Introduction
In the central nervous system (CNS), the final
extent of functional impairment following ischemic and traumatic
injury depends on not only how severe is the damage caused by the
primary lesion but also which chemicals are released and, in turn,
how they stimulate chain reactions and mediate responses in the
adjacent areas, while the disease or injury is spreading (1,2).
Mostly, secondary degeneration processes take place in the neurons
and glia away from the primary injury site, which initially remain
intact and functional but may die at later time (3–5). For
example, glaucomatous optic neuropathy involves injury of retinal
ganglion cell (RGC) axons at a localized region of the optic nerve
frequently to mechanical insult induced by intraocular pressure
(IOP) elevation (6,7). Subsequently, secondary RGC
degeneration leads to widespread loss of vision over an extended
period of time (8). To develop
therapies to halt this progressive damage and preserve function,
understanding the mechanism of secondary degeneration and the
responses of the neural tissue is essential (9).
It is challenging to study primary and secondary RGC
degeneration separately in glaucomatous or traumatic optic
neuropathy. A partial optic nerve model has been developed to
distinguish secondary degeneration from primary RGC degeneration
morphologically, as well as their temporal and spatial variation
(10,11). An area of the retina that is
expected to demonstrate dramatic loss of RGCs less than two weeks
after the partial transection or direct trauma to the optic nerve.
This process is considered as primary degeneration. In other
retinal areas, locations beyond the initial injury site, delayed
and gradual loss of RGCs occurs after some weeks and this process
is considered as secondary degeneration (12). Investigations of secondary
degeneration have showed that RGCs die by apoptosis as a result of
activation of proteins of the bcl-2 family and caspase families and
the release of reactive oxygen species (ROS) from the transected
site which is proposed to be a key upstream pathway leading to the
cell death cascades (13–15). Differences between the pathways for
primary and secondary RGC degeneration and differential drug
effects have been recognized (14–16).
However, the exact factors contributing to the death of the
non-transected RGCs or whether any protective mechanism can
counteract the damaging insults remains to be elucidated (17,18).
An investigation of the regional expression of proteins may help
provide an understanding of the specific pathway leading to
secondary RGC degeneration. In this study, by utilizing the partial
optic nerve injury model, which separates primary and secondary
degeneration, it is possible to evaluate the degree of damage both
the RGC axon in the optic nerve and the RGC body in the retina, and
to locate the sites of primary and secondary RGC degeneration. To
explore the differences between protein expressions at different
locations, the retina was divided into 4 quadrants for the
investigation. To determine whether the retinal proteins were
regulated during primary or secondary RGC degeneration
two-dimensional fluorescence difference gel electrophoresis (DIGE)
was performed and the identities of differentially expressed
proteins were confirmed by tandem mass spectrometry (MS).
Materials and methods
Animals
The use of animals in the study was approved by the
Animal Research Committee of the University of California, Los
Angeles. The procedures were performed in compliance with the ARVO
Statement for the Use of Animals in Ophthalmic and Vision Research.
Three month old male Wistar rats weighting 300–350 g were housed
with standard food and water provided ad libitum in animal
research facility of the University of California Los Angeles.
Lighting was turned on at 3 am and off at 3 pm. The animals were
kept for at least one week in this environment before surgical
procedures. Animals were sacrificed by carbon dioxide overdose at
various time points after pONT with cervical dislocation as
secondary euthanasia method to ensure the complete euthanasia.
Partial optic nerve transection
(pONT)
Previously published procedures for pONT were
modified and performed in animals anesthetized with isoflurane gas
and topical proparacaine 1% eye drops (11). An incision was made in the temporal
conjunctiva rather than the superior conjunctiva due to easier
access to the nerve behind the globe without excessive retraction
of the globe. A diamond knife for radial keratotomy was used to
incise the optic nerve to a depth of one third of its diameter 2–3
mm behind the eye. A metal guard limited penetration and variation
of incision distance. Prior to the procedure, the diameter of
control optic nerves of adult Wistar rats (n=20) from histological
optic nerve cross sections previously collected was estimated. The
averaged diameter was found to be approximately 0.87 mm and,
therefore, the depth of the blade was adjusted to 0.28 mm. A
partial cross section of the optic nerve was made carefully, so as
not to damage the adjacent blood supply. All eyes were examined
ophthalmoscopically to ensure complete retinal blood flow. The
conjunctival incision was sutured and topical ophthalmic ointment
(tobramycin, Tobrex; Alcon, Fort Worth, TX, USA) was applied
immediately after the surgical procedures and then twice daily for
2 days. Surgical procedures were performed on one eye of each rat,
the contralateral eye serving as an untreated control.
Experimental design
To evaluate primary and secondary RGC loss after
pONT, 30 animals were included and randomly divided into three
groups: Normal (n=10), 1 week (n=8) and 8 weeks (n=12).
Immunohistochemistry using Rbpms antibody and topographical
quantification of RGCs in the retinal wholemount was performed,
while the optic nerve segments 1–2 mm behind the globe were
collected for the grading of the injury. To explore early
differentially expressed proteins after pONT, an additional 4
animals (numbers: 1824, 1825, 1826 and 1827) were sacrificed 2
weeks after pONT. Each retina was carefully and equally divided
into 4 quadrants including superior, temporal, inferior, and nasal
under a dissection microscope, frozen immediately in liquid
nitrogen, and analyzed separately using a DIGE approach according
to our published protocol (19).
The protein candidates with significant changes were identified
using tandem MS/MS mass spectrometry.
Rbpms immunohistochemistry on retinal
wholemounts and RGC quantification
Animals were deeply anesthetized with intramuscular
injections of 80 mg/kg sodium pentobarbital and then transcardially
perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. After
enucleation and post-fixation for 1 h, the retinas were dissected
and processed with anti-Rbpms antibodies as described previously
(20). Briefly, all retinas were
bisected for better staining results than for the whole retina
methods. The samples were incubated with 10% fetal bovine serum for
1 h to block nonspecific staining, and then immersed in Rbpms
antibody in PBS containing 1% Triton, 0.5% BSA, and 0.9% sodium
chloride (PBS-T-BSA) overnight at 4°C. After washing in PBS-T-BSA,
the retinas were incubated with secondary Alexa Fluor 488 goat
anti-rabbit IgG antibody (1/1,000) overnight at 4°C. With radial
cuts, the retinas were mounted on a glass slide and air dried.
