Introduction
Glaucoma is the most common cause of irreversible
blindness worldwide, accounting for ~15% of all cases and ≥500,000
new cases annually in both developed and developing countries
(1–3). It is characterized by the development
of a specific pattern of visual field loss and optic neuropathy.
Patients are usually treated using filtration surgery which serves
to reduce intraocular pressure in the eye (4). Other treatments include topical
medication, laser treatment and surgical modalities. Of the
treatments available, surgery is the most effective (5,6).
Glaucoma filtration surgery may stimulate conjunctival fibroblast
proliferation, migration, differentiation and promote ECM
secretion, which are important processes in wound healing and scar
formation (7–9). However, glaucoma filtration surgery
is not always effective, and excessive scarring of the filtering
bleb after surgery is a major problem following surgery.
Scar formation at the filtering bleb after glaucoma
filtration surgery is a multifactorial process; human conjunctival
fibroblasts (HConFs) play an essential role in subconjunctival
wound healing (10). In addition,
the cytokine transforming growth factor (TGF)-β is an important
factor in regulating wound healing and scar formation (10). The binding of TGF-β to its
heterodimeric receptor activates intracellular signaling cascades,
including the canonical SMAD pathway and the mitogen-activated
protein kinase (MAPK) pathway, and leads to fibrosis (11). The PI3K/AKT signaling pathway,
which regulates survival, was also reported to function in
regulating cell migration, proliferation and apoptosis (12,13).
The PI3K/AKT pathway can be activated by TGF-β and also plays an
important role in modulating ECM synthesis (14).
All-trans-retinoic acid (ATRA), a derivative of
vitamin A, can inhibit TGF-β and inhibit fibrosis (15). In a previous study, it was reported
that ATRA inhibited TGF-β-induced HConF-mediated collagen gel
contraction via the SMAD signaling pathway (16). However, the effects of ATRA on
TGF-β-induced HConF migration, proliferation and ECM synthesis
remain unclear. In the present study, HConFs were cultured to
investigate the effects of ATRA on the proliferation, migration,
apoptosis and ECM synthesis in conjunctival fibroblasts. The aim of
the present study was to improve understanding of the mechanism of
action of ATRA. The mechanistic insights provided by this study may
be applicable to preventing scar formation following glaucoma
filtration surgery.
Materials and methods
Materials
ATRA and TGF-β (Sigma-Aldrich; Merck KGaA) were
prepared as 0.4 and 50 ng/ml stock solutions in DMSO, respectively,
and were stored below −20°C in the dark. ATRA and TGF-β were
diluted to 10–200 µM and 1 ng/ml, respectively, in DMEM
(Sigma-Aldrich; Merck KGaA) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) on the day of the experiment. The highest
concentration of DMSO in the test solutions was 0.1%. To exclude
the possibility that proliferation may be inhibited by DMSO, all
cells were exposed to a final DMSO concentration of 0.1% in DMEM
containing 10% FBS.
Cell culture
Primary HConFs (ScienCell Research Laboratories,
Inc.) were cultured according to the manufacturer's instructions;
three individual lots of HConFs were obtained. The HConFs were
maintained and cultured at 37°C in fibroblast medium (ScienCell
Research Laboratories, Inc.) containing 2% FBS, fibroblast growth
supplement (ScienCell Research Laboratories, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin in a 5% CO2
humidified incubator. The HConFs were characterized by a
spindle-shaped morphology and were positively stained with
anti-fibronectin antibodies.
Cell proliferation assay
A HConF proliferation assay was performed as
previously described (17).
Briefly, HConF suspensions (0.5×104 cells/well) were
cultured in fibroblast medium in a 96-well plate (200 µl/well) for
24 h at 37°C. ATRA (0.01–10 µM) was added to the fibroblast medium
and the cells were cultured for a further 48 h at 37°C in the
absence or presence of TGF-β (1 ng/ml), with 0.1% DMSO as the
control. The concentrations of ATRA and TGF-β used were determined
according to a previous study (15). Subsequently, each well was
incubated with 10 µl Cell Counting Kit-8 solution (BestBio; Sigma)
for a further 3 h at 37°C. The absorbance was measured at 450 nm
using an automated microplate reader (model 3001–1387; Thermo
Fisher Scientific, Inc.).
Apoptosis assay
HConF apoptosis was determined using an Annexin
V-FITC apoptosis kit (BestBio) as previously described (17). Briefly, the HConFs
(5×105 cells/well) were plated in 6-well plates and
cultured for 48 h. After rinsing twice with PBS, the cells were
resuspended in 400 µl 1X binding buffer [10 mM HEPES, 140 mM sodium
chloride, 2.5 mM calcium chloride (pH 7.4)], into which 5 µl of
Annexin V-FITC was added. After 15 min incubation at 2–8°C in the
dark, 10 µl of propidium iodide (BestBio) was added to the cells
prior to further incubation for 5 min at 2–8°C in the dark. Within
15 min after staining, the cells were analyzed using a flow
cytometer (Cytomics FV 500) and CytExpert 2.0 software (both
Beckman Coulter, Inc.). The apoptotic rate was calculated as the
sum of the percentage of early + late apoptotic cells.
Wound healing assay
HConFs were seeded at a density of 5×105
cells/well in 6-well plates. The culture medium for the HConFs was
replaced after 24 h with serum-free medium, and then cells were
incubated for a further 2 h at 37°C. Next, a sterile 20-µl pipette
tip was used to scratch a line in the confluent cell monolayer,
after which the cells were washed three times with PBS. The scratch
wound healed after 24 h of incubation in the following conditions:
1 µM ATRA + 1 ng/ml TGF-β, 1 ng/ml TGF-β and fibroblast medium
without ATRA and TGF-β. The effects on HConF migration were
evaluated by measuring the area of the wound from the images
captured at a magnification of ×100 at 0 and 24 h using a light
microscope (Olympus Corporation); analysis was performed using
ImageJ version 1.5 (National Institutes of Health).
Transwell migration assay
A chemomigration assay was performed using Transwell
plates (pore size, 8 µm) as previously described (18). Briefly, the cells in the upper
Transwell chamber (1×105) were suspended in 200 µl of
DMEM, and DMEM containing 20% FBS was added to the lower chamber.
Following a 12-h incubation at 37°C, the medium with non-migrated
cells in the upper chamber was removed with a cotton swab. The
cells that had migrated to the lower chamber were fixed with 4%
paraformaldehyde for 30 min at 37°C and then stained with 0.5%
crystal violet for 10 min at 37°C. The cells were counted at ×200
magnification using a light microscope in six different fields of
view.
Cell treatment
HConFs were incubated in fibroblast medium in 6-well
plates for 24 h. Then, the medium was replaced with the serum-free
medium. Serum-starved cells were incubated for 6 h in the presence
or absence of ATRA (1 µM), and then for 48 h in the presence or
absence of TGF-β (1 ng/ml). Then, cell lysates were analyzed via
western blotting.
Western blot analysis
Western blot analysis was performed as previously
described (17,19,20).
Cells were washed with pre-cooled PBS and lysed with RIPA buffer
(Nanjing KeyGen Biotech Co., Ltd.) containing the protease
inhibitor phenylmethylsulfonyl fluoride and the phosphatase
inhibitor sodium orthovanadate (Nanjing KeyGen Biotech Co., Ltd.)
on ice for 30 min. Subsequently, the cells were gently scraped from
the plate and centrifuged at 1,500 × g for 12 min at 4°C. Protein
concentrations were measured using the bicinchoninic acid method. A
total of 20 µg/sample of protein was separated via 6–15% SDS-PAGE
and then transferred onto PVDF membranes. Membranes were blocked
with 5% non-fat dry milk for 1 h at 37°C before overnight
incubation at 4°C with mouse monoclonal anti-collagen I (1:2,500;
cat. no. ab88147; Abcam), rabbit polyclonal anti-fibronectin
(1:1,500; cat. no. ab137720; Abcam), mouse antibodies against PI3K
p85α (1:500; cat. no. sc-1637), phosphorylated (p)-PI3K p85α
(1:500; cat. no. sc-12929), AKT (1:500; cat. no. sc-5298) and p-AKT
(1:500; cat. no. sc-293125; all from Santa Cruz Biotechnology,
Inc.), and mouse monoclonal anti-GAPDH (1:1,000; cat. no. A01020;
Abbkine Scientific Co., Ltd.). Each membrane was incubated with
anti-rabbit (1:5,000; cat. no. A0208) or anti-mouse (1:5,000; cat.
no. A0216) horseradish peroxidase-conjugated secondary antibodies
(both from Beyotime Institute of Biotechnology) for 1 h at room
temperature. The blots were visualized using ECL reagent (Beyotime
Institute of Biotechnology) and the bands were analyzed using
ImageJ software.
Inhibition of TGF-β-induced collagen I
and fibronectin expression in HConFs using a PI3K inhibitor
HConFs were incubated in fibroblast medium in 6-well
plates for 24 h, then the medium was replaced with serum-free
medium. Serum-starved cells were incubated with 10 µM LY294002
(Calbiochem; Merck KGaA) for 1 h at 37°C. Subsequently, the cells
were treated with 1 ng/ml TGF-β for 48 h at 37°C. The effects of
the PI3K inhibitor on the TGF-β-induced expression of collagen I
and fibronectin in HConF were investigated via western blot
analysis.
Statistical analysis
Data analysis was performed using SPSS version 19.0
(IBM Corp.). The data are presented as the mean ± SD. Each
experiment was repeated at least three times. Statistical analysis
was performed using Student's t-test or one-way ANOVA; following
ANOVA, the least significant difference test was used for pairwise
comparisons between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
ATRA inhibits TGF-β-induced HConF
proliferation
The effect of ATRA on HConF proliferation was
examined. The cells were incubated for 48 h with 0.01–10 µM ATRA in
the absence or presence of 1 ng/ml TGF-β with 0.1% DMSO as the
control. ATRA was revealed to inhibit HConF proliferation in a
concentration-dependent manner (P<0.05; Fig. 1A). HConFs were divided into three
treatment groups: The ATRA group (1 µM ATRA + 1 ng/ml TGF-β), the
TGF-β group (1 ng/ml TGF-β) and the control group (0.1% DMSO). ATRA
inhibited TGF-β-induced HConF proliferation by 77.50±1.88%
(P<0.01 vs. TGF-β; Fig.
1B).
ATRA induces HConF apoptosis
The effect of ATRA on HConF apoptosis was examined
using flow cytometry. A significantly increased number of apoptotic
HConFs were observed in the ATRA group compared with the control
group (53.25±1.2 vs. 22.5±1.1%, respectively; P<0.01). The TGF-β
group showed a significantly decreased apoptotic rate compared with
the control group (14.75±1.4%; P<0.01; Fig. 2).
ATRA inhibits TGF-β-induced cell
migration
The migratory ability of the HConFs was evaluated
using in vitro wound healing and Transwell assays. Similar
results were observed for the two assays. The ATRA group exhibited
a ~30% reduction in wound healing compared with the control group
(48.9±1.34 vs. 71.30±1.55%, respectively; P<0.05). Wound healing
was increased in the TGF-β group compared with the control group
(90.50±1.22%; P<0.05; Fig. 3A).
Reduced HConF migration was observed for the ATRA group (14.85±1.13
cells; P<0.01), whereas significantly increased migration was
noted for the TGF-β group compared with the control group
(135.55±1.12 cells vs. 28.65±1.02 cells, respectively; P<0.01;
Fig. 3B) in the Transwell
migration assays.
ATRA inhibits TGF-β-induced collagen I
and fibronectin expression
The effect of ATRA on TGF-β-induced ECM production
was investigated. Western blotting analysis and densitometric
analysis revealed that exposure of HConFs to 1 ng/ml TGF-β for 48 h
induced the production of collagen I and fibronectin, whereas 1 µM
ATRA inhibited TGF-β-induced synthesis of collagen I and
fibronectin by HConFs (P<0.01; Fig.
4).
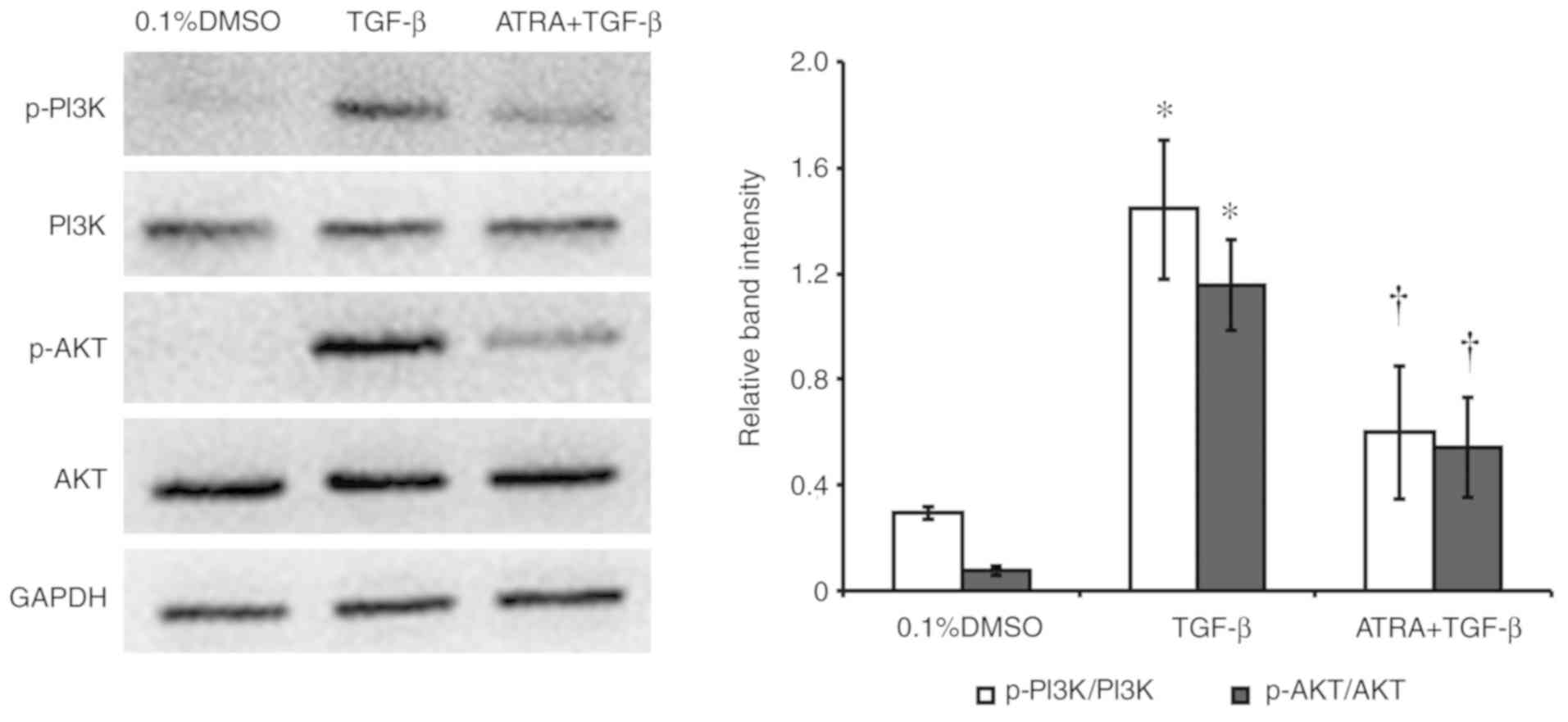
ATRA inhibits the PI3K/AKT
pathway
The effect of ATRA on TGF-β-induced PI3K and AKT
phosphorylation in HConFs was examined (Fig. 5). Western blotting analysis and
densitometric analysis revealed that the exposure of HConFs to 1
ng/ml TGF-β for 48 h induced the phosphorylation/activation of PI3K
and AKT. Treatment with 1 µM ATRA inhibited TGF-β-induced PI3K and
AKT phosphorylation, indicating that ATRA inhibited the
TGF-β-induced PI3K/AKT signaling pathway (P<0.05; Fig. 5).
Inhibition of TGF-β-induced collagen I
and fibronectin expression in HConFs by a PI3K inhibitor
The effect of the PI3K inhibitor LY294002 on the
TGF-β-induced expression of collagen I and fibronectin in HConF was
investigated. Cells were incubated with 10 µM LY294002 for 1 h
before treatment with 1 ng/ml TGF-β for 48 h. Western blotting
analysis and densitometric analysis revealed that LY294002
significantly inhibited TGF-β-induced expression of collagen I and
fibronectin in HConFs (P<0.05; Fig.
6). The TGF-β-induced expression of collagen I and fibronectin
in HConFs was inhibited by 27 and 47% in the presence of LY294002,
respectively. The inhibitor had no effect on collagen I and
fibronectin expression in the absence of TGF-β (data not
shown).
Discussion
In the present study, it was revealed that ATRA
inhibited TGF-β-induced migration, proliferation and ECM synthesis
in HConFs. Furthermore, ATRA was found to promote apoptosis and
inhibit the TGF-β-induced phosphorylation of PI3K and AKT in
HConFs. In addition, the present study showed that the PI3K
inhibitor LY294002 attenuated the TGF-β-induced expression of
collagen I and fibronectin.
Preventing conjunctival scarring remains a challenge
in clinical ophthalmology. TGF-β is a key factor implicated in
postoperative scarring as it stimulates the migration,
proliferation and differentiation HConFs, and promotes ECM
deposition and remodeling (21).
ATRA, a derivative of vitamin A, has been shown to regulate ECM
expression and serve an important role in fibrotic diseases through
the inhibition of TGF-β1 (22).
Moreover, ATRA was found to serve a protective role against
collagen accumulation, cell injury and proliferation in various
types of fibrosis, including liver fibrosis, pulmonary fibrosis and
kidney fibrosis (22–25). In a previous study, it was
demonstrated that ATRA inhibited TGF-β-induced HConF-mediated
collagen gel contraction by attenuating the formation of actin
stress fibers and focal adhesions, as well as by inhibiting MAPK,
c-Jun and SMAD signaling (16). In
the present study, it was demonstrated that ATRA inhibited the
TGF-β-induced migration and proliferation of HConFs, inhibited ECM
synthesis and increased the apoptosis in HConFs. Collectively,
these results suggested that ATRA attenuates conjunctival scarring
by modulating the function of HConFs and ECM synthesis.
The PI3K/AKT pathway modulates cell proliferation,
differentiation, apoptosis, motility, survival and glucose
metabolism (26). PI3K generates
3′-phosphorylated phosphoinositides, including
phosphatidylinositol-3,4-bisphosphate and
phosphatidylinositol-3,4,5-triphosphate, which then recruit target
proteins to the plasma membrane (27). AKT is a serine/threonine kinase
that acts as an effector of PI3K (27). It has been reported that activation
of the PI3K/AKT signaling pathway may induce ECM secretion in
several cell types (28,29). LY294002 and AKT small interfering
RNA were reported to significantly reduce TGF-β1-induced α-smooth
muscle actin expression, a marker of fibroblasts, in conjunctival
fibroblasts, indicating that these changes were mediated by the
PI3K/AKT pathway (30). In
addition, LY294002 could inhibit the proliferation and migration of
conjunctival fibroblasts (31,32).
In the present study, it was shown that ATRA promoted apoptosis and
inhibited proliferation, migration and ECM synthesis. In addition,
it was demonstrated that LY294002 could inhibit TGF-β-induced
expression of collagen I and fibronectin, similar to ATRA; this is
consistent with the results of a previous study (33). Hence, it was deduced that the
inhibitory effects of ATRA on HConFs are likely mediated by
inhibition of the PI3K/AKT signaling pathway.
TGF-β-mediated signaling can occur via
SMAD-dependent or SMAD-independent pathways; the SMAD-independent
pathway includes the MAPK and PI3K/AKT pathways (34). TGF-β activates the SMAD pathway via
the phosphorylation of Smad2 and Smad3, which then leads to the
formation of a Smad2/3 complex; the Smad2/3 complex and Smad4 are
subsequently translocated to the nucleus in order to regulate the
expression of genes associated with fibroblast proliferation,
migration and ECM deposition (35). In our previous study, a role for
SMAD signaling was found in the ATRA-mediated inhibition of
TGF-β-induced, HConF-mediated collagen gel contraction (16). Therefore, it was hypothesized that
the SMAD signaling pathway is also involved in the inhibitory
effects of ATRA on proliferation, migration and ECM synthesis in
HConFs.
In conclusion, the findings of the present study
revealed the mechanism of action of ATRA. ATRA was found to
modulate PI3K/AKT signaling and impact on TGF-β-induced apoptosis,
proliferation, migration and ECM synthesis. Given the significant
global impact of glaucoma and the inadequacy in its treatment
methods, conjunctival scarring at the surgical site after glaucoma
filtration surgery (36,37) and the adverse side effects of
anti-scarring anti-metabolite medication, such as mitomycin-C and
5-fluorouracil, which can potentially lead to blindness (38–41),
there is a requirement for improved therapeutic approaches. From
the present study, it is proposed that ATRA may be a promising
compound capable of modulating scar formation after glaucoma
filtration surgery. However, as the present study was performed
in vitro, future research should be directed towards
characterizing the in vivo effects of ATRA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81770889), the Natural
Science Foundation of Guangdong Province (grant no. 2017A030313774)
and the Health Programs of Finance Department of Jilin Province
(grant no. 3D5177783429).
Availability of data and materials
All the data generated and analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YL and YZ conceived and designed the experiments. LL
and XW performed the experiments. LL, XW, and YZ analyzed the data.
LL and YL wrote the manuscript. All the authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thylefors B and Négrel AD: The global
impact of glaucoma. Bull World Health Org. 72:323–326.
1994.PubMed/NCBI
|
|
2
|
Foster A and Johnson GJ: Magnitude and
causes of blindness in the developing world. Int Ophthalmol.
14:135–140. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quigley HA: Number of people with glaucoma
worldwide. Br J Ophthalmol. 80:389–393. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sommer A: Doyne lecture. Glaucoma: Facts
and figures. Eye (Lond). 10:295–301. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jay JL: Rational choice of therapy in
primary open angle glaucoma. Eye (Lond). 6:243–247. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Migdal C, Gregory W and Hitchings R:
Long-term functional outcome after early surgery compared with
laser and medicine in open-angle glaucoma. Ophthalmology.
101:1651–1657. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang L, Crowston JG, Cordeiro MF, Akbar
AN and Khaw PT: The role of the immune system in conjunctival wound
healing after glaucoma surgery. Surv Ophthalmol. 45:49–68. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atreides SP, Skuta GL and Reynolds AC:
Wound healing modulation in glaucoma filtering surgery. Int
Ophthalmol Clin. 44:61–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Desjardins DC, Parrish RK II, Folberg R,
Nevarez J, Heuer DK and Gressel MG: Wound healing after filtering
surgery in owl monkeys. Arch Ophthalmol. 104:1835–1839. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saika S, Yamanaka O, Okada Y, Tanaka S,
Miyamoto T, Sumioka T, Kitano A, Shirai K and Ikeda K: TGF beta in
fibroproliferative diseases in the eye. Front Biosci (Schol Ed).
1:376–390. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cong M, Iwaisako K, Jiang C and Kisseleva
T: Cell Signals Influencing Hepatic Fibrosis. Int J Hepatol.
2012:1585472012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sang H, Li T, Li H and Liu J: Gab1
regulates proliferation and migration through the PI3K/Akt
signaling pathway in intrahepatic cholangiocarcinoma. Tumour Biol.
36:8367–8377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou H, Li D, Shi C, Xin T, Yang J, Zhou
Y, Hu S, Tian F, Wang J and Chen Y: Effects of exendin-4 on bone
marrow mesenchymal stem cell proliferation, migration and apoptosis
in vitro. Sci Rep. 5:128982015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng R, Xiong Y, Zhu F, Ma Z, Liao W, He
Y, He J, Li W, Yang J, Lu Q, et al: Fenofibrate attenuated
glucose-induced mesangial cells proliferation and extracellular
matrix synthesis via PI3K/AKT and ERK1/2. PLoS One. 8:e768362013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wen X, Li Y, Hu K, Dai C and Liu Y:
Hepatocyte growth factor receptor signaling mediates the
anti-fibrotic action of 9-cisretinoic acid in glomerular mesangial
cells. Am J Pathol. 167:947–957. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Kimura K, Orita T, Teranishi S,
Suzuki K and Sonoda KH: Inhibition by all-trans-retinoic acid of
transforming growth factor-β-induced collagen gel contraction
mediated by human tenon fibroblasts. Invest Ophthalmol Vis Sci.
55:4199–4205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun L, Dong Y, Zhao J, Yin Y, Tong B,
Zheng Y and Xin H: NPPB modulates apoptosis, proliferation,
migration and extracellular matrix synthesis of conjunctival
fibroblasts by inhibiting PI3K/AKT signaling. Int J Mol Med.
41:1331–1338. 2018.PubMed/NCBI
|
|
18
|
Marshall J: Transwell(®)
invasion assays. Methods Mol Biol. 769:97–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Ko JA, Yanai R, Kimura K, Chikama
T, Sagara T and Nishida T: Induction by latanoprost of collagen gel
contraction mediated by human tenon fibroblasts: Role of
intracellular signaling molecules. Invest Ophthalmol Vis Sci.
49:1429–1436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Yanai R, Lu Y, Kimura K and Nishida
T: Promotion by fibronectin of collagen gel contraction mediated by
human corneal fibroblasts. Exp Eye Res. 83:1196–1204. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cordeiro MF: Role of transforming growth
factor beta in conjunctival scarring. Clin Sci (Lond). 104:181–187.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye Y and Dan Z: All-trans retinoic acid
diminishes collagen production in a hepatic stellate cell line via
suppression of active protein-1 and c-Jun N-terminal kinase signal.
J Huazhong Univ Sci Technolog Med Sci. 30:726–733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong Z, Tai W, Yang Y, Zhang T, Li Y, Chai
Y, Zhong H, Zou H and Wang D: The role of all-trans retinoic acid
in bleomycin-induced pulmonary fibrosis in mice. Exp Lung Res.
38:82–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morath C, Dechow C, Lehrke I, Haxsen V,
Waldherr R, Floege J, Ritz E and Wagner J: Effects of retinoids on
the TGF-beta system and extracellular matrix in experimental
glomerulonephritis. J Am Soc Nephrol. 12:2300–2309. 2001.PubMed/NCBI
|
|
25
|
Zhou TB, Drummen GP and Qin YH: The
controversial role of retinoic acid in fibrotic diseases: Analysis
of involved signaling pathways. Int J Mol Sci. 14:226–243. 2013.
View Article : Google Scholar
|
|
26
|
Chen J: The IL-23/IL-17 axis may be
important in obesity-asso-ciated cancer by way of the activation of
multiple signal pathways. Int J Obes (Lond). 34:1227–1229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Y, Shi X, Sheng K, Han G, Li W, Zhao
Q, Jiang B, Feng J, Li J and Gu Y: PI3K/Akt signaling transduction
pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol Med
Rep. 19:783–791. 2019.PubMed/NCBI
|
|
28
|
Liu Y, Li W, Liu H, Peng Y, Yang Q, Xiao
L, Liu Y and Liu F: Inhibition effect of small interfering RNA of
connective tissue growth factor on the expression of extracellular
matrix molecules in cultured human renal proximal tubular cells.
Ren Fail. 36:278–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin D, Zhang GM, Xu X and Wang LY: The
PI3K/Akt signaling pathway mediates the high glucose-induced
expression of extracellular matrix molecules in human retinal
pigment epithelial cells. J Diabetes Res. 2015:9202802015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chung EJ, Sohn YH, Kwon SH, Jung SA and
Lee JH: Lithium chloride inhibits TGF-β1-induced myofibroblast
transdifferentiation via PI3K/Akt pathway in cultured fibroblasts
from Tenon's capsule of the human eye. Biotechnol Lett.
36:1217–1224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo X, Yang Y, Liu L, Liu X, Xu J, Wu K
and Yu M: Pirfenidone induces G1 arrest in human Tenon's
fibroblasts in vitro involving AKT and MAPK signaling pathways. J
Ocul Pharmacol Ther. 33:366–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carducci A, Scafetta G, Siciliano C,
Carnevale R, Rosa P, Coccia A, Mangino G, Bordin A, Vingolo EM,
Pierelli L, et al: GMP-grade platelet lysate enhances proliferation
and migration of tenon fibroblasts. Front Biosci (Elite Ed).
8:84–99. 2016.PubMed/NCBI
|
|
33
|
Li N, Cui J, Duan X, Chen H and Fan F:
Suppression of type I collagen expression by miR-29b via PI3K, Akt,
and Sp1 pathway in human Tenon's fibroblasts. Invest Ophthalmol Vis
Sci. 53:1670–1678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang YE: Non-Smad pathways in TGF-beta
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wen J, Lin X, Gao W, Qu B, Ling Y, Liu R
and Yu M: MEK inhibition prevents TGF-β1-induced myofibroblast
trans differentiation in human tenon fibroblasts. Mol Med Rep.
19:468–476. 2019.PubMed/NCBI
|
|
36
|
Addicks EM, Quigley HA, Green WR and Robin
AL: Histologic characteristics of filtering blebs in glaucomatous
eyes. Arch Ophthalmol. 101:795–798. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hitchings RA and Grierson I: Clinico
pathological correlation in eyes with failed fistulizing surgery.
Trans Ophthalmol Soc U K. 103:84–88. 1983.PubMed/NCBI
|
|
38
|
Mielke C, Dawda VK and Anand N:
Intraoperative 5-fluorouracil application during primary
trabeculectomy in Nigeria: A comparative study. Eye (Lond).
17:829–834. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wong TT, Khaw PT, Aung T, Foster PJ, Htoon
HM, Oen FT, Gazzard G, Husain R, Devereux JG, Minassian D, et al:
The singapore 5-fluorouracil trabeculectomy study: Effects on
intraocular pressure control and disease progression at 3 years.
Ophthalmology. 116:175–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shin DH, Ren J, Juzych MS, Hughes BA, Kim
C, Song MS, Yang KJ and Glover KB: Primary glaucoma triple
procedure in patients with primary open-angle glaucoma: The effect
of mitomycin-C in patients with and without prognostic factors for
filtration failure. Am J Ophthalmol. 125:346–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perkins TW, Gangnon R, Ladd W, Kaufman PL
and Heatley GA: Trabeculectomy with mitomycin C: Intermediate-term
results. J Glaucoma. 7:230–236. 1998. View Article : Google Scholar : PubMed/NCBI
|