Introduction
Abnormalities in monoamine synthesis and
transmission have, for a long time, been implicated in the
pathogenesis of depressive disorder (1). Tyrosine hydroxylase (TH) and
tryptophan hydroxylase (TPH) are rate-limiting enzymes in
catecholamine and serotonin biosyntheses, respectively and their
level is closely linked to the etiology and development of
depression. A previous clinical study showed that the TH
immunoreactivity was lower (~30%) in the locus coeruleus of suicide
victims (2). It was found that
chronic mild stress resulted in depressive behavior in rats with
decreased TH mRNA expression in the locus coeruleus in both sexes
(3). In the brain of male C57BL/6
mice, it was found that chronic fluoxetine treatment increased
locus coeruleus TH (4). Meanwhile,
it was evidenced that TPH activity was significantly decreased in
the hippocampus of mice following the induction of chronic stress
over 20 days (5). In TPH-2
knockout and TPH1/TPH2 double knockout mice, central serotonin
(5-HT) levels were markedly reduced with associated depressive
behaviors (6). Previous studies
have reported that fluoxetine and sertraline contribute to an
increase in TPH expression (7–9).
However, further studies focusing on the association between the
serotonergic/noradrenergic systems and the mechanisms of action of
antidepressants are still required.
It was recently suggested that the
indoleamine-2,3-dioxygenase (IDO) pathway of tryptophan metabolism
may be involved in the onset of depression (10,11).
IDO is an extra-hepatic rate-limiting enzyme, which catalyzes the
metabolism of tryptophan along the kynurenine pathways (12). The activation of IDO leads to
tryptophan depletion and the accumulation of kynurenine
pathway-induced neurotoxic metabolites, including kynurenic acid,
quinolinic acid and 3-hydroxy kynurenine; both of these changes are
considered to be associated with the progression of depression
(13,14). In lipopolysaccharide
injection-induced depressive mice, upregulation of IDO expression
may be observed in the brainstem, as well as an increased
kynurenine/tryptophan ratio in the serum (15). On the other hand, neurotoxic
metabolites of IDO extensively disturb neurotransmission by
releasing oxidative stress mediators. In fact, the brain is
particularly vulnerable to oxidative/nitrosative stress in
neurodegenerative disorders. Stefanescu et al (16), reported increased malondialdehyde
(MDA) content with decreased superoxide dismutase (SOD) activity in
the serum of depressed patients (particularly in patients with
recurrent symptoms), which could be reversed by treatment with
venlafaxine (VLX) and citalopram.
Type A monoamine oxidase (MAO-A) is an enzyme
associated with monoamine transmitter metabolism, and plays a vital
role in the onset, development and treatment of depression
(17). The MAO-A level in the
brain is determined prior to birth, and MAO-A and its major
substrate, 5-HT, regulate the development of the neuronal
architecture (18). A clinical
study of 17 depressed individuals concluded that MAO-A levels in
the brain were elevated during untreated major depression and that
this was the primary monoamine-lowering process (19). Following exposure to chronic social
defeat stress, a significant increase was observed in rat serum
corticosterone (glucocorticoids) levels, which was correlated with
the upregulation of MAO-A (20).
In fact, the reversible inhibitor of MAO-A moclobemide has shown an
antidepressant effect in the treatment of a wide spectrum of
depressive disorders (21).
Selective serotonin and noradrenaline reuptake
inhibitors (SNRIs) are a major class of antidepressants, and their
therapeutic effect is generally attributed to an increase in the
availability of monoamines in the synapses between neurons
(22). The efficiency of the drugs
takes up to 4–6 weeks to manifest, and the understanding of this
mechanism remains unclear. Whether the drugs regulate the key
enzymes associated with the synthesis and metabolism of monoamine
neurotransmitters needs to be further evaluated. In the present
study, the expression of TH, TPH, IDO and MAO-A, as well as the
oxidative stress levels in response to treatment with VLX (a
classical SNRI), were measured in a rat model of CUS-induced
depression.
Materials and methods
Animals
A total of 80 6-week-old male Sprague-Dawley rats
(Animal Experimental Center, Chongqing Medical University) weighing
180–200 g were housed in groups of 4 rats/cage. Before the
experiment, rats were kept at 25±2°C and a relative humidity of
55±5% in a quiet, airy and clean environment (12-h light/dark
cycle) with ad libitum feeding and drinking. The animals
were allowed to habituate to the room for 7 days and received
health checks daily. Rats were anesthetized with 10% chloral
hydrate [30 mg/0.1 kg; intraperitoneal (i.p.)] before being
sacrificed. All experimental procedures were approved by the Ethics
Committee of Chongqing Medical University.
Experimental design
A total of 80 screened rats were randomly divided
into four groups (n=20): The control group; chronic unpredictable
stress (CUS) group; VLX group; and VLX+CUS group. VLX (Wuhan
Shengtianyu Technology Co. Ltd.) was dissolved in 0.5% sodium
carboxymethyl cellulose (CMC-Na) solution to obtain a concentration
of 2 mg/ml. Animals in the VLX and VLX+CUS groups were treated with
VLX (20 mg/kg·day) by gavage once a day for 28 days. Control and
CUS group rats were treated with same volume of CMC-Na.
CUS paradigm
CUS and VLX+CUS group rats were exposed to various
stressors for 28 days, and the matched control rats did not receive
any stressors. The stressors were little-modified from a method
described previously (23),
including 1-min nip-tail, 5-min forced swimming in ice water, 24-h
cage tilting at 45°, 24-h wet bedding, 24-h food and water
deprivation, 5-min thermal environment at 47°C, 3-min electric
shock at 45V, 2-h loud noise, overnight illumination, and
alterations of the light-dark cycle (2-h light/dark cycle). On
average, rats were individually exposed to the stressors once a
day. The same stressors were never performed in succession within 4
days.
Open-field test (OFT)
The locomotive activity was measured by OFT
following a previously published method (24), in quiet and semi-dark conditions. A
self-made wooden box (100 cm ×100 cm ×40 cm; 4 cm ×4 cm square
grids in the bottom) with a black inner surface was used in this
experiment. Each rat was carefully placed in the center of the
bottom of the box sequentially and allowed to freely explore for 5
min. Ambulation and rearing scores were collected by two
experienced observers. The box was cleaned thoroughly between
tests.