Topographical analysis of immunolabeled cells was performed as
previously described (20,21). The percentage of cell loss was
defined as the decreased number of cells in the experimental eye as
a percentage of the density in the contralateral control eye of the
same animal.
Optic nerve injury analysis
To semi-quantify the axonal injury, a reliable
method of grading optic nerve injury was adopted (22,23).
The tissues were fixed, processed, and embedded in acrylic resin.
One-micrometer-thick sections were cut and stained with 1%
toluidine blue. The areas of the optic nerve cross sections were
measured by Image J software (NIH, Bethesda, MD, USA). Each section
was manually divided into two areas (transected and non-transected)
for grading separately. Optic nerve injury was assessed by two
independent masked observers using a graded scale ranging from 1
(normal; no degenerated axon was noted) to 5 (total degeneration;
all axons showed degenerated organelles, axonal content, and myelin
sheath).
Homogenization and protein
extraction
Each frozen retinal sample was homogenized with 100
µl lysis buffer containing 30 mM Tris-HCl (pH 8.5), 7 M urea, 2 M
thiourea, 2% CHAPS, 1% ASB14 and Complete, Mini protease inhibitor
(1 tablet per 10 ml buffer) in a liquid nitrogen cooled Teflon
freezer mill (Mikro-Dismembrator Braun Biotech, Melsungen,
Germany). A tungsten carbide (9 mm) grinding ball was placed inside
the chamber to help shatter the tissue into powder form at low
temperature during dismembration. The homogenate was incubated for
30 min on ice and then centrifuged at 16,100 g for 20 min at 4°C.
The supernatant was collected, and protein concentration determined
by PlusOne 2-D Quant kit (GE Healthcare, Life Science, Marlborough,
MA, USA).
Protein sample labeling with CyDyes™
for DIGE
Lysine labeling protocol, which referred to as
‘minimal labeling’ was utilized in this study. As previously
described (19,24), soluble protein lysates from each
specific retinal region (Superior/Temporal/Inferior/Nasal) of the
treated and control eyes were randomly labeled with either Cy3 or
Cy5 Dye. An additional pooled protein lysate (a mixture of equal
amounts of proteins from all the four regions of both the treated
and control eyes) was labeled with Cy2 as an internal standard. To
avoid the bias from dye labeling efficiency, dye-swapping was
adopted in the treated and control retina.
Two-dimensional gel electrophoresis
& image analysis
Isoelectric focusing (IEF) was performed using
linear immobilised pH gradient (IPG) strips (17 cm, pH 3–10 NL) as
previously described (19). The
Cy2, Cy3, and Cy5-labeled images were acquired on a Typhoon model
9400 Variable Mode Imager (GE Healthcare, Life Science) at
excitation/emission values of 488/520, 532/580, 633/670 nm
respectively with a resolution of 100 µm. After scanning, 2D gels
were fixed and further stained with MS compatible silver stain for
spot visualization (19,24,25).
The images of the scanned gels were cropped using the ImageQuant™
V5.0 to remove extraneous areas of the gel images. Image analysis
was then performed using DeCyder Differential Analysis Software,
version 6.0 (Amersham Biosciences Corp.). Spot detection and
quantification were performed using the differential in-gel
analysis (DIA) mode and images from different gels were matched
using the biological variance analysis (BVA) mode so pair-wise
image analysis among all gels could be performed. The automatic
matching of protein spots across different gels was further
confirmed by manual scanning to avoid potential artifacts. Protein
spots with an expression change >1.2-fold and Student's Paired
t-test, P≤0.05 were defined as differentially expressed proteins as
previously defined (19).
Protein identification using tandem
MS/MS mass spectrometry
For protein identification using tandem MS was
performed following the previously used protocol (19). In brief, differentially expressed
protein spots were excised from the post silver stained gels after
DIGE. Trypsin digested peptides were extracted from the gel plugs
with ultra-sonication. The peptides were separated using an
Ultimate 3000 nano liquid chromatography system (LC Packings;
Dionex) coupled to a HCTultra ion trap mass spectrometer (Bruker
Daltonics, Leipig, Germany) equipped with an online nanospray
source. The peptides were detected in the positive ion mode and
fragmented by collision-induced dissociation. Precursor selection
was set as 300–1,500 m/z. Two most abundant precursor ions were
selected for MS/MS. Three scans were averaged to obtain an MS/MS
mass spectrum.
MS/MS Ion Search was performed using generated an
mgf file format obtained through individual online submission on
the Matrixscience website (http://www.matrixscience.com). Rattus was
selected as the primary taxonomy in the SwissProt (AA) database.
For negative identification, a homology search using
Rodentia (Rodents) was performed. Trypsin was designated as
the enzyme and only one missed cleavage was allowed.
Carbamidomethylation of cysteines was set as fixed modification and
oxidation of methionine residues as variable modification. The mass
tolerances were 0.6 Da for both protein (MS) and peptide (MS/MS).
For all mass lists, no restrictions were applied for both the
protein isoelectric point and molecular weight and ESI-TRAP was
selected as instrument type. The proteins were considered
identified when the peptide ion score was above the threshold value
(typically >25, beyond green shading) in the Peptide score
distribution. To minimize false positive results, only proteins
with at least three non-duplicated peptides with significant hits
were considered.
Statistical analysis
The data was averaged and presented as the mean ±
SD. A repeated measures ANOVA was conducted to compare optic nerve
injury grading and RGC density in different time points after pONT.
All measurements at four distances and four quadrants from each
animal were included in the overall ANOVA model, and the effects of
quadrants and distances were controlled by multi-factors ANOVA
model. When comparisons of RGC density among groups were performed
within each quadrant, all measurements at four distances from each
animal were included in the ANOVA model and the distance effect was
controlled by multi-factors ANOVA model. One-way ANOVA followed by
Tukey's test was applied for the analysis of differences in RGC
degeneration between experimental and contralateral eyes were
analyzed by paired t-test. P<0.05 was considered statistically
significant. All statistical analyses were performed using SPSS
version 16 (IBM Corporation, Armonk, NY, USA).
Results
Primary and secondary RGC axonal
damage after pONT
The optic nerve of one eye was partially transected
while the other eye was used as control. A schematic diagram shows
the location of pONT (Fig. 1A). As
shown in Fig. 1B, each section was
divided manually into two areas: Transected and non-transected
regions (arrows) for axonal injury analysis (Fig. 1B). The grading of optic nerve
injury in transected and non-transected regions at 8 weeks was
compared to those at 1 week and the controls. For the transected
region, the averaged grading at both time points was 5 (severe
degeneration). Fig. 1C
demonstrates that the injury in the non-transected region was
substantial compared to the contralateral control at both time
points (P=0.007 at 1 week and 0.004 at 8 weeks) and the grading was
significantly increased from 1.45 at 1 week (n=8) to 1.8 at 8 weeks
after pONT (P=0.048; n=12).
 | Figure 1.Semi-quantification of optic nerve
injury after pONT. (A) Schematic diagram of pONT procedures in
adult rats. A rectangle indicates the area transected by the
diamond knife with the depth set at 0.28 mm, which corresponds to
approximately one third of the optic nerve diameter. (B) Micrograph
of optic nerve cross section 2 weeks after pONT. Area of transected
optic nerve region filled up with degenerated axons was manually
circled as indicated by arrows. Both areas of transected (temporal)
and non-transected (nasal) optic nerve were analyzed by optic nerve
injury grading. (C) Analysis of optic nerve injury in
non-transected region. Axonal damage of the optic nerve was graded
on a scale of 1 to 5 (Grade: 1=no injury; 5=severe injury). The
injury in the non-transected (nasal) region was significant at 1
week and 8 weeks, when compared to the control. The injury was
significantly increased from 1 to 8 weeks #P<0.01 and
*P<0.05 as indicated. RE, right eye; LE, left eye; S, superior;
N, nasal; I, inferior; T, temporal; CTL, control; pONT, partial
optic nerve transection. |
Primary and secondary RGC somal loss
after pONT
To quantify the extent of primary and secondary loss
of RGC bodies, the whole retinas collected at 1 and 8 weeks after
pONT were subjected to the established procedures of Rbpms
immunohistochemistry, which is widely regarded as a specific RGC
marker. Fig. 2A illustrates the
sampling scheme for topographical quantification of RGC bodies.
Fig. 2B shows the dramatic loss of
Rbpms-labeled RGC bodies, whilst predominantly in the temporal
region, it was also observed in parts of the superior and inferior
retina at 8 weeks after pONT, with mild to moderate loss of RGC
bodies in the nasal region and portions of the superior and
inferior retina. The percentage loss of RGC bodies in the
experimental eye compared to the corresponding retinal quadrant in
the untreated control is shown in Fig.
2C. Approximately 14.6, 21.1 and 28.2% reduction of RGC numbers
in the superior, temporal, and inferior retinal quadrants
respectively was noted at 1 week after pONT. These RGC losses were
statistically significant, when compared to contralateral control
(Fig. 2B; P=0.047, 0.037, 0.024).
In contrast, no noticeable change was observed in the nasal
quadrant at this time point (2.4% P=0.65). At 8 weeks after pONT,
the percentage losses at superior, temporal, inferior, and nasal
quadrants were 41.28±7.92, 72.3±5.99, 53.64±6.1, and 43.63±7.74%
respectively (all P-values <0.001) when compared to controls.
The losses of RGC bodies at all these four quadrants increased
between 1 to 8 weeks (P<0.01). In summary, the retinal sample
collected from the temporal quadrant was the primary site of RGC
degeneration, while the nasal quadrant suffered later RGC loss
during secondary RGC degeneration.
 | Figure 2.Quantitative analysis of surviving
RGCs after pONT. (A) Sampling for topographic counting of RGC
bodies on retinal whole mounts after immunohistochemistry using
Rbpms antibody. Triangle indicates the site of pONT. (B)
Representative composite fluorescence micrograph showing regional
loss of Rbpms-labeled RGC bodies on retinal flat mount 8 weeks
after pONT. The loss of RGCs in the temporal quadrant was
remarkable, while partial RGC loss was observed in superior and
inferior quadrants. (C) Patterns of RGC body loss after pONT. There
was significant loss of RGC bodies in temporal, superior, and
inferior quadrants was noted at one week after pONT compared to
control. However, there was no significant RGC body loss in the
nasal quadrant. At 8 weeks after pONT, the losses of RGC bodies in
all retinal quadrants were significant when compared to control.
The decreases in the numbers of surviving RGCs in all retinal
quadrants at 8 weeks was significant when compared to corresponding
regions at 1 week. #P<0.01, $P<0.001
and *P<0.05 as indicated. S, superior; N, nasal; I, inferior; T,
temporal; RGCs, retinal ganglion cells; pONT, partial optic nerve
transection. |
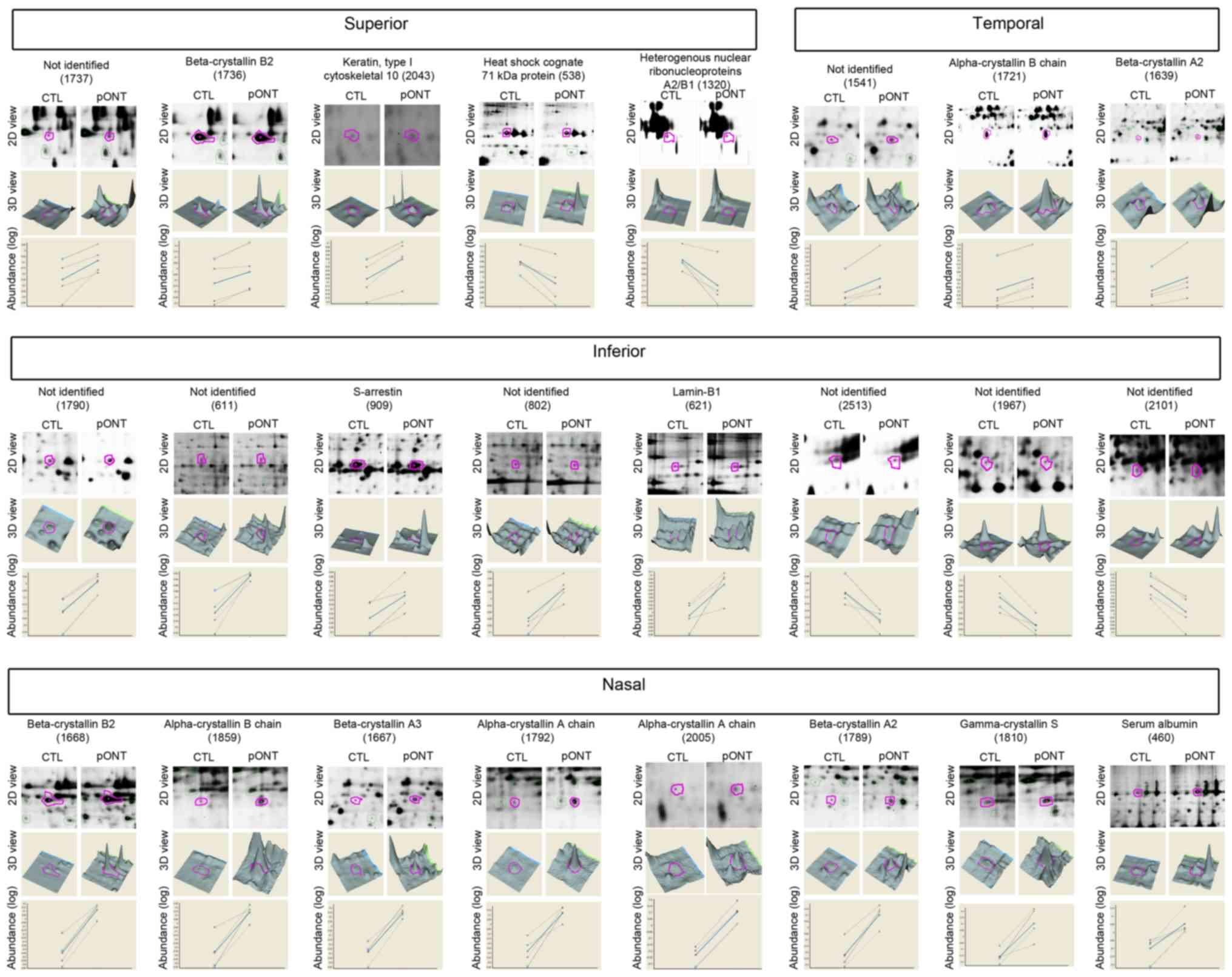
Regional protein expression after
pONT
To determine the protein changes associated with
development of secondary loss of RGCs, the retinas at two weeks
after pONT were collected. To target the mechanisms of primary and
secondary RGC degeneration, each retina was divided into 4 retinal
quadrants and proteins from each quadrant were extracted and
analyzed separately by DIGE. The protein profile of each retinal
quadrant of experimental eye was compared to their corresponding
retinal quadrant in the contralateral control eye. In total, 24
proteins were found to be increased or decreased by more than 20%
(Fig. 3; t-test, P<0.05) at two
weeks after pONT. The identities and fold changes of these
regulated proteins were determined by tandem mass spectrometry and
listed in Table I. Eight proteins
were recorded as ‘unidentified’, mainly due to poor MS signals and
low peptide abundance. There were 4, 2, 2 and 8 protein spots
representing differentially expressed proteins in superior,
temporal, inferior and nasal quadrant respectively. The
two-dimension (2D) view and 3-dimension (3D) view of these
regulated proteins in the gel are shown in Fig. 3. The measurement of standardized
abundance of each sample (dotted line; n=4) was recorded and
averaged (solid line) for comparison. Upregulation of α-crystallin
B chain (P23928) and β-crystallin A2 (Q9JJV1) was observed in both
temporal and nasal quadrants, with a higher fold change occurring
in the nasal quadrant (α-crystallin B chain=3.51 fold; β-crystallin
A2=2.13) than the temporal (α-crystallin B chain=1.42 fold;
β-crystallin A2=1.23) quadrant. More β-crystallin B2 upregulation
was observed in the nasal (3.84 fold) than the superior (1.27-fold)
quadrant. Two gel spots corresponding to the same αA crystallin
chain (P24623) were found in the nasal quadrant. Among the four
sets of comparisons, 10 out of 16 identified spots were found to be
crystallins, belonging to six crystallin members. Other identified
spots were keratin (type I cytoskeletal 10; Q61FW6), heat shock
cognate 71 kDa protein (P63018), S-arrestin (P15887), lamin-B1
(P70615), and serum albumin (P02770).
 | Table I.Protein identification by tandem mass
spectrometry in 4 retinal quadrants at 2 weeks after pONT. |
Table I.
Protein identification by tandem mass
spectrometry in 4 retinal quadrants at 2 weeks after pONT.
| Quadrant | Spota | Ratio
(pONT/CTL)b | P-value
(t-test) | Protein
[species] |
Accessionc | MudPIT
scored | MW
(kDa)e | Cal.
PIf | S.C.%g | Non-duplicate
peptide matchesh |
|---|
| Superior |
|
|
|
|
|
|
|
|
|
|
|
| 1737 | 1.49 | 0.039 | Not
identified |
|
|
|
|
|
|
|
| 1736 | 1.27 | 0.037 | β-crystallin B2
[Rattus norvegicus] | P62697 | 129 | 23.38 | 6.50 | 17 | 4 |
|
| 2043 | 1.27 | 0.016 | Keratin, type I
cytoskeletal 10 [Rattus norvegicus] | Q6IFW6 | 115 | 56.51 | 5.10 | 6 | 5 |
|
| 538 | −1.21 | 0.040 | Heat shock cognate
71 kDa protein [Rattus norvegicus] | P63018 | 174 | 70.87 | 5.37 | 10 | 7 |
|
| 1320 | −1.22 | 0.041 | Heterogeneous
nuclear ribonucleoproteins A2/B1 [Rattus norvegicus] | A7VJC2 | 272 | 37.48 | 8.97 | 22 | 9 |
| Temporal |
|
|
|
|
|
|
|
|
|
|
|
| 1541 | 1.48 | 0.044 | Not
identified |
|
|
|
|
|
|
|
| 1721 | 1.42 | 0.0057 | α-crystallin B
chain [Rattus norvegicus] | P23928 | 273 | 20.09 | 6.76 | 25 | 6 |
|
| 1639 | 1.23 | 0.043 | β-crystallin A2
[Mus musculus] | Q9JJV1 | 183 | 22.23 | 6.30 | 16 | 3 |
| Inferior |
|
|
|
|
|
|
|
|
|
|
|
| 1790 | 1.65 | 0.0040 | Not
identified |
|
|
|
|
|
|
|
| 611 | 1.34 | 0.022 | Not
identified |
|
|
|
|
|
|
|
| 909 | 1.26 | 0.020 | S-arrestin [Rattus
norvegicus] | P15887 | 78 | 44.95 | 5.75 | 6 | 3 |
|
| 802 | 1.23 | 0.023 | Not
identified |
|
|
|
|
|
|
|
| 621 | 1.22 | 0.049 | Lamin-B1 [Rattus
norvegicus] | P70615 | 176 | 66.61 | 5.16 | 14 | 9 |
|
| 2513 | −1.20 | 0.023 | Not
identified |
|
|
|
|
|
|
|
| 1967 | −1.26 | 0.017 | Not
identified |
|
|
|
|
|
|
|
| 2101 | −1.39 | 0.0047 | Not
identified |
|
|
|
|
|
|
| Nasal |
|
|
|
|
|
|
|
|
|
|
|
| 1668 | 3.84 | 0.0042 | β-crystallin B2
[Rattus norvegicus] | P62697 | 103 | 23.38 | 6.50 | 26 | 5 |
|
| 1859 | 3.51 | 0.016 | α-crystallin B
chain [Rattus norvegicus] | P23928 | 327 | 20.09 | 6.76 | 37 | 10 |
|
| 1667 | 2.76 | 0.0021 | β-crystallin A3
[Rattus norvegicus] | P14881 | 179 | 25.27 | 6.17 | 11 | 3 |
|
| 1792 | 2.67 | 0.018 | α-crystallin A
chain [Rattus norvegicus] | P24623 | 102 | 22.45 | 6.35 | 10 | 3 |
|
| 2005 | 2.17 | 0.0018 | α-crystallin A
chain [Rattus norvegicus] | P24623 | 36 | 22.45 | 6.35 | 15 | 3 |
|
| 1789 | 2.13 | 0.0039 | β-crystallin A2
[Mus musculus] | Q9JJV1 | 77 | 22.23 | 6.30 | 16 | 3 |
|
| 1810 | 1.87 | 0.021 | γ-crystallin S
[Rattus norvegicus] | P0C5E9 | 292 | 20.94 | 6.95 | 37 | 12 |
|
| 460 | 1.44 | 0.037 | Serum albumin
[Rattus norvegicus] | P02770 | 133 | 68.73 | 6.09 | 13 | 7 |
Discussion
Direct injury to the optic nerve leads to RGC axonal
damage at the initial lesion site and body loss, followed by
widespread loss in the neighboring area. The present study divided
the retinal sample into portions and observed primary and secondary
loss of RGCs after pONT in these quadrants and explored potential
protein candidates associated with primary and secondary RGC
degeneration. In particular, using proteomic analysis (DIGE) and
protein identification (tandem MS), it was possible to demonstrate
differential regulation of retinal proteins in the area (nasal
region) adjacent to the primary injury site (temporal region) two
weeks after pONT, by which time the RGC bodies have not
disappeared. The present finding indicates a dynamic regulation of
crystallin proteins in both over timely and topographically.
Previous studies performed by the team have determined that αA and
αB provides pro-survival effects to RGCs against optic nerve injury
(26,27). The current novel data, further
support the hypothesis that not only the injured neurons react to
injury, but also the intact RGCs in neighboring neuronal tissue
respond to the insult and probably initiate self-defense function
(28,29).
Primary and secondary RGC degeneration can be
clearly differentiated in the retina and optic nerve at times up to
8 weeks after pONT. Histologically, the demarcation line between
two types of RGC degeneration is easily seen in the retinal
whole-mount immunostained with Rbpms antibody and in the optic
nerve cross-section stained with toluidine blue. For the level of
retina, approximately 72, and 44% of RGCs were lost at primary
(temporal) and secondary (nasal) RGC degeneration sites
respectively at 8 weeks after pONT. This is consistent with the
findings of Levkovitch-Verbin et al (11) who reported approximately 41 and 35%
fewer RGCs in the primary RGC degeneration zone (superior) and
secondary degeneration zone (inferior) respectively 9 weeks after
pONT, but 63% loss in the primary zone at 8 days, other studies
showing a greater extent of RGC body loss in the primary injury
site than the secondary zone. The discrepancy between the
percentages of RGC loss may be due to different RGC labeling
techniques. Delivery of Fluorogold to the superior colliculus on
both sides only pre-labels the RGCs with axons projecting to
superior colliculi and a higher percent of labeling requires
proficient surgical skill. In addition, the shrunken retrogradely
labeled cells that are not morphologically RGC-like would be
excluded. On the other hand, whether the expression of Rbpms
proteins and intensity of immunolabeling is altered by different
types of injuries should also be addressed. Our previous study
demonstrated that Rbpms is a specific marker for RGCs under various
adverse conditions such as NMDA-induced excitotoxicity, optic nerve
axotomy and experimental glaucoma with IOP elevation. The use of an
RGC marker is considered useful for quantifying RGC in disease
states (20,21) which is supported by its use in many
studies (30–33). Therefore, we believe this model,
combined with careful dissection is suitable for exploring
potential protein candidates involved in secondary mechanisms.
The present study characterized the differential
regulation of retinal proteins in relation to their locations with
significant loss of RGC bodies and axons after pONT. In contrast,
optic nerve axotomy is known to result in an acute loss of RGCs. In
rodents, the complete axotomy of the optic nerve causes over 90% of
RGCs to disappear in 2 weeks (34). The mechanisms leading to total RGC
degeneration have been widely studied. A few of researchers have
established the procedures of pONT in rats, which provide for
spatial separation of primary from secondary degeneration (8,11,35–38),
allowing investigation of the mechanism of secondary RGC
degeneration. This model is useful to assess the sequences of
events contributing specifically to secondary degenerative events
in both RGC body and axons, and is relevant to the optic nerve
degeneration in some ocular diseases, such as glaucomatous,
traumatic, and ischemic optic neuropathy (15). Commencing 5 min to 3 days after
pONT, early events, including increased expression of manganese
superoxide dismutase, and decreased catalase activity, and calcium
accumulation, lead to the over production of ROS and changes in
mitochondrial morphology and function (13,39).
After several days to months, there is an infiltration of
macrophages and activation of microglia, which are involved in
secondary RGC degeneration, while swelling in myelinated axons and
myelin sheath thickening causes the following visual dysfunction
(39,40). However, the biochemical data may
not be able to determine the pathways of primary and secondary
degeneration if the entire retina is analyzed. Chiha et al
(41) initiated studies to
separate the injured and non-injured retina for microarray and
their results indicated complex primary injury gene regulation and
delayed response in two different locations. To the best of our
knowledge, this report is the first to demonstrate the regional
protein regulation in the retina after pONT and, in particular,
that stress proteins are selectively induced in the region for
secondary RGC degeneration.
Collectively, we have demonstrated the upregulation
of a group of crystallin proteins at a non-injured location rather
than the injured area at two weeks after pONT. The upregulated
crystallin proteins included αA, αB, βA2, βA3, and βB2. These
proteins generally function as chaperones and protect cells.
Coincidently, our previous study found that the genes for these
crystallin members are predominantly expressed in the RGC layer
cells and to a lesser extent, in the inner nuclear layer cells
(26). It is tempting to link the
increased expression of crystallin proteins with the number of
surviving RGCs and their protective response to stress. The
expression levels of crystallins vary with the nature of insult and
degree of damage such as intraocular pressure elevation (26) and complete optic nerve axotomy
(27). This explains why αB and
βA2 crystallin were increased approximately to 1.4- and 1.2-fold in
the injured region, compared with 3.5- and 2.1-fold in the
non-injured region. Our experimental design allowing
location-specific tissue sampling facilitates study of local
regulation of proteins. In addition, the simultaneous upregulation
of both α and β crystallin protein suggests a common mechanism
regulating the expression of these genes and proteins in the
retinas (28,29). The temporal and spatial regulation
of crystallin genes are believed to be modulated by different
arrangements of developmentally regulated transcription factors
such as Pax-6, RORα, and heat shock factor 2 and 4, and other
transcription factors. β/γ crystallins are implicated in RGC axonal
regeneration through an autocrine, inflammation-induced, or
astrocyte-derived CNTF-mediated mechanism. The upstream signaling
of crystallins induction leading to RGC survival and regeneration
of healthy and intact neurons should be further investigated.
Our study has limitations in that the exact role of
each protein has not been determined, but it does provide
fundamental data to understand how RGC degeneration progresses. An
increased level of serum albumin in the non-injured region (nasal)
may be due to one or a combination of the following sources: i)
retinal vasculature, ii) vitreous, or iii) de novo synthesis
within the retina. In normal conditions, the blood-retina barrier
provides tight junctions between the endothelial cells and retinal
pigment epithelium. It is possible that there is a local breakdown
in the blood-retina barrier as a result of primary RGC degeneration
or synthesis of intracellular albumin in response to localized
oxidative injury similar to that reported experimental glaucoma in
monkey (42). In the current
study, a mixed protein response was observed in the superior and
inferior regions. Protein expression of keratin type I, S-arrestin,
and Lamin-B1 was increased, whereas heat shock cognate 71 kDa
protein and heterogeneous nuclear ribonucleoproteins A2/B1 were
downregulated. The protein changes at these two regions may reflect
the dramatic alteration in structures and RNA/protein synthesis at
the transition zone between primary and secondary degeneration but
this is yet to be confirmed.
The primary injury in the CNS leads to neuronal loss
at the initial lesion site and widespread loss in the neighboring
area. In the optic nerve, traumatic and ischemic insults cause the
RGCs to die progressively, which involves secondary degeneration.
Although numerous studies have demonstrated direct neuroprotective
and neuroregenerative ability of α and β crystallins (43–48),
little is known about the stress response and self-defense
mechanism of crystallins in the neighboring areas next to the
injury site. The present proteomics analysis revealed specific
protein regulation in the portion of the retina corresponding to
the transected and non-transected optic nerve. The majority of
regulated proteins are crystallin family members. Whether this
regional induction of crystallins functions to protect neurons
against the noxious effects from primary injury requires further
investigation.
Acknowledgements
The authors would like to thank Dr Joseph Caprioli
(University of California Los Angeles, Los Angeles, USA) for
providing helpful discussion and Dr Maureen Valerie Boost (Hong
Kong Polytechnic University, Hong Kong, P.R. China) for her
diligent proofreading of the article.
Funding
The present study was supported by the Henry G Leong
Endowed Professorship Fund (to CHT), General Research Funds [grant
nos. BQ46K(CHT), PolyU 251006/14M (TCL) and PolyU5605/13M(HHLC)],
PolyU Internal Grants [grant nos. G-YBBU(CHT), G-YBBS(HHLC),
G-YBGS(HHLC), Z-0GF(HHLC), G-YBHJ(CWD), G-SB26(CWD), G-YBQX(TCL)
and G-YBXH(TCL)] and the Gerald Oppenheimer Family Foundation (to
JMKK).
Availability of data and materials
The datasets used and/or analyze during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL was a major contributor in proteomic approach and
analysis, and contributed equally with JMKK to experimental design
and all data analyses. KKL contributed to design, performed
biochemical assays and proteomic experiments, analyzed and
interpreted the proteomic data. HC, CWD and CHT contributed to
experimental design and interpreted the proteomic data. JMKK
contributed to conception and design, generation of animal model,
acquisition of samples, analysis of RGC body and axonal
degeneration, and was a major contributor in writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The use of animals in the study was approved by the
Animal Research Committee of the University of California, Los
Angeles. The procedures were performed in compliance with the ARVO
Statement for the Use of Animals in Ophthalmic and Vision
Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bazan NG, Rodriguez-deTurco EB and Allan
G: Mediators of injury in neurotrauma: Intracellular signal
transduction and gene expression. J Neurotrauma. 12:791–814. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crowe MJ, Bresnahan JC, Shuman SL, Masters
JN and Beattie MS: Apoptosis and delayed degeneration after spinal
cord injury in rats and monkeys. Nat Med. 3:73–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dusart I and Schwab ME: Secondary cell
death and the inflammatory reaction after dorsal hemisection of the
rat spinal cord. Eur J Neurosci. 6:712–724. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Faden AI: Pharmacological treatment of
central nervous system trauma. Pharmacol Toxicol. 78:12–17. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu D and McAdoo DJ: An experimental model
combining microdialysis with electrophysiology, histology and
neurochemistry for exploring mechanisms of secondary damage in
spinal cord injury: Effect of potassium. J Neurotrauma. 10:349–362.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quigley HA: Neuronal death in glaucoma.
Prog Retin Eye Res. 18:39–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quigley HA, Addicks EM, Green WR and
Maumenee AE: Optic nerve damage in human glaucoma. II. The site of
injury and susceptibility to damage. Arch Ophthalmol. 99:635–649.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoles E and Schwartz M: Degeneration of
spared axons following partial white matter lesion: Implications
for optic nerve neuropathies. Exp Neurol. 153:1–7. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoles E and Schwartz M: Potential
neuroprotective therapy for glaucomatous optic neuropathy. Surv
Ophthalmol. 42:367–372. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levkovitch-Verbin H, Quigley HA,
Kerrigan-Baumrind LA, D'Anna SA, Kerrigan D and Pease ME: Optic
nerve transection in monkeys may result in secondary degeneration
of retinal ganglion cells. Invest Ophthalmol Vis Sci. 42:975–982.
2001.PubMed/NCBI
|
|
11
|
Levkovitch-Verbin H, Quigley HA, Martin
KR, Zack DJ, Pease ME and Valenta DF: A model to study differences
between primary and secondary degeneration of retinal ganglion
cells in rats by partial optic nerve transection. Invest Ophthalmol
Vis Sci. 44:3388–3393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davis BM, Guo L, Brenton J, Langley L,
Normando EM and Cordeiro MF: Automatic quantitative analysis of
experimental primary and secondary retinal neurodegeneration:
Implications for optic neuropathies. Cell Death Discov.
2:160312016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fitzgerald M, Bartlett CA, Harvey AR and
Dunlop SA: Early events of secondary degeneration after partial
optic nerve transection: An immunohistochemical study. J
Neurotrauma. 27:439–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levkovitch-Verbin H, Dardik R, Vander S
and Melamed S: Mechanism of retinal ganglion cells death in
secondary degeneration of the optic nerve. Exp Eye Res. 91:127–134.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li HY, Ruan YW, Ren CR, Cui Q and So KF:
Mechanisms of secondary degeneration after partial optic nerve
transection. Neural Regen Res. 9:565–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levkovitch-Verbin H, Spierer O, Vander S
and Dardik R: Similarities and differences between primary and
secondary degeneration of the optic nerve and the effect of
minocycline. Graefes Arch Clin Exp Ophthalmol. 249:849–857. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li HY, Hong X, Huang M and So KF:
Voluntary running delays primary degeneration in rat retinas after
partial optic nerve transection. Neural Regen Res. 14:728–734.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Hare Doig RL, Chiha W, Giacci MK, Yates
NJ, Bartlett CA, Smith NM, Hodgetts SI, Harvey AR and Fitzgerald M:
Specific ion channels contribute to key elements of pathology
during secondary degeneration following neurotrauma. BMC Neurosci.
18:622017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Lam CS, Tse DY, To CH, Liu Q,
McFadden SA, Chun RK, Li KK, Bian J and Lam C: Early quantitative
profiling of differential retinal protein expression in
lens-induced myopia in guinea pig using fluorescence difference
two-dimensional gel electrophoresis. Mol Med Rep. 17:5571–5580.
2018.PubMed/NCBI
|
|
20
|
Kwong JM, Caprioli J and Piri N: RNA
binding protein with multiple splicing: A new marker for retinal
ganglion cells. Invest Ophthalmol Vis Sci. 51:1052–1058. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwong JM, Quan A, Kyung H, Piri N and
Caprioli J: Quantitative analysis of retinal ganglion cell survival
with Rbpms immunolabeling in animal models of optic neuropathies.
Invest Ophthalmol Vis Sci. 52:9694–9702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishii Y, Kwong JM and Caprioli J: Retinal
ganglion cell protection with geranylgeranylacetone, a heat shock
protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis
Sci. 44:1982–1992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia L, Cepurna WO, Johnson EC and Morrison
JC: Patterns of intraocular pressure elevation after aqueous humor
outflow obstruction in rats. Invest Ophthalmol Vis Sci.
41:1380–1385. 2000.PubMed/NCBI
|
|
24
|
Lam TC, Li KK, Lo SC, Guggenheim JA and To
CH: Application of fluorescence difference gel electrophoresis
technology in searching for protein biomarkers in chick myopia. J
Proteome Res. 6:4135–4149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lam TC, Li KK, Lo SC, Guggenheim JA and To
CH: A chick retinal proteome database and differential retinal
protein expressions during early ocular development. J Proteome
Res. 5:771–784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piri N, Song M, Kwong JM and Caprioli J:
Modulation of alpha and beta crystallin expression in rat retinas
with ocular hypertension-induced ganglion cell degeneration. Brain
Res. 1141:1–9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Munemasa Y, Kwong JM, Caprioli J and Piri
N: The role of alphaA- and alphaB-crystallins in the survival of
retinal ganglion cells after optic nerve axotomy. Invest Ophthalmol
Vis Sci. 50:3869–3875. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piri N, Kwong JM and Caprioli J:
Crystallins in retinal ganglion cell survival and regeneration. Mol
Neurobiol. 48:819–828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piri N, Kwong JM, Gu L and Caprioli J:
Heat shock proteins in the retina: Focus on HSP70 and alpha
crystallins in ganglion cell survival. Prog Retin Eye Res.
52:22–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma TP, Liu Y, Wordinger RJ, Pang IH
and Clark AF: Neuritin 1 promotes retinal ganglion cell survival
and axonal regeneration following optic nerve crush. Cell Death
Dis. 6:e16612015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Chan A, Qin Y, Kwong JMK, Caprioli
J, Levinson R, Chen L and Gordon LK: Programmed cell death-1 is
expressed in large retinal ganglion cells and is upregulated after
optic nerve crush. Exp Eye Res. 140:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Obara EA, Hannibal J, Heegaard S and
Fahrenkrug J: Loss of Melanopsin-expressing retinal ganglion cells
in severely staged glaucoma patients. Invest Ophthalmol Vis Sci.
57:4661–4667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joachim SC, Renner M, Reinhard J, Theiss
C, May C, Lohmann S, Reinehr S, Stute G, Faissner A, Marcus K, et
al: Protective effects on the retina after ranibizumab treatment in
an ischemia model. PLoS One. 12:e01824072017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clarke DB, Bray GM and Aguayo AJ:
Prolonged administration of NT-4/5 fails to rescue most axotomized
retinal ganglion cells in adult rats. Vision Res. 38:1517–1524.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fitzgerald M, Payne SC, Bartlett CA, Evill
L, Harvey AR and Dunlop SA: Secondary retinal ganglion cell death
and the neuroprotective effects of the calcium channel blocker
lomerizine. Invest Ophthalmol Vis Sci. 50:5456–5462. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chu PH, Li HY, Chin MP, So KF and Chan HH:
Effect of lycium barbarum (wolfberry) polysaccharides on preserving
retinal function after partial optic nerve transection. PLoS One.
8:e813392013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li HY, Ruan YW, Kau PW, Chiu K, Chang RC,
Chan HH and So KF: Effect of Lycium barbarum (Wolfberry) on
alleviating axonal degeneration after partial optic nerve
transection. Cell Transplant. 24:403–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li H, Liang Y, Chiu K, Yuan Q, Lin B,
Chang RC and So KF: Lycium barbarum (wolfberry) reduces secondary
degeneration and oxidative stress, and inhibits JNK pathway in
retina after partial optic nerve transection. PLoS One.
8:e688812013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Payne SC, Bartlett CA, Harvey AR, Dunlop
SA and Fitzgerald M: Myelin sheath decompaction, axon swelling, and
functional loss during chronic secondary degeneration in rat optic
nerve. Invest Ophthalmol Vis Sci. 53:6093–6101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Payne SC, Bartlett CA, Savigni DL, Harvey
AR, Dunlop SA and Fitzgerald M: Early proliferation does not
prevent the loss of oligodendrocyte progenitor cells during the
chronic phase of secondary degeneration in a CNS white matter
tract. PLoS One. 8:e657102013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chiha W, LeVaillant CJ, Bartlett CA,
Hewitt AW, Melton PE, Fitzgerald M and Harvey AR: Retinal genes are
differentially expressed in areas of primary versus secondary
degeneration following partial optic nerve injury. PLoS One.
13:e01923482018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carter-Dawson L, Zhang Y, Harwerth RS,
Rojas R, Dash P, Zhao XC, WoldeMussie E, Ruiz G, Chuang A, Dubinsky
WP and Redell JB: Elevated albumin in retinas of monkeys with
experimental glaucoma. Invest Ophthalmol Vis Sci. 51:952–959. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ying X, Zhang J, Wang Y, Wu N, Wang Y and
Yew DT: Alpha-crystallin protected axons from optic nerve
degeneration after crushing in rats. J Mol Neurosci. 35:253–258.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu N, Yu J, Chen S, Xu J, Ying X, Ye M, Li
Y and Wang Y: α-Crystallin protects RGC survival and inhibits
microglial activation after optic nerve crush. Life Sci. 94:17–23.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Böhm MR, Prokosch V, Brückner M, Pfrommer
S, Melkonyan H and Thanos S: βB2-crystallin promotes axonal
regeneration in the injured optic nerve in adult rats. Cell
Transplant. 24:1829–1844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shao WY, Liu X, Gu XL, Ying X, Wu N, Xu HW
and Wang Y: Promotion of axon regeneration and inhibition of
astrocyte activation by alpha A-crystallin on crushed optic nerve.
Int J Ophthalmol. 9:955–966. 2016.PubMed/NCBI
|
|
47
|
Anders F, Teister J, Liu A, Funke S, Grus
FH, Thanos S, von Pein HD, Pfeiffer N and Prokosch V: Intravitreal
injection of β-crystallin B2 improves retinal ganglion cell
survival in an experimental animal model of glaucoma. PLoS One.
12:e01754512017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang YH, Yin ZQ and Wang Y: Synergistic
effect of olfactory ensheathing cells and alpha-crystallin on
restoration of adult rat optic nerve injury. Neurosci Lett.
638:167–174. 2017. View Article : Google Scholar : PubMed/NCBI
|