blood
collection to prevent platelet activation. The initial
centrifugation of the PRP, as described below, was performed <2
h after collection. Aspirin (0.5 g; Sigma-Aldrich; Merck KGaA) was
dissolved in 1 ml DMSO, sterilized by filtration through a
bacterial filter (0.22 µm), and the filtrate was added to each unit
of PRP.
Separation and extraction of PMPs
PRP was dispensed into a 15 ml centrifuge tube and
washed using 5 ml of 4.2 mM ethylene diamine tetra-acetic acid per
tube. The tubes were centrifuged at 150 × g for 15 min at 22°C and
the pellet containing the red blood cells was discarded. The
supernatant was further centrifuged at 1,000 × g for 10 min at 22°C
and the supernatant was discarded. The pellet containing the
platelets was resuspended in 1 ml of Hepes-NaCl2 buffer
(10 mM HEPES, 0.85% NaCl2, pH 7.4) and transferred to a
1.5 ml tube. Each tube contained 10–12 million platelets. Thrombin
(Sigma-Aldrich; Merck KGaA) was added at a final concentration of
0.1 IU/ml to tubes to activate the platelets, each tube was mixed
gently and evenly, and subsequently placed in a 37°C cell incubator
for 90 min. Other tubes were vortexed at room temperature for 1 min
(oscillation frequency 2,800 beats/min) to activate platelets. The
suspension was centrifuged at 3,200 × g for 15 min at 4°C and the
supernatant was transferred to a new 1.5 ml tube. The supernatant
was further centrifuged at 20,000 × g for 90 min at 4°C and the
supernatant was discarded, leaving the pellet which contained the
PMPs.
Flow cytometry
Data was analyzed by CellQuest v5.1 software (BD
Bioscience). Each tube of PMPs was resuspended in 200 µl binding
buffer (BD Bioscience) and mixed evenly. Polystyrene microspheres
with a diameter of 1 µm (Sigma-Aldrich; Merck KGaA) were diluted
1:1,000 with PBS and 5 µl was added to each tube. As a control, one
tube of 200 µl binding buffer with 5 µl microspheres was left
blank. The solution was then incubated at 4°C for 15 min in the
dark with 20 µl CD63-phycoerythrin (PE; cat. no. 556020; BD
Bioscience), CD61-PE (cat. no. 555754; BD Bioscience),
CD62P-allophycocyanin (APC; cat. no. 550888; BD Bioscience),
CD40L-PE (cat. no. 555702; BD Bioscience), CD41-APC (cat. no.
303710; BioLegend, Inc.) or 5 µl Annexin V-FITC antibodies (BD
Bioscience).
TEM
Platelets with PMPs were fixed in 1 ml 2%
paraformaldehyde and 2% glutaraldehyde at 4°C for 6 h. After
fixing, the samples were centrifuged at 1,000 × g for 10 min at 4°C
and supernatant containing the fixative was discarded. The
precipitate was washed five times with PBS and centrifuged again at
1,000 × g at 4°C for 10 min. The precipitate was resuspended in
plasma (supernatant obtained from the second centrifugation) and
centrifuged at 1,000 × g at 4°C for 30 min. The majority of the
supernatant (plasma) was discarded, leaving a small volume. The
samples were fixed using 1% osmium tetroxide at 4°C for 2 h, rinsed
once with PBS and centrifuged at 1,000 × g for 10 min at 4°C.
Gradient dehydration was performed as follows: 70% acetone for 15
min, 80% acetone for 15 min, 90% acetone for 15 min, and 100%
acetone for 10 min (×2). The pellet was embedded in a transparent
capsule no. 3 (Electron Microscopy room of Second Military Medical
University) with epoxy resin and oven-dried at 45°C for 12 h and
60°C for 36 h. The embedded block was sliced into 70 nm thick
sections with an ultramicrotome and placed on a copper mesh covered
with polyvinyl formal film. Melted wax was dripped on to a sterile
Petri dish to form a wax plate, a few drops of lead dye solution
were dropped on to the wax and the copper mesh with the sample was
placed on top of the lead dye droplet and incubated for 15 min at
room temperature. The copper mesh was taken from the lead dye
solution, washed three times with distilled water, dried with
filter paper and observed by TEM. The specimens were examined using
an electron microscope (HT7700; Hitachi, Ltd.) at an operating
voltage of 100 kV (magnification, ×1,000–5,000). Images were
analyzed using ImageJ v1.8.0 software (National Institutes of
Health).
