Introduction
Patients who are admitted to the intensive care unit
(ICU) following successful resuscitation have a poor prognosis and
a high risk of postresuscitation disease, a condition that includes
multiple life threatening disorders, including neurological
failure. The mechanisms underlying postresuscitation disease have
been suggested to involve whole-body ischemia and reperfusion
syndrome, which triggers a systemic inflammatory response and is
characterized by high levels of circulating cytokines and
inflammatory mediators, similar to patients with sepsis (1). Therefore, strategies to limit the
high levels of circulating cytokines and thereby improving patient
outcomes following resuscitation have become a focus of an
increasing number of studies.
Efferent signals transmitted by the vagus nerve via
the neurotransmitter acetylcholine may attenuate local and systemic
inflammation, and this regulatory activity has been termed ‘the
cholinergic anti-inflammatory pathway’ (CAP) (2). Vagus nerve stimulation (VNS) may
exert a protective effect against acute cerebral, cardiac and
kidney ischemia reperfusion injury (IRI) by suppressing
inflammation and apoptosis via the alpha 7 nicotinic acetylcholine
receptor (α7nAChR) (3–5). It has recently been demonstrated that
VNS may improve survival in rat models of cardiopulmonary
resuscitation (CPR) (6),
indicating that VNS may be an alternative therapeutic strategy for
CPR with a neural interface approach.
Despite preclinical evidence demonstrating the
benefits of VNS for IRI, this therapy has not yet been implemented
in a clinical setting. A major reason is the lack of an established
technique that makes VNS easier to administer and less invasive in
a clinical setting. In addition, numerous cholinergic pharmacologic
agonists, including nicotine and acetylcholine exhibit a narrow
therapeutic index, which limits their clinical use (7). A study by Gigliotti et al
(8) described a
non-pharmacological, noninvasive, ultrasound-based method to
prevent renal IRI in mice. The results of this study demonstrated
that a blockade or genetic deficiency of α7nAChR abrogated the
protective effect of ultrasound (US), suggesting the involvement of
the CAP (8). This study
highlighted the possibility of a novel approach to prevent IRI
following CPR. Therefore, the aim of the present study was to
determine whether US, as a clinical applicable noninvasive
approach, would improve the outcomes following resuscitation in a
CPR rat model. It was hypothesized that US would exert a protective
effect against IRI induced by CPR predominantly through the
activation of CAP via α7nAChR.
Materials and methods
Animal preparation
Male Sprague-Dawley rats (aged 13–16 weeks and
weighing 350–400 g) were purchased from Experimental Animal
Research Center of Hubei Province [Laboratory Animal Use
Certificate NO. SCXK (E) 2015-0018] and were housed in a
temperature-controlled room with a 12 h light/dark cycle. All
animals had ad libitum access to standard food and water.
All protocols in the animal model were conducted in strict
accordance with the guidelines of the Ministry of Science and
Technology of the People's Republic of China for Animal Care and
Use, which conformed to the Guide for the Care and Use of
Laboratory Animals published by the United States of America
National Institutes of Health (9).
The study was approved by the Medical Ethics Committee of Huazhong
University of Science and Technology (approval no. 2017-S2191).