Forced swim test (FST)
The FST was conducted according a reported method
(25) by placing rats individually
into a vertical transparent acrylic cylinder (60 cm height × 30 cm
diameter). The cylinder was filled with water at a depth of 35 cm
(at 23±2°C) which kept the rat upright and unable to touch the
bottom nor jump out of the cylinder. Each rat was forced to swim
for 6 min, and the immobile time during the first 3, last 3 and
total 6 min was recorded by an observer blinded to the treatments.
Immobility was defined as a state of floating (>2 sec, no
climbing or swimming) with necessary stroke to keep the head above
water.
Western blot analysis
On the 29th day, the animals were sacrificed by
rapid decapitation and their heads were immediately snap frozen in
liquid nitrogen for few seconds. In each rat, the cortex and
hippocampi were rapidly dissected on an ice-cold surface, and
frozen in liquid nitrogen before protein extraction. Protein
samples were extracted using a Membrane and Cytosol Protein
Extraction kit (cat. no. P0033; Beyotime Institute of
Biotechnology) following homogenization. A bicinchoninic acid kit
(cat. no. P0011; Beyotime Institute of Biotechnology) was used to
measure the protein concentration. Protein samples were boiled in
SDS-PAGE buffer for 5 min. A mass of 50 µg/lane total protein was
separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes. The membrane was blocked with 5% milk in TBST
(TBS with 0.05% Tween-20) for 1 h at room temperature. Following
blocking, the membrane was washed with TBST, then incubated with
anti-TH (cat. no. AB152) and anti-TPH (cat. no. AB1541) antibodies
separately (1:1,000 dilution; EMD Millipore) overnight at 4°C, and
β-actin (cat. no. TA-09, 1:2,000 dilution, OriGene Technologies,
Inc.) was established as loading control. After washing, the
membranes were incubated with peroxidase-conjugated secondary
antibodies (cat. no. A0208, 1:1,000 dilution, Beyotime Institute of
Biotechnology; cat. no. D110174, 1:5,000 dilution, Sangon Biotech
Co., Ltd.) for 1 h at room temperature. The membranes were
developed using an enhanced chemiluminescence western blot
detection system (Pierce; Thermo Fisher Scientific, Inc.). Images
were acquired and analyzed using Quantity One version 4.5.2
software (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Tissue samples were homogenized for RNA isolation
using TRIzol® (Thermo Fisher Scientific, Inc.) in
combination with RNeasy Minikits (Qiagen GmbH). RT was conducted
using iScript cDNA synthesis kit (Bio-Rad Laboratories) for 5 min
at 25°C, 30 min at 42°C, and 5 min at 85°C. qPCR was performed
using SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.) in a
Real-Time PCR detection system (Bio-Rad Laboratories).
Thermocycling conditions were an initial step of 30 sec at 95°C and
40 cycles of 5 sec at 95°C, 30 sec at 55°C, and 1 min at 72°C, with
a final extension of 10 min at 72°C. β-actin was included as a
loading control in each analysis. Quantification was conducted via
the 2−ΔΔCq method (26). Primer sequences were: β-actin
(NM_031144.3), forward 5′-CGTAAAGACCTCTATGCCAACA-3′ and reverse
5′-TAGGAGCCAGGGCAGTAATC-3′; TH (NM_012740.3), forward
5′-AGAGGACAGCATCCCACAGC-3′ and reverse 5′- ATCACGGGCGGACAGTAGA-3′;
TPH (NM_173839.2), forward 5′-TTTGTAGCCAACATTCCTCA-3′ and reverse
5′-ACTATTGAAAGTAGAAACCACCTC-3′; IDO (NM_023973.1), forward
5′-TGATGTCCTTCTGGGAATAAA-3′ and reverse
5′-AGCCTCCTTCAAGTCTTCATT-3′; and MAO-A (NM_033653.1), forward
5′-GCCAGCCAGTAGGTAGGAT-3′ and reverse
5′-CTTGGACTCGGGTTCTTCA-3′.
Determination of SOD activity and MDA
content
Rat blood samples were collected after rapid
decapitation and deposited at 4°C for 30 min. Serum was obtained
after centrifugation at 1,000 × g for 10 min at 4°C (Thermo Fisher
Scientific, Inc.). The cortex and hippocampi of each rat were
isolated rapidly on ice. Tissue samples were weighed and then
homogenized separately with an electric tissue homogenizer (IKA
Werke GmbH & Co. KG) in ice-cold homogenization buffer. The
homogenate was centrifuged at 5,000 × g for 10 min at 4°C. The
supernatant was carefully obtained for the determination of SOD
activity and MDA content according to the instructions of the assay
kit (Nanjing Jiancheng Bioengineering Institute).
Statistical analysis
Data from behavioral and biochemical studies are
presented as the mean ± SEM. Experiments were repeated ≥5 times.
All statistical analyses were conducted using GraphPad 5.0
(GraphPad Software, Inc.) via one-way ANOVA followed by the
Bonferroni test for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
Behavioral changes in the OFT and
FST
In the OFT at the 3rd week, CUS rats displayed a
significant decrease in both ambulation and rearing score, compared
with control rats (P<0.01, Fig.
1A; P<0.05, Fig. 1C).
Similarly, in the OFT at the 4th week, decreased ambulation and
rearing scores were also observed in the CUS group compared with
the control group (P<0.05, Fig.
1B; P<0.01, Fig. 1D).
Administration of VLX prevented the decrease in ambulation and
rearing score in the VLX+CUS rats at the 4th week (P<0.05 vs.
CUS rats; Fig. 1B and D).