Statistical analysis
SPSS v19.0 statistical software (IBM Corp.) was used
for data analysis. All data are expressed as the mean ± standard
error of the mean. A two-tailed Student's t-test was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
PMPs can be separated by gradient
centrifugation
There is no standard protocol to extract PMPs as far
as the authors are aware. However, a protocol for the isolation of
PMPs from blood samples was recommended (Fig. 1A). In the present study, gradient
centrifugation was used to extract PMPs, with some adjustments.
During this process, red blood cells and the majority of platelet
fragments were removed from the sample and the resulting
precipitate containing the PMPs could be observed at the base of
the tube (Fig. 1B). However, it
should be noted that a portion of microparticles were lost during
the gradient centrifugation.
Flow cytometry results of PMPs
The distribution ranges of the particles with
different diameters were divided (Fig.
2A): R1 means 1.0 µm polystyrene microspheres; R2 means
particles smaller than 1.0 µm; R3 means particles larger than 1.0
µm. Flow cytometry analysis of PMPs demonstrated that platelets
were markedly sensitive to both physical and chemical stimuli.
Pretreatment with aspirin effectively reduced the activation of
platelets; however, the attenuation of activation was not complete
(Fig. 2B and C). PRP without
aspirin pretreatment derived a portion of PMPs, which were lost
during the gradient centrifugation, then after totally being
activated, the PMPs finally obtained were obviously fewer than PRP
with aspirin pretreatment (Fig.
2D). Treatment with thrombin or vortexing stimulated the
release of a large number of microparticles from the PMPs. The
majority of the precipitates obtained by gradient centrifugation
contained particles <1 µm in diameter, although it was possible
that platelet fragments or large vesicles were also present
(Fig. 2E and F).
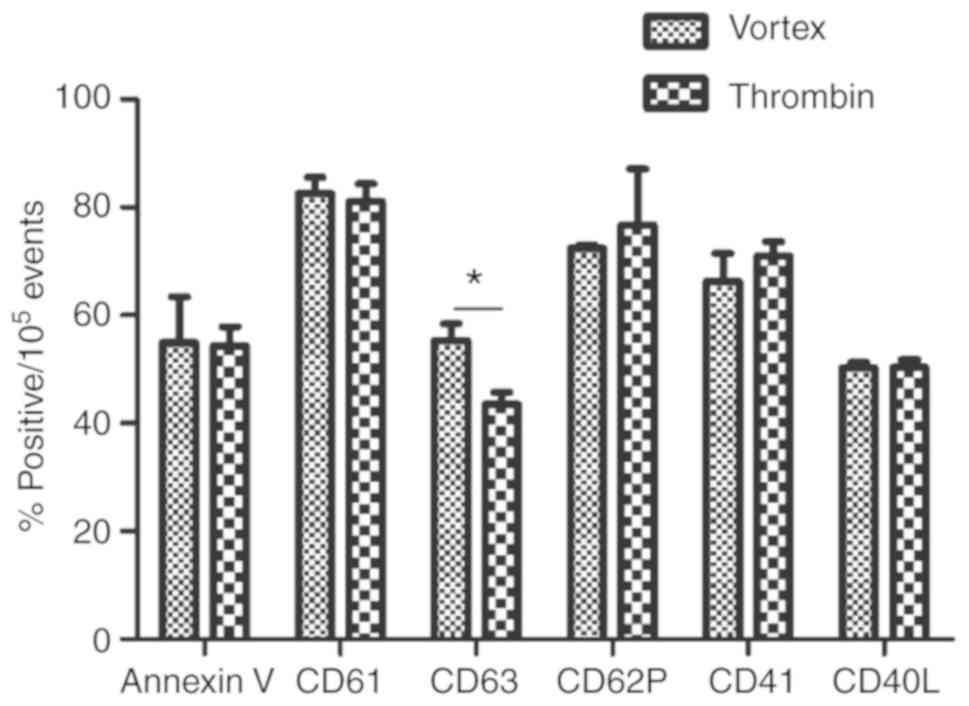
Labeling with Annexin V or the five platelet surface
markers (CD61, CD62P, CD63, CD40L and CD41) demonstrated that the
targets were present on the surface of the microparticles at high
levels in the PMPs obtained from platelets stimulated by thrombin
or vortexing. There was no significant difference in the expression
of surface markers found between PMPs from the thrombin-stimulated
platelets compared with the vortex-stimulated platelets, except in
the case of CD63, which was significantly higher in the PMPs from
vortex-activated platelets (55.38±5.27% vs. 43.50±3.86%; P<0.05;
Fig. 3).