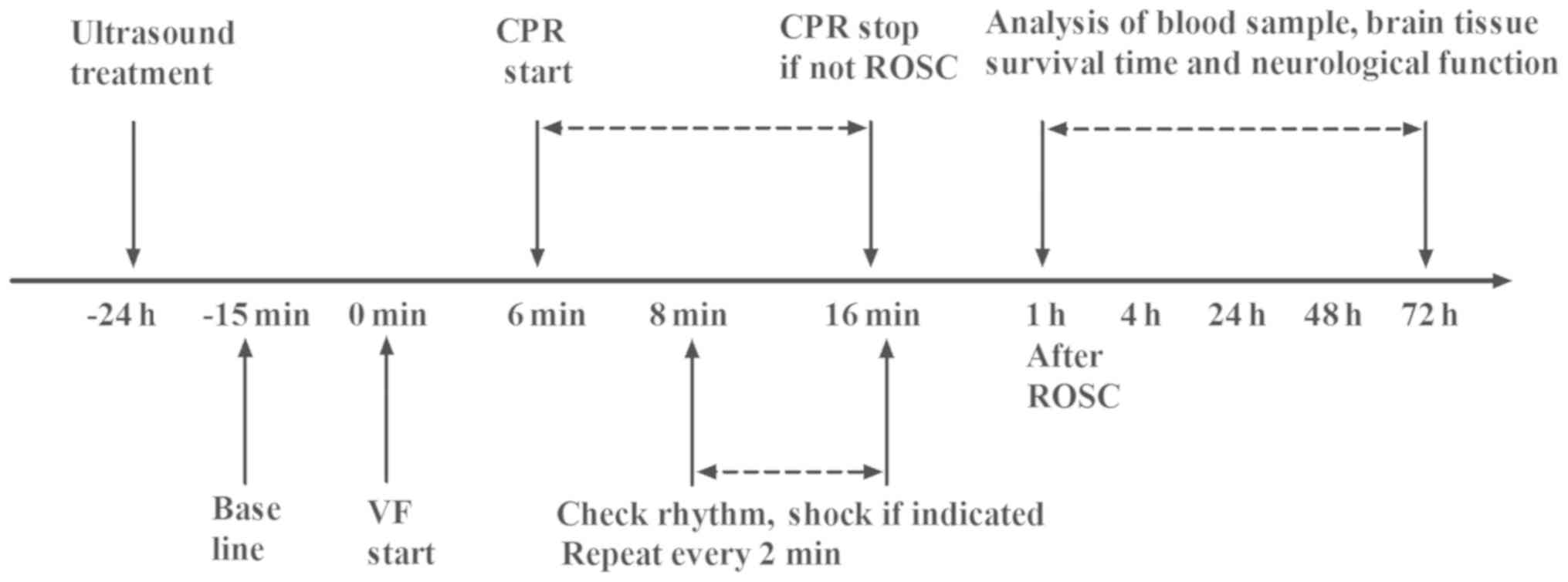
Experimental protocols
To determine the effect of US on survival following
CPR, the rats were randomly assigned to one of five groups
(n=8/group) as follows: i) The CPR group, where the rats underwent
6 min of untreated VF followed by CPR and defibrillation; ii) the
US group, in which the treatment was identical to the CPR group,
with the exception that rats were exposed to US 24 h prior to CPR;
iii) the MLA group, in which the treatment was identical to the US
group, with the exception that the α7nAChR antagonist MLA (4 mg/kg)
was administered at 30 min prior to US and VF respectively; iv) the
GTS group, in which the treatment was identical to the CPR group,
with the exception that the α7nAChR agonist GTS-21 (4 mg/kg) was
injected at 30 min prior to VF; and v) the SHAM group, in which the
rats were exposed to surgical preparation without CPR and US
application. All animals were observed closely for 72 h, following
which they were euthanized with an intraperitoneal injection of
sodium pentobarbital (150 mg/kg). At necropsy, the organs were
inspected for gross abnormalities, including evidence of traumatic
injuries resulting from cannulation, airway management or
precordial chest compression.
US treatment protocol
A previous study demonstrated that US may activate
CAP by stimulating the kidney (8).
Therefore, the present study elected to perform the same US
protocol as was performed in the study of Gigliotti et al
(8). For US exposure, the rats
were anesthetized with an intraperitoneal injection of
pentobarbital sodium (45 mg/kg) 1 day prior to establishing the
CA/CPR model. The rats were subsequently placed on the operating
table in a supine position with their abdominal and back fur
shaved. Pre-warmed ultrasound gel was placed on their depilated
skin for US treatment. A clinical Philips IU Elite Ultrasound
machine coupled with a L9-3 probe was used for US treatment.
Firstly, the left kidney was localized using conventional B-mode
image. Then, pulsed high power US was delivered to the left kidney
(contrast general mode) under burst mode at a mechanical index of
0.72. The US with a pulse length of 1 sec was applied every 6 sec
for a total of 2 min. The right kidney was subsequently exposed to
US using the same procedure. Following US treatment, the animals
were permitted to recover from the anesthetic in a
temperature-controlled incubator.