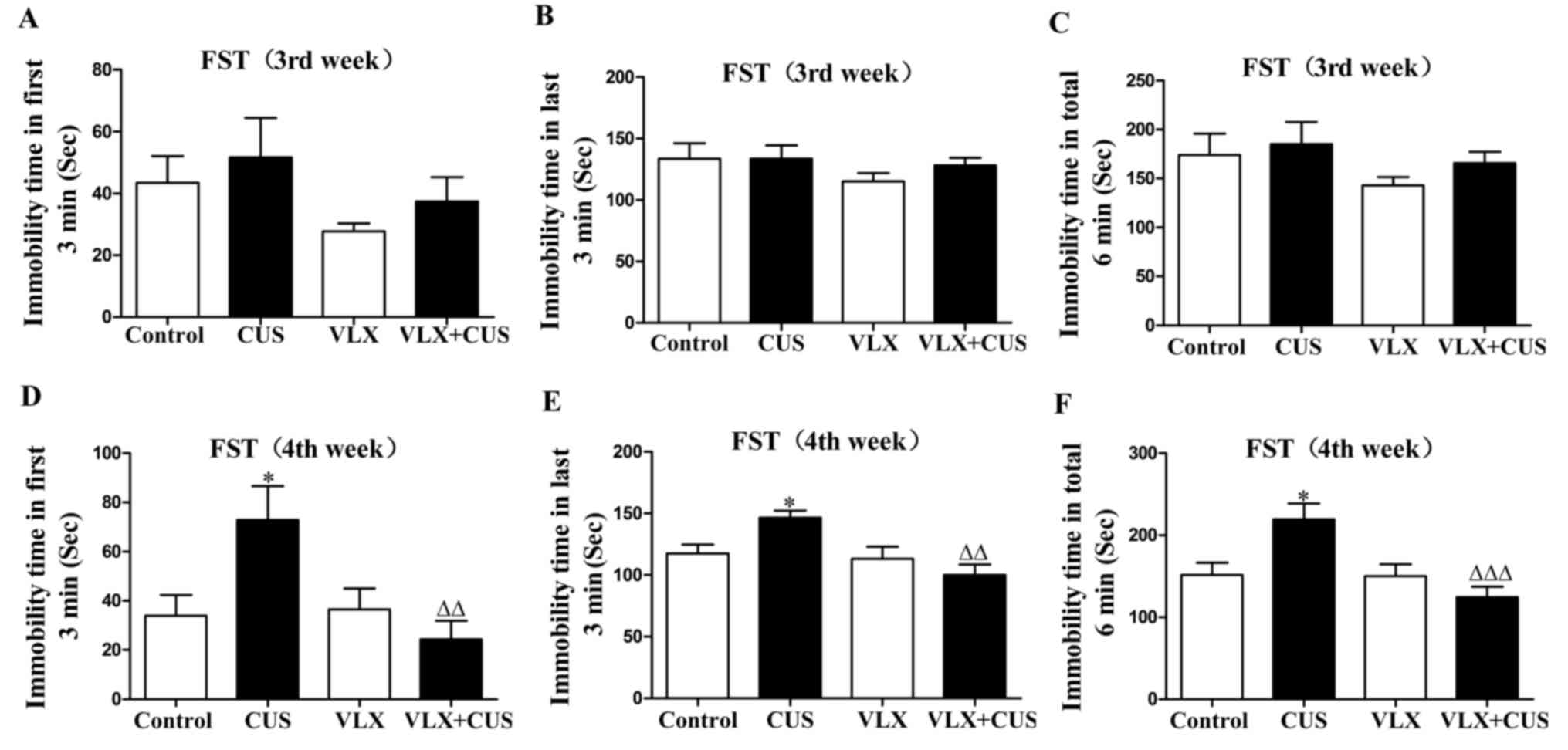
In the FST, the immobility time of the CUS rats was
significantly prolonged at the 4th week (P<0.05 vs. control
rats; Fig. 2D-F). VLX treatment
inhibited this increase in immobility time in the VLX+CUS rats at
the 4th week, compared with the CUS rats (P<0.01, Fig. 2D and E; P<0.001, Fig. 2F).
TH and TPH mRNA expression in the
cortex and hippocampus
TH mRNA expression in the hippocampus, and TPH mRNA
expression in both the cortex and hippocampus were significantly
decreased in the CUS rats (P<0.05 vs. control rats; Fig. 3B-D). VLX administration clearly
prevented the decrease in TH mRNA in the cortex and hippocampus in
the VLX+CUS rats (P<0.05 vs. CUS rats; Fig. 3A and B). VLX treatment tended to
upregulate TPH mRNA expression in the cortex and hippocampus in
VLX+CUS rats, but there was no significant difference (P>0.05
vs. CUS rats; Fig. 3C and D).
TH and TPH protein expression in the
cortex and hippocampus
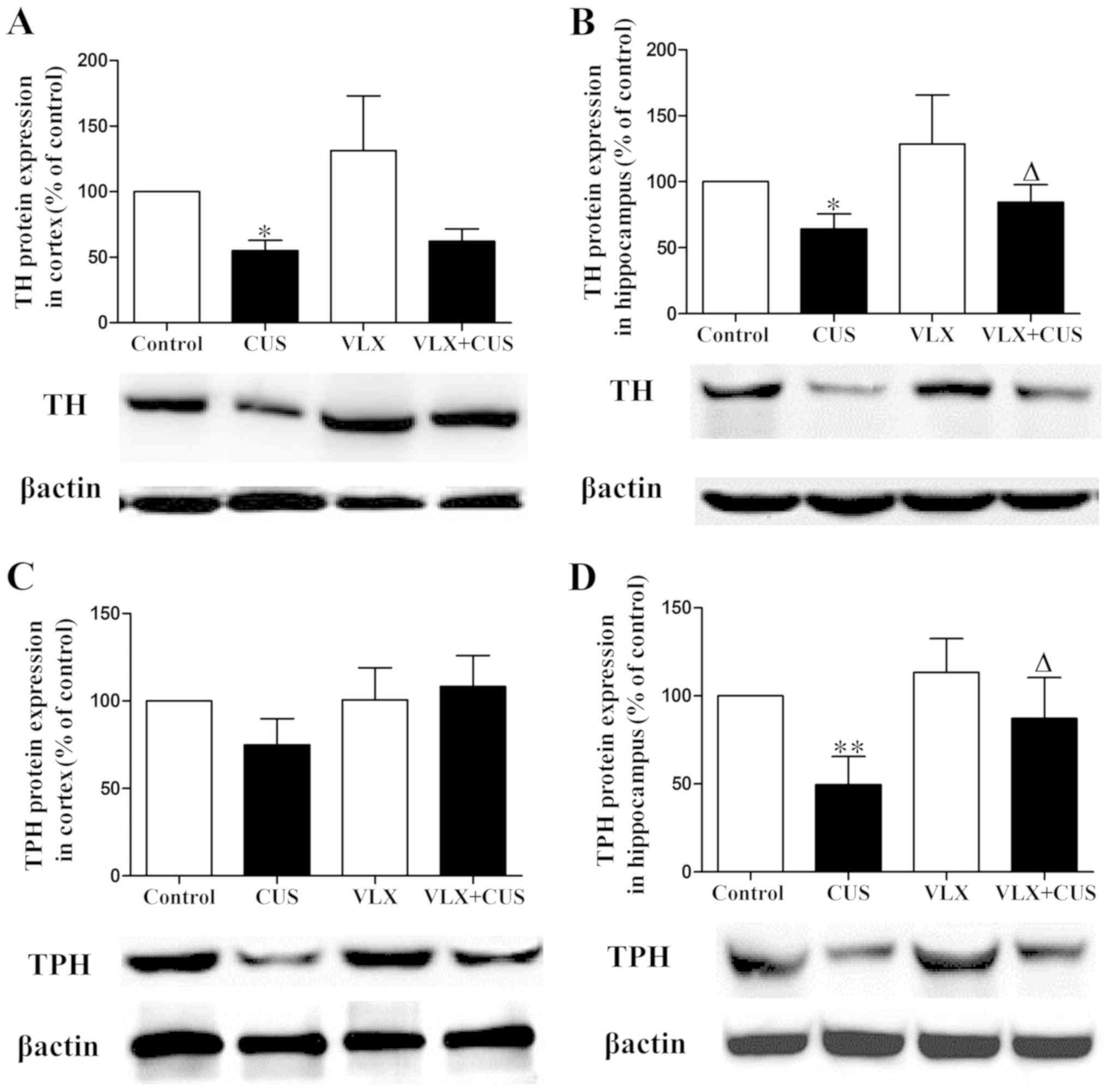
Meanwhile, TH protein expression was clearly
decreased in the cortex and hippocampus of CUS rats (P<0.05 vs.