TEM of platelets and PMPs
Platelets which had not been pretreated with aspirin
displayed an increased level of activation after centrifugation
compared with aspirin-treated platelets and the untreated platelets
possessed extended pseudopodia compared with the aspirin-treated
platelets (Fig. 4A and B). The
distal portion of the pseudopodia gradually separated from the
platelet body to form a membrane chain (Fig. 4C). Eventually, the membrane chain
broke and the microparticles were secreted. Treatment with thrombin
and vortexing activated platelets resulting in the release of PMPs
(Fig. 4D and E). PMPs have
different shapes and complex ultrastructures, and their contents,
membrane structure and electron density vary. In a similar way to
medicine capsules, PMPs contain biologically active substances such
as α-granules, glycogen granules and mitochondria (Fig. 4F).
Discussion
Microparticles secreted by platelets are involved in
the regulation of many physiological and pathophysiological
processes in the body, including coagulation, vasomotor regulation,
cell proliferation, differentiation, apoptosis, inflammation, the
immune response and the transmission of signals between cells
(1,6). Studies have demonstrated that
different kinds of cells can secrete microparticles, including red
blood cells, lymphocytes, platelets, endothelial cells and tumor
cells; however, the microparticles derived by platelets account for
70–90% of microparticles present in the circulatory system
(7–9). Extracting PMPs is a difficult and
labor-intensive process. A two-step gradient centrifugation method
is recommended to extract PMPs from blood samples, although a
standardized approach is not available. In theory, the longer and
faster the final centrifugation step, the more particles of smaller
sizes can be obtained, although there may be an upper limit. In
addition, increasing the number of gradient layers will
concurrently increase the purity of the sample, with the caveat
that there will also be an increase in the loss of the desired
product. Therefore, determining the optimal combination of
centrifugal speed, duration and number of gradient layers, to
maximize the purity and minimize the loss of microparticles
requires further investigation.
In the present study, adjustments were made to the
most frequently used two-step gradient centrifugation method. PRP
was obtained from the blood station at Changhai Hospital affiliated
to The Second Military Medical University, and met China's national
quality standards, reducing the proportion of other cells obtained.
Residual red blood cells were removed from the PRP in the first
centrifugation step, further improving the purity of the platelets.
The precipitation of the platelets was achieved by centrifugation,
after which the platelets were activated using thrombin or by
vortexing. To increase the purity of PMPs further, two additional
centrifugation steps were used. However, there was still a small
proportion of unwanted particles >1 µm in diameter in the final
precipitate, possibly platelet fragments or large vesicles. PMPs
are particles with a 0.1–1 µm diameter that are secreted by
activated or apoptotic platelets (3). Therefore, a 1 µm diameter was set as
the upper limit for microparticles in the flow cytometry
experiments performed in the present study. Platelets can be
activated with ease, with activation resulting in the release of a
large number of microparticles (10). Platelets continue to release
microparticles when they are inactive, although at a lower level
(10). Previous studies have
demonstrated that collagen, thrombin, lipopolysaccharide, viruses,
immune complexes, temperature changes and shear stress could all
activate platelets, resulting in the release of microparticles
(7,11–15).
To minimize the loss of microparticles during transportation and
centrifugation, antiplatelet activation or anticoagulant drugs can
be added before further activation of platelets, of which aspirin
is probably the most economical (16). A previous clinical study reported
that aspirin significantly reduced the number of PMPs in
circulation in patients with coronary heart disease (17). The present study additionally
demonstrated that although aspirin did not completely prevent
platelets from being activated, it markedly inhibited the secretion
of PMPs. In the PRP samples not pre-treated with aspirin, there was
a notable increase in the release of microparticles following
centrifugation. This fraction of microparticles was discarded with
the supernatant during the gradient centrifugation, and the final
number of microparticles extracted was markedly decreased compared
with the aspirin pre-treated samples.
Murphy and Gardner (18) studied the effects of temperature on
platelets and demonstrated that platelets maintained in
vitro at 22°C had an improved structure and function. Bode
and Knupp (19)
demonstrated that platelets lost more glycoproteins and formed more
microparticles at 4°C. Therefore, platelets in the present study
were extracted at 22°C and the final ultracentrifugation step was
performed at 4°C to obtain the PMPs.