Cardiac arrest (CA) model
protocol
Rats were fasted overnight; however, they were
permitted ad libitum access to water during the preparation
period. The rats were anesthetized with an intraperitoneal
injection of pentobarbital sodium (45 mg/kg). In order to maintain
the depth of anesthesia, additional doses (10 mg/kg) were
administered approximately once per hour. Following trachea
intubation, the rats were mechanically ventilated with a tidal
volume of 0.65 ml/100 g, a respiratory rate of 70/min, and a
fraction of inspired oxygen (FiO2) of 0.21 using the
ALC-V8S ventilator (Shanghai Alcott Biotechnology Co., Ltd.). A
conventional lead II ECG was continuously recorded by subcutaneous
needle electrodes. PE50 catheters were inserted into the left
femoral artery and vein in order to measure arterial pressure and
to establish an intravenous infusion passage. Arterial blood
pressure and ECG were recorded using the BL-420F data acquisition
and analysis system (Chengdu Taimeng Software Co., Ltd.). The core
temperature was monitored by a rectal temperature probe and
maintained at 36.5±0.5°C during the experiment by a heating lamp
and heating pad. The basal parameters of the rats were recorded 15
min prior to the induction of ventricular fibrillation (VF). VF was
induced by a modified method based on transcutaneous electrical
epicardium stimulation, as described previously (10). Electrical stimulation was performed
using an electrical stimulator from the BL-420F Biofunctional
Experimental System with current mode, continuous single
stimulation pattern, a delay of 100 msec, a width of 1 msec, a
frequency of 50 Hz, an initial intensity of 1 mA and a stimulation
time of 3 min. A stimulation time of 3 min was selected in order to
prevent spontaneous defibrillation. The non-intervention time was 3
min. Therefore, the total cardiac arrest time was 6 min. At 6 min
following the initiation of VF, CPR was initiated. The mechanical
ventilation was changed to a tidal volume of 0.65 ml/100 g, a
respiratory rate (RR) of 100 times/min, and a FiO2 of 1.0. Chest
compression was applied at a frequency of ~200 times/min (with the
assistant of a metronome). The depths of compressions were
initially adjusted to maintain an aortic diastolic pressure between
26 and 28 mmHg (11). Adrenaline
{20 µg/kg, [Grand Pharma (China) Co., Ltd.]} was intravenously
administered immediately following the initiation of CPR and was
repeated once every 3 min if necessary. At 2 min after CPR,
defibrillation was performed with 4 J if the ECG indicated VF. If
the rats achieved restoration of spontaneous circulation (ROSC)
with the return of supraventricular rhythm or a systolic pressure
above 60 mmHg for 10 min after defibrillation, the rescue
measurements were stopped and investigators monitored the ECG and
hemodynamic parameters. If not, CPR was repeated and defibrillation
was performed again 2 min following CPR. If animals did not
achieved ROSC after 10 min with the above treatment, CPR was
terminated and this process was considered a failure. Following
resuscitation the hemodynamic parameters were continuously
monitored and recorded for 4 h. All catheters, including the
endotracheal tube, were removed at 4 h post-ROSC. Then animals were
subsequently returned to their cages for close observation
(Fig. 1).
Evaluation of neurological defect
score (NDS)
Neurological function was evaluated according to the
method of NDS at 24 h intervals for a total of 72 h. The
neurological deficits were scored from 0 (no observed neurologic
deficit) to 500 (mortality or brain death) (12). NDS was examined and confirmed by
two independent investigators who were blinded to the study.
Measurement of serum tumor necrosis
factor-α (TNF-α) and interleukin-6 (IL-6) levels
Blood samples were obtained from the rats at 1, 4
and 72 h post ROSC through the femoral vein. The samples were
centrifuged at 2,963 × g at 4°C for 15 min and the supernatant was
collected and then stored at −80°C. ELISAs were performed according
to the manufacturers' protocol with rat-TNF-α Immunoassay (cat. no.
RTA00; R&D Systems Europe, Ltd.) and rat-IL-6 ELISA kit (cat.
no. CSB-E04640r; Cusabio Technology LLC). Briefly, polystyrene
96-well microtitel immunoplates were coated with the
affinity-purified polyclonal rat anti-TNF-α antibody. Then the
samples and the TNF-α standard solutions were distributed in each
plate, and the plates were washed and incubated with anti-TNF-α
galactosidase for 2 h at room temperature. The plates were
subsequently washed and incubated with substrate solution for 30
min at room temperature. Following this incubation, the optical
density was measured using an ELISA reader. This method was also
employed to examine the levels of IL-6 according to the
aforementioned protocol and with relevant modifications.
Histopathological analysis
After 3 days, the rats were sacrificed and the
hippocampus was rapidly removed from the cerebrum. The left half of
the hippocampus was stored at −80°C and used later for western blot
analysis. The right half of the hippocampus was fixed in 4%
paraformaldehyde overnight at 4°C and embedded in paraffin for
immunohistochemical analysis. The present study focused on the
hippocampal CA1 region