control rats; Fig. 4A and B). TPH
protein expression in CUS rats was significantly reduced in the
hippocampus (P<0.01 vs. control rats; Fig. 4D) and tended to decrease in the
cortex (P>0.05 vs. control rats; Fig. 4C). VLX administration prevented the
decrease in TH and TPH protein expression in the hippocampus of
VLX+CUS rats (P<0.05 vs. CUS rats; Fig. 4B and D). VLX treatment tended to
increase TPH protein expression in the cortex of VLX+CUS rats, but
no significant difference was observed (P>0.05 vs. CUS rats;
Fig. 4C).
mRNA expression of IDO and MAO-A
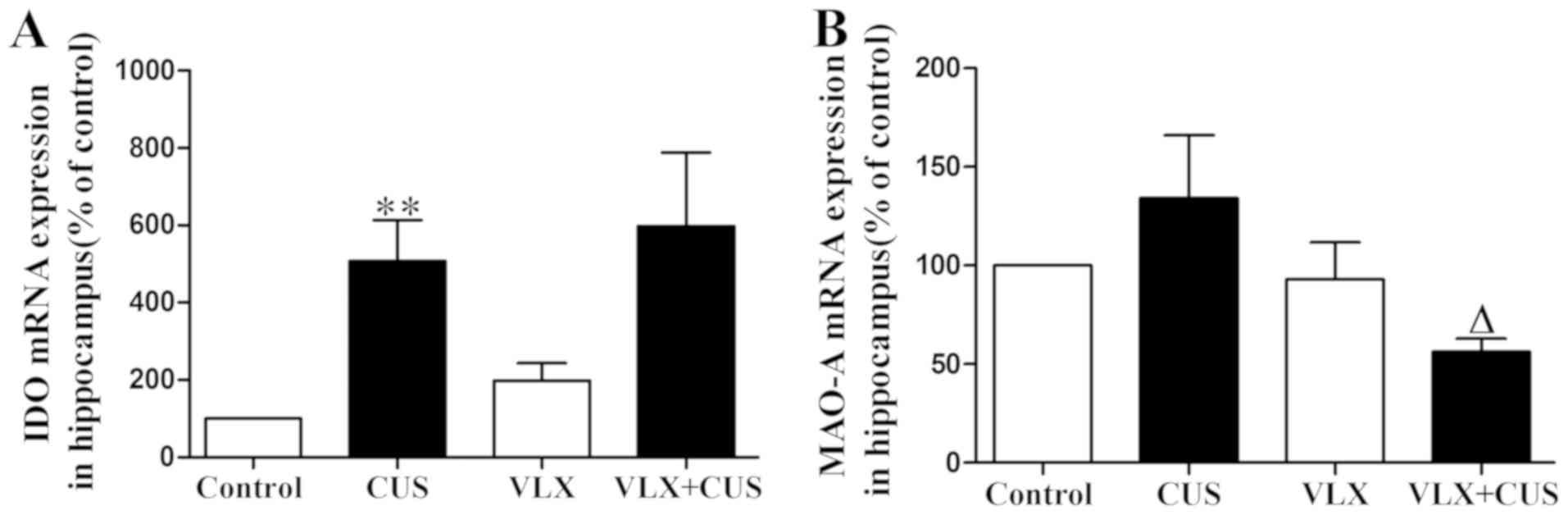
In CUS rats, IDO mRNA expression was significantly
elevated and MAO-A mRNA expression tended to be upregulated
compared with control rats (P<0.01, Fig. 5A; P>0.05, Fig. 5B). Administration of VLX inhibited
the increase in MAO-A expression but failed to have an effect on
IDO overexpression in VLX+CUS rats, compared with CUS rats
(P>0.05, Fig. 5A; P<0.05,
Fig. 5B).
Alteration in SOD activity and MDA
content
Compared with control rats, the CUS rats showed
significantly lower SOD activity in the serum, cortex and
hippocampus (P<0.05; Fig.
6A-C), as well as a higher MDA content in the cortex and
hippocampus (P<0.05, Fig. 6E and
F). There was no significant difference in MDA content of serum
between CUS and control rats (P>0.05, Fig. 6D).VLX intervention prevented the
decrease in SOD activity in the serum and cortex in VLX+CUS rats
(P<0.05 vs. CUS rats; Fig. 6A and
B). VLX also inhibited the increase in MDA content in the
cortex and hippocampus in VLX+CUS rats, compared with CUS rats
(P<0.05, Fig. 6E; P<0.01,
Fig. 6F). However, VLX failed to
decrease the MDA content of serum significantly in VLX+CUS rats
(P>0.05 vs. CUS rats, Fig.
6D).
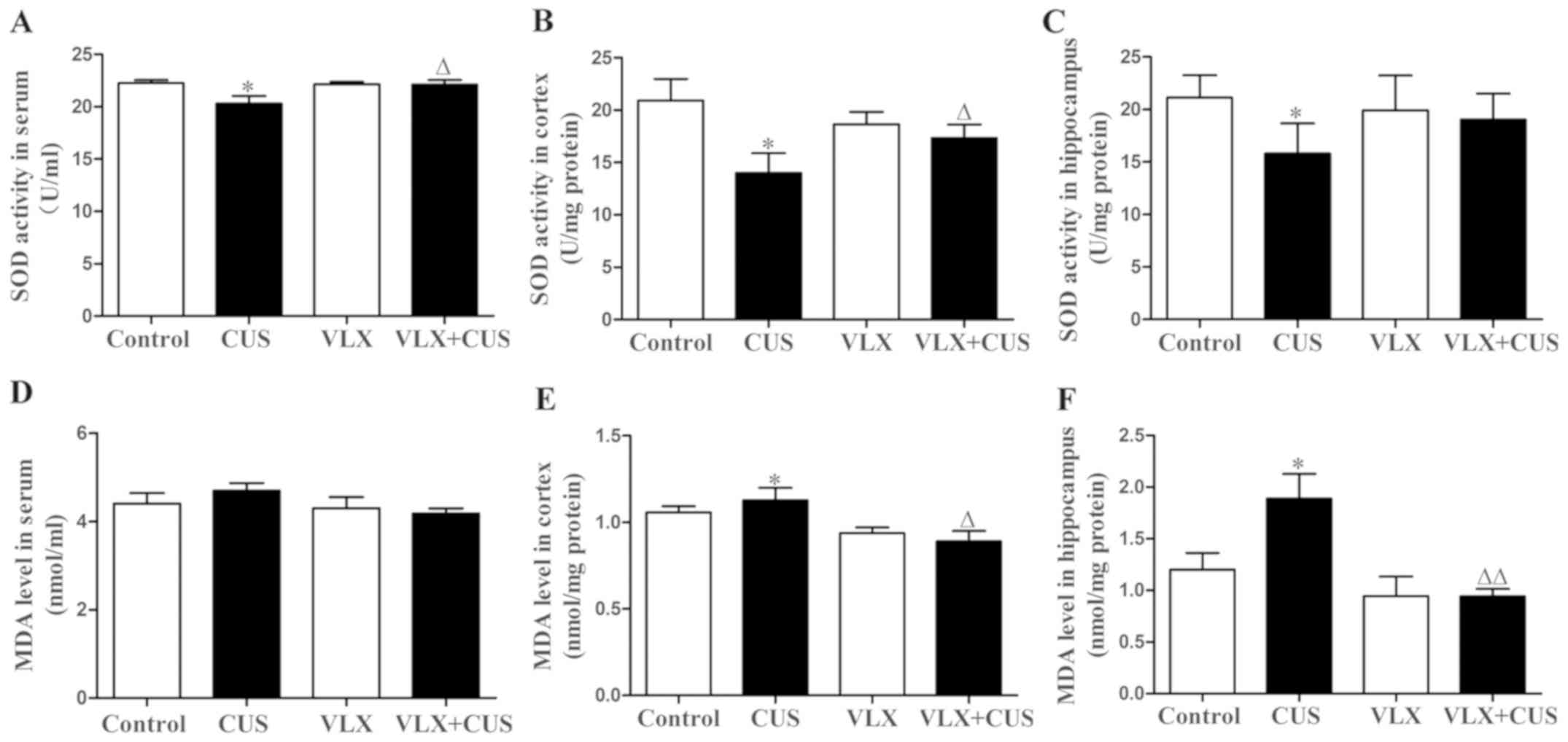 | Figure 6.Effect of CUS on SOD activity and MDA
content in the rat serum, cortex and hippocampus, and the effect of
intervention with VLX. SOD activity in the (A) serum, (B) cortex
and (C) hippocampus was assessed. The MDA content of the (D) serum,
(E) cortex and (F) hippocampus was measured. Mean ± SEM, n=10.
*P<0.05 vs. control; ΔP<0.05,
ΔΔP<0.01 vs. CUS. CUS, chronic unpredictable stress;
VLX, venlafaxine; SOD, superoxide dismutase; MDA,
malondialdehyde. |
Discussion
In the present study, it was shown that exposure to
CUS for 4 weeks not only induced depression-like behaviors, but
also resulted in a general downregulation of TH and TPH, as well as
an upregulation of IDO and MAO-A. The results indicated that
chronic stress-induced depression-like behavior may be associated
with abnormalities in the synthesis and metabolism of monoaminergic
transmitters. Moreover, CUS induced increased MDA content and
decreased SOD activity, suggesting neuronal damage induced by
oxidative stress. Chronic VLX treatment (20 mg/kg·day) for 4 weeks
alleviated depression-like behavior in CUS rats in the OFT and FST.
When the dose of VLX used in this study is converted to a human
dose, it is 195 mg/day based on the body surface area normalization
method (27). This converted dose
is in the normal range of human dosages (the recommended maximum
dosage of VLX is 225 mg/day orally) (28). The dose of VLX in this study was
also reported effective in previous studies (29,30).
In addition, it was shown that chronic VLX treatment not only
mitigated oxidative stress, but also prevented the decrease in TH
and TPH and inhibited the overexpression of IDO and MAO-A,
suggesting that its antidepressant effect may involve mitigating
oxidative stress and augmenting monoamine neurotransmitter
synthesis.
The dysregulation of TH and TPH has been linked to
the pathogenesis of depression, due to their roles in synthesis of
monoaminergic transmitters. As previously reported, decreased TH
protein and mRNA expression in the hippocampus may be observed in
mice with depression caused by repeated injections of
corticosterone (31). Following
daily administration (5 mg/kg, i.p.) of fluoxetine for 2 weeks, the
TH mRNA levels markedly increase in the rat locus coeruleus
(32). In addition, evidence
suggests that, when repeatedly separated from their pups, maternal
rats become depressed, exhibiting lower expression of 5-HT and TPH
in the dorsal raphe (33). Rats
receiving fluoxetine treatment exhibit an increase in TPH
expression in the dorsal raphe (7). Similarly, in the present study, the
mRNA and protein TH and TPH expression in the cortex and
hippocampus was generally decreased in the CUS rats, which was
prevented by VLX treatment. These data showed that CUS might induce
depressive symptoms by decreasing the synthesis of monoamine
neurotransmitters. It is also possible that VLX exerted an
antidepressant effect not only through the short-term reuptake
inhibition of serotonin and noradrenalin, but also via long-term
promotion of monoamine neurotransmitter synthesis. On the contrary,
it has been reported that chronic restraint stress induces anxiety
and depression-like behaviors accompanied by an increased TH mRNA
expression in the nucleus accumbens, with fluoxetine treatment able
to reverse this (34). Moreover,
the protein expression of TPH was found to be increased by chronic
social stress and normalized by citalopram (35). Interestingly, another study showed
that chronic fluoxetine treatment raised TPH immunoreactivity in
treated adolescent rats, while it reduced TPH immunoreactivity in
treated adult rats in total dorsal raphe nuclei (36). Based on previous studies and the
present results, these discrepancies in the expression of TH and
TPH may be due to the variety of depression models, disparities in
animal age, diversity of brain regions, and differences among the
antidepressants used. However, further research is required to
determine how SNRIs mediate the TH and TPH expression.
Tryptophan metabolism mediated by the kynurenine
pathway may directly affect the synthesis of 5-HT. During this
process, IDO catalyzes the conversion of tryptophan to kynurenine,
usually via oxidative stress and cell-mediated immune activation
(37). The upregulation of IDO is
considered to be a biomarker of depressive disorder. A previous
study reported that IDO was upregulated in the cortex following
exposure to mild CUS, with markedly increased tumor necrosis factor
(TNF)-α expression in the plasma and cerebral cortex (38). In a clinical study, it was shown
that peripheral interferon (IFN)-α treatment led to IDO activation,
ultimately causing depressive symptoms (39). It was shown that imipramine reduced
IDO expression in both the hippocampus and raphe nuclei of rats
exposed to chronic mild stress; however, these effects were not
statistically significant (40).
The present study showed that CUS led to a large increase in IDO
expression in the hippocampus, with a generally increased MDA
content and decreased SOD activity, indicating that CUS may
inactivate 5-HT synthesis, partly through the IDO-induced
kynurenine pathway of tryptophan metabolism. Interestingly,
although VLX treatment clearly reversed the changes in oxidative
stress parameters, it did not have an effect on IDO overexpression,
suggesting that the antidepressant effect of VLX may involve the
prevention of the harmful effects of oxidative stress, but not the
correction of IDO overexpression. This result was similar to that
of a previous study, which found that neither VLX alone, nor VLX in
combination with agomelatine, had an effect on IDO activity in mice
with chronic stress (41). This
confirmed that the antidepressant effects of VLX were not mediated
by its effect on the kynurenine pathway, at least in the present
model.
Furthermore, an increasing body of evidence suggests
that the monoamine metabolic enzyme MAO-A is a key regulator of
depressive disorder (42,43). 5-HT, an MAO-A substrate, has been
known to be essential for neuronal plasticity and central to the
pathogenesis of depression and age-related neurological diseases
(44). MAO-A is not only present
by heredity, but also by environmental factors, including hormonal
factors and stress. An early study showed that salivary MAO-A
activity was closely linked to stress (45). A previous study showed that the
MAO-A expression was increased in the losing male mice following
repeated experiences of social defeat, as compared with the winners
and controls (46). It was also
reported that MAO-A binding is elevated in postpartum or
perimenopausal depression (47,48).
In fact, MAO-A activity can be inhibited by several types of
antidepressants, including fluoxetine and VLX, suggesting that a
decrease in MAO-A activity may be linked to the effects of the
drugs on serotonergic, noradrenergic and dopaminergic
neurotransmission (49). The
present study showed that the MAO-A mRNA expression was generally
elevated in the hippocampi of CUS rats, suggesting that chronic
stress may cause depressive symptoms via the disruption of the
metabolism of monoamine neurotransmitters. Long-term venlafaxine
intervention clearly inhibited MAO-A overexpression, indicating
that its antidepressant effect may involve weakening the metabolic
abnormalities of monoamine neurotransmitters.
In conclusion, depression is usually the outcome of
interactions between genetic and environmental factors, and its
pathophysiology involves alterations of key enzymes responsible for
monoamine neurotransmitter synthesis and metabolism. The
therapeutic effect of VLX appears to upregulate the TH and TPH and
decrease the MAO-A expression, indicating that strategies that
facilitate monoamine neurotransmitter synthesis and correct
metabolic disturbance may be beneficial for human depressive
disorder. Moreover, VLX treatment mitigated oxidative stress,
suggesting its importance in the protection of neurons from
invasion, which is essential for neuroplasticity. However, VLX
failed to have an effect on IDO overexpression, highlighting the
need for more comprehensive and individualized treatments focusing
on human depression. This article studied alterations in certain
enzymes involved in the synthesis and metabolism of monoamine
neurotransmitters. However, future research is required to explain
the exact mechanism.
Acknowledgements
Not applicable.
Funding
The present study was partly supported by a grant
from The National Natural Science Foundation of China (grant no.
31400881).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The work presented here was carried out by all
authors in collaboration. DL and Q-XZ designed the research scheme.
DL, X-YH, L-JW carried out the experiments. DL, H-JX, PS and X-PC
were involved in data collection and analyzed the data. DL wrote
the manuscript. Q-XZ revised the manuscript and took overall
responsibility.
Ethics approval and consent to
participate
All experimental procedures were approved by The
Ethics Committee of Chongqing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hamon M and Blier P: Monoamine
neurocircuitry in depression and strategies for new treatments.
Prog Neuropsychopharmacol Biol Psychiatry. 45:54–63. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biegon A and Fieldust S: Reduced tyrosine
hydroxylase immunoreactivity in locus coeruleus of suicide victims.
Synapse. 10:79–82. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dunčko R, Kiss A, Škultétyová I, Rusnák M
and Jezová D: Corticotropin-releasing hormone mRNA levels in
response to chronic mild stress rise in male but not in female rats
while tyrosine hydroxylase mRNA levels decrease in both sexes.
Psychoneuroendocrinology. 26:77–89. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heydendael W and Jacobson L: Widespread
hypothalamic-pituitary-adrenocortical axis-relevant and
mood-relevant effects of chronic fluoxetine treatment on
glucocorticoid receptor gene expression in mice. Eur J Neurosci.
31:892–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Avgustinovich DF, Alekseenko OV,
Bakshtanovskaia IV, Koriakina LA, Lipina TV, Tenditnik MV, Bondar'
NP, Kovalenko IL and Kudriavtseva NN: Dynamic changes of brain
serotonergic and dopaminergic activities during development of
anxious depression: Experimental study. Usp Fiziol Nauk. 35:19–40.
2004.(In Russian). PubMed/NCBI
|
|
6
|
Savelieva KV, Zhao S, Pogorelov VM, Rajan
I, Yang Q, Cullinan E and Lanthorn TH: Genetic disruption of both
tryptophan hydroxylase genes dramatically reduces serotonin and
affects behavior in models sensitive to antidepressants. PLoS One.
3:e33012008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang FZ, Wu Y, Zhang WG, Cai YY and Shi
SX: Estradiol or fluoxetine alters depressive behavior and
tryptophan hydroxylase in rat raphe. Neuroreport. 21:309–312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shishkina GT, Kalinina TS and Dygalo NN:
Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by
chronic fluoxetine treatment correlates with its antidepressant
effect. Neuroscience. 150:404–412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SW, Park SY and Hwang O: Up-regulation
of tryptophan hydroxylase expression and serotonin synthesis by
sertraline. Mol Pharmacol. 61:778–785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin Y, Wang N, Zhang X, Han X, Zhai X and
Lu Y: IDO and TDO as a potential therapeutic target in different
types of depression. Metab Brain Dis. 33:1787–1800. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christmas DM, Potokar J and Davies SJ: A
biological pathway linking inflammation and depression: Activation
of indoleamine 2,3-dioxygenase. Neuropsychiatr Dis Treat.
7:431–439. 2011.PubMed/NCBI
|
|
12
|
Samelson-Jones BJ and Yeh SR: Interactions
between nitric oxide and indoleamine 2,3-dioxygenase. Biochemistry.
45:8527–8538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Myint AM and Kim YK: Cytokine-serotonin
interaction through IDO: A neurodegeneration hypothesis of
depression. Med Hypotheses. 61:519–525. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dantzer R, O'Connor JC, Freund GG, Johnson
RW and Kelley KW: From inflammation to sickness and depression:
When the immune system subjugates the brain. Nat Rev Neurosci.
9:46–56. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dobos N, deVries EF, Kema IP, Patas K,
Prins M, Nijholt IM, Dierckx RA, Korf J, den Boer JA, Luiten PG and
Eisel UL: The role of indoleamine 2, 3- dioxygenase in a mouse
model of neuroinflammation-induced depression. J Alzheimers Dis.
28:905–915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stefanescu C and Ciobica A: The relevance
of oxidative stress status in first episode and recurrent
depression. J Affect disord. 143:34–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naoi M, Maruyama W and Shamoto-Nagai M:
Type A monoamine oxidase and serotonin are coordinately involved in
depressive disorders: From neurotransmitter imbalance to impaired
neurogenesis. J Neural Transmission (Vienna). 125:53–66. 2018.
View Article : Google Scholar
|
|
18
|
Buckholtz JW and Meyer-Lindenberg A: MAOA
and the neurogenetic architecture of human aggression. Trends
Neurosci. 31:120–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meyer JH, Ginovart N, Boovariwala A,
Sagrati S, Hussey D, Garcia A, Young T, Praschak-Rieder N, Wilson
AA and Houle S: Elevated monoamine oxidase a levels in the brain:
An explanation for the monoamine imbalance of major depression.
Arch Gen Psychiatry. 63:1209–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grunewald M, Johnson S, Lu D, Wang Z,
Lomberk G, Albert PR, Stockmeier CA, Meyer JH, Urrutia R, Miczek
KA, et al: Mechanistic role for a novel glucocorticoid-KLF11
(TIEG2) protein pathway in stress-induced monoamine oxidase A
expression. J Biol Chem. 287:24195–24206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonnet U: Moclobemide: Therapeutic use and
clinical studies. CNS Drug Rev. 9:97–140. 2010. View Article : Google Scholar
|
|
22
|
Elhwuegi AS: Central monoamines and their
role in major depression. Prog Neuropsychopharmacol Biol
Psychiatry. 28:435–451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye Y, Wang G, Wang H and Wang X:
Brain-derived neurotrophic factor (BDNF) infusion restored
astrocytic plasticity in the hippocampus of a rat model of
depression. Neurosci Lett. 503:15–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grundmann O, Lv Y, Kelber O and Butterweck
V: Mechanism of St. John's wort extract (STW3-VI)during chronic
restraint stress is mediated by the interrelationship of the
immune, oxidative defense, and neuroendocrine system.
Neuropharmacology. 58:767–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Makino M, Kitano Y, Komiyama C and
Takasuna K: Human interferon-alpha increases immobility in the
forced swimming test in rats. Psychopharmacology (Berl).
148:106–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watanabe Y, Asami Y, Hirano Y, Kuribayashi
K, Itamura R and Imaeda T: Factors impacting the efficacy of
venlafaxine extended release 75–225 mg/day in patients with major
depressive disorder: Exploratory post hoc subgroup analyses of a
randomized, double-blind, placebo-controlled study in Japan.
Neuropsychiatr Dis Treat. 14:1261–1272. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szkutnik-Fiedler D, Kus K, Balcerkiewicz
M, Grześkowiak E, Nowakowska E, Burda K, Ratajczak P and Sadowski
C: Concomitant use of tramadol and venlafaxine–evaluation of
antidepressant-like activity and other behavioral effects in rats.
Pharmacol Rep. 64:1350–1358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yilmaz N, Demirdas A, Yilmaz M, Sutcu R,
Kirbas A, Cure MC and Eren I: Effects of venlafaxine and
escitalopram treatments on NMDA receptors in the rat depression
model. J Membr Biol. 242:145–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Ma R, Shen J, Su H, Xing D and Du
L: A mouse model of depression induced by repeated corticosterone
injections. Eur J Pharmacol. 581:113–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brady LS, Gold PW, Herkenham M, Lynn AB
and Whitfield HJ Jr: The antidepressants fluoxetine, idazoxan and
phenelzine alter corticotropin-releasing hormone and tyrosine
hydroxylase mRNA levels in rat brain: Therapeutic implications.
Brain Res. 572:117–125. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sung YH, Shin MS, Cho S, Baik HH, Jin BK,
Chang HK, Lee EK and Kim CJ: Depression-like state in maternal rats
induced by repeated separation of pups is accompanied by a decrease
of cell proliferation and an increase of apoptosis in the
hippocampus. Neurosci Lett. 470:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X, Seese RR, Yun K, Peng T and Wang
Z: The role of galanin system in modulating depression, anxiety,
and addiction-like behaviors after chronic restraint stress.
Neuroscience. 246:82–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abumaria N, Rygula R, Hiemke C, Fuchs E,
Havemann-Reinecke U, Rüther E and Flügge G: Effect of chronic
citalopram on serotonin-related and stress-regulated genes in the
dorsal raphe nucleus of the rat. Eur Neuropsychopharmacol.
17:417–429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klomp A, Václavů L, Meerhoff GF, Reneman L
and Lucassen PJ: Effects of chronic fluoxetine treatment on
neurogenesis and tryptophan hydroxylase expression in adolescent
and adult rats. PLoS One. 9:e976032014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maes M, Leonard BE, Myint AM, Kubera M and
Verkerk R: The new ‘5-HT’ hypothesis of depression: Cell-mediated
immune activation induces indoleamine 2,3-dioxygenase, which leads
to lower plasma tryptophan and an increased synthesis of
detrimental tryptophan catabolites (TRYCATs), both of which
contribute to the onset of depression. Prog Neuropsychopharmacol
Biol Psychiatry. 35:702–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu YN, Peng YL, Liu L, Wu TY, Zhang Y,
Lian YJ, Yang YY, Kelley KW, Jiang CL and Wang YX: TNFα mediates
stress-induced depression by upregulating indoleamine 2,
3-dioxygenase in a mouse model of unpredictable chronic mild
stress. Eur Cytokine Netw. 26:15–25. 2015.PubMed/NCBI
|
|
39
|
Raison CL, Dantzer R, Kelley KW, Lawson
MA, Woolwine BJ, Vogt G, Spivey JR, Saito K and Miller AH: CSF
concentrations of brain tryptophan and kynurenines during immune
stimulation with IFN-alpha: Relationship to CNS immune responses
and depression. Mol Psychiatry. 15:393–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mohamed BM, Aboul-Fotouh S, Ibrahim EA,
Shehata H, Mansour AA, Yassin NA, El-Eraky W and Abdel-Tawab AM:
Effects of pentoxifylline, 7-nitroindazole, and imipramine on tumor
necrosis factor-α and indoleamine 2,3-dioxygenase enzyme activity
in the hippocampus and frontal cortex of chronic
mild-stress-exposed rats. Neuropsychiatr Dis Treat. 9:697–708.
2013.PubMed/NCBI
|
|
41
|
Thomas J, Khanam R and Vohora D:
Augmentation of antidepressant effects of venlafaxine by
agomelatine in mice are independent of kynurenine pathway.
Neurochem Int. 99:103–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schulze TG, Müller DJ, Krauss H, Scherk H,
Ohlraun S, Syagailo YV, Windemuth C, Neidt H, Grässle M,
Papassotiropoulos A, et al: Association between a functional
polymorphism in the monoamine oxidase A gene promoter and major
depressive disorder. Am J Med Genet. 96:801–803. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Duncan J, Johnson S and Ou XM: Monoamine
oxidases in major depressive disorder and alcoholism. Drug Discov
Ther. 6:112–122. 2012.PubMed/NCBI
|
|
44
|
Mattson MP, Maudsley S and Martin B: BDNF
and 5-HT: A dynamic duo in age-related neuronal plasticity and
neurodegenerative disorders. Trends Neurosci. 27:588–594. 2004.
View Article : Google Scholar
|
|
45
|
Doyle A, Hucklebridge F, Evans P and Clow
A: Salivary monoamine oxidase A and B inhibitory activities
correlate with stress. Life Sci. 59:1357–1362. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Filipenko ML, Beilina AG, Alekseyenko OV,
Dolgov VV and Kudryavtseva NN: Repeated experience of social
defeats increases serotonin transporter and monoamine oxidase A
mRNA levels in raphe nuclei of male mice. Neurosci Lett. 321:25–28.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sacher J, Wilson AA, Houle S, Rusjan P,
Hassan S, Bloomfield PM, Stewart DE and Meyer JH: Elevated brain
monoamine oxidase A binding in the early postpartum period. Arch
Gen Psychiatry. 67:468–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rekkas PV, Wilson AA, Lee VW, Yogalingam
P, Sacher J, Rusjan P, Houle S, Stewart DE, Kolla NJ, Kish S, et
al: Greater monoamine oxidase a binding in perimenopausal age as
measured with carbon 11-labeled harmine positron emission
tomography. JAMA Psychiatry. 71:873–879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fisar Z, Hroudová J and Raboch J:
Inhibition of monoamine oxidase activity by antidepressants and
mood stabilizers. Neuro Endocrinol Lett. 31:645–656.
2010.PubMed/NCBI
|