The mechanism of microparticle production is not
fully understood and may be related to the asymmetric loss of
proteasomes and membrane phospholipids (20,21).
A previous study demonstrated that during normal blood flow,
activated platelets form a membrane chain downstream of the blood
flow, and eventually the membrane chain breaks, releasing
microparticles (22). In the
present study, platelets were observed to form a membrane chain
following activation. Eventually the membrane chain broke and
microparticles were released. There are a number of methods used to
identify PMPs and their associated markers. After activation, the
intracellular calcium concentration of platelets is elevated, and
non-selective ion channels on the cell surface and on the
mitochondria, in addition to the stimulation of some enzymes,
promote the flipping of phosphatidylserine; when present on the
extracellular facing side of the cell membrane, phosphatidylserine
subsequently acts as a signal for phagocytosis on apoptotic cells
(23–25). Therefore, Annexin V is commonly
used as a marker for detecting microparticles. However, studies
have shown that ~50% of the microparticles in circulation do not
present phosphatidylserine on their surface (26). The use of Annexin V alone as a
marker of microparticles may, therefore, underestimate the number
of microparticles present (26).
In the present study, Annexin V positive particles accounted for
~50% of the particles obtained. Therefore, the use of Annexin V
alone as a marker for microparticles may be inadequate and may
result in a large underestimation of the number of
microparticles.
A limitation of using flow cytometry to detect
microparticles is the accuracy of detection for sub-200 nm
particles (27). TEM is the most
accurate and reliable method for identifying microparticles;
however, the preparation of samples is a time-consuming process and
requires high quality specimens. In order to determine whether the
extracted microparticles are derived from platelets, it is also
necessary to identify platelet surface-specific molecular markers.
In the present study, CD41, CD61, CD62P, CD63 and CD40L were used
in combination with Annexin V to perform a single step detection of
microparticles from platelets activated by vortexing or thrombin
treatment. Differences in handling and storage methods may result
in changes to the surface markers present on PMPs (28,29).
The present results demonstrated that all six markers were
expressed on the surface of PMPs, with CD63 expression found to be
significantly higher in microparticles derived from
vortex-stimulated platelets compared with microparticles from
thrombin-stimulated platelets. In a previous study, CD63 was used
as a biomarker for the detection of exosomes (30). However, in the present study,
exosomes were not able to be detected by flow cytometry. Brisson
et al (31) demonstrated
that the majority of larger extracellular vesicles, up to 1 µm in
diameter, also expressed CD63. Whether the difference in CD63
levels on PMPs obtained from the two different stimulation methods
was a result of differences in the presence of large vesicles is
unknown and requires further study. Yuana et al (32) studied microparticles in fresh
plasma using electron microscopy. The results demonstrated that
microparticles existed in various shapes, including round,
drop-shaped, tubular and cup-shaped. In the present study, based on
the results of TEM, PMPs also displayed a variety of shapes, sizes,
contents, ultrastructure and electron densities. After activation,
platelets secrete particles and tend to disintegrate (33). In the present study, the diameter
of microparticles was 200–600 nm. Other differences observed in the
TEM images include the presence of either a single or double
layered membrane, α-granule content, the presence of glycogen
granules and the presence of mitochondria. The majority of the
particles observed were circular, oval or almost round. The size of
microparticles is associated with their contents. Microparticles
containing organelles typically have a larger diameter and
irregularly shaped particles may be a result of the handling
process (32,33). To the best of our knowledge, a
certain shape or size of PMP has not been attributed to a
particular function. Difficulties in isolating specific types of
PMPs has hampered progress in understanding differences in
function.
In conclusion, high purity PMPs may be obtained by
gradient centrifugation, although a small fraction of platelet
fragments or large vesicles may remain. A higher purity of PMPs can
be achieved if a 1 µm filter is used. At present, the use of flow
cytometry to detect PMPs based on Annexin V may lead to inaccurate
results. TEM is more accurate in identifying microparticles;
however, the technical limitations, labor-intensive preparation
process and considerably lower throughput make TEM less convenient.
Determining the best method to use towards identifying PMPs may be
best decided on a per case basis; it may be possible to use TEM on
a small sample of purified PMPs to confirm the results of flow
cytometry. Difficulties in identifying PMPs may be a result of the
diversity of PMPs, and this diversity may additionally underlie the
range of functions attributed to PMPs. Therefore, further studies
are required to elucidate the function of PMPs and to improve the
methods for their identification.
Acknowledgements
The authors would like to thank Dr Li Su (College of
Pharmacy, Second Military Medical University) and Dr Xiao-Yan Fan
(Second Military Medical University) for their contributions to
flow cytometry and TEM.
Funding
The present study was supported by a grant from The
National Natural Science Foundation of China (grant no.
81570208).
Availability of data and materials
All data generated and/or analyzed in the present
study is included in the published article.
Authors' contributions
JZ, XXZ, BZ and JG conceived and designed the
experiments. JG, CF and XS performed the experiments. JG and SZ
analyzed the data and wrote the manuscript. All authors read and
approved the final manuscript
Ethics approval and consent to
participate
Patients provided informed consent for the
collection of blood samples and the protocol used in the present
was approved by the Ethics Committee of Changhai Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burnouf T, Goubran HA, Chou ML, Devos D
and Radosevic M: Platelet microparticles: Detection and assessment
of their paradoxical functional roles in disease and regenerative
medicine. Blood Rev. 28:155–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Semple JW, Italiano JE Jr and Freedman J:
Platelets and the immune continuum. Nat Rev Immunol. 11:264–274.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clark SR, Thomas CP, Hammond VJ,
Aldrovandi M, Wilkinson GW, Hart KW, Murphy RC, Collins PW and
O'Donnell VB: Characterization of platelet aminophospholipid
externalization reveals fatty acids as molecular determinants that
regulate coagulation. Proc Natl Acad Sci USA. 110:5875–5880. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mooberry MJ and Key NS: Microparticle
analysis in disorders of hemostasis and thrombosis. Cytometry A.
89:111–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Montoro-García S, Shantsila E, Marín F,
Blann A and Lip GY: Circulating microparticles: New insights into
the biochemical basis of microparticle release and activity. Basic
Res Cardiol. 106:911–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kailashiya J: Platelet-derived
microparticles analysis: Techniques, challenges and
recommendations. Anal Biochem. 546:78–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burnier L, Fontana P, Kwak BR and
Angelillo-Scherrer A: Cell-derived microparticles in haemostasis
and vascular medicine. Thromb Haemost. 101:439–451. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horstman LL and Ahn YS: Platelet
microparticles: A wide-angle perspective. Crit Rev Oncol Hematol.
30:111–142. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
VanWijk MJ, VanBavel E, Sturk A and
Nieuwland R: Microparticles in cardiovascular diseases. Cardiovasc
Res. 59:277–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cauwenberghs S, Feijge MA, Harper AG, Sage
SO, Curvers J and Heemskerk JW: Shedding of procoagulant
microparticles from unstimulated platelets by integrin-mediated
destabilization of actin cytoskeleton. FEBS Lett. 580:5313–5320.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reininger AJ, Heijnen HF, Schumann H,
Specht HM, Schramm W and Ruggeri ZM: Mechanism of platelet adhesion
to von Willebrand factor and microparticle formation under high
shear stress. Blood. 107:3537–3545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sims PJ, Faioni EM, Wiedmer T and Shattil
SJ: Complement proteins C5b-9 cause release of membrane vesicles
from the platelet surface that are enriched in the membrane
receptor for coagulation factor Va and express prothrombinase
activity. J Biol Chem. 263:18205–18212. 1988.PubMed/NCBI
|
|
13
|
Johnson L, Reade MC, Hyland RA, Tan S and
Marks DC: In vitro comparison of cryopreserved and liquid
platelets: Potential clinical implications. Transfusion.
55:838–847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown GT and McIntyre TM:
Lipopolysaccharide signaling without a nucleus: Kinase cascades
stimulate platelet shedding of proinflammatory IL-1β-rich
microparticles. J Immunol. 186:5489–5496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boilard E, Paré G, Rousseau M, Cloutier N,
Dubuc I, Lévesque T, Borgeat P and Flamand L: Influenza virus H1N1
activates platelets through FcgammaRIIA signaling and thrombin
generation. Blood. 123:2854–2863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan Y, Liang H, Liu H, Li D, Chen X, Li L,
Zhang CY and Zen K: Platelet-Secreted MicroRNA-223 promotes
endothelial cell apoptosis induced by advanced glycation end
products via targeting the insulin-like growth factor 1 receptor. J
Immunol. 192:437–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bulut D, Becker V and Mügge A:
Acetylsalicylate reduces endothelial and platelet-derived
microparticles in patients with coronary artery disease. Can J
Physiol Pharmacol. 89:239–244. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murphy S and Gardner FH: Effect of storage
temperature on maintenance of platelet viability-deleterious effect
of refrigerated storage. N Engl J Med. 280:1094–1098. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bode AP and Knupp CL: Effect of cold
storage on platelet glycoprotein 1B and vesiculation. Transfusion.
34:690–696. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta N, Li W, Willard B, Silverstein RL
and McIntyre TM: Proteasome proteolysis supports stimulated
platelet function and thrombosis. Arterioscler Thromb Vasc Biol.
34:160–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morel O, Jesel L, Freyssinet JM and Toti
F: Cellular mechanisms underlying the formation of circulating
microparticles. Arterioscler Thromb Vasc Biol. 31:15–26. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tersteeg C, Heijnen HF, Eckly A,
Pasterkamp G, Urbanus RT, Maas C, Hoefer IE, Nieuwland R, Farndale
RW, Gachet C, et al: FLow-induced PRotrusions (FLIPRs): A
platelet-derived platform for the retrieval of microparticles by
monocytes and neutrophils. Circ Res. 114:780–791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mattheij NJ, Gilio K, van Kruchten R, Jobe
SM, Wieschhaus AJ, Chishti AH, Collins P, Heemskerk JW and Cosemans
JM: Dual mechanism of integrin alphaIIbβ3 closure in procoagulant
platelets. J Biol Chem. 288:13325–13336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bettache N, Gaffet P, Allegre N, Maurin L,
Toti F, Freyssinet JM and Bienvenüe A: Impaired redistribution of
aminophospholipids with distinctive cell shape change during
Ca2+-induced activation of platelets from a patient with
Scott syndrome. Br J Haematol. 101:50–58. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choo HJ, Saafir TB, Mkumba L, Wagner MB
and Jobe SM: Mitochondrial calcium and reactive oxygen species
regulate agonist-initiated platelet phosphatidylserine exposure.
Arterioscler Thromb Vasc Biol. 32:2946–2955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arraud N, Linares R, Tan S, Gounou C,
Pasquet JM, Mornet S and Brisson AR: Extracellular vesicles from
blood plasma: Determination of their morphology, size, phenotype
and concentration. J Thromb Haemost. 12:614–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nolan JP: Flow cytometry of extracellular
vesicles: Potential, Pitfalls, and Prospects. Curr Protoc Cytom.
73:1–16. 2015.PubMed/NCBI
|
|
28
|
Flaumenhaft R, Dilks JR, Richardson J,
Alden E, Patel-Hett SR, Battinelli E, Klement GL, Sola-Visner M and
Italiano JE Jr: Megakaryocyte-derived microparticles: Direct
visualization and distinction from platelet-derived microparticles.
Blood. 113:1112–1121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rank A, Nieuwland R, Delker R, Köhler A,
Toth B, Pihusch V, Wilkowski R and Pihusch R: Cellular origin of
platelet-derived microparticles in vivo. Thromb Res. 126:e255–e259.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baranyai T, Herczeg K, Onódi Z, Voszka I,
Módos K, Marton N, Nagy G, Mäger I, Wood MJ, El Andaloussi S, et
al: Isolation of exosomes from blood plasma: Qualitative and
quantitative comparison of ultracentrifugation and size exclusion
chromatography methods. PLoS One. 10:e01456862015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brisson AR, Tan S, Linares R, Gounou C and
Arraud N: Extracellular vesicles from activated platelets: A
semiquantitative cryo-electron microscopy and immuno-gold labeling
study. Platelets. 28:263–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuana Y, Koning RI, Kuil ME, Rensen PC,
Koster AJ, Bertina RM and Osanto S: Cryo-electron microscopy of
extracellular vesicles in fresh plasma. J Extracell Vesicles.
22013.doi: 10.3402/jev.v2i0.21494.
|
|
33
|
Ponomareva AA, Nevzorova TA, Mordakhanova
ER, Andrianova IA, Rauova L, Litvinov RI and Weisel JW:
Intracellular origin and ultrastructure of platelet-derived
microparticles. J Thromb Haemost. 15:1655–1667. 2017. View Article : Google Scholar : PubMed/NCBI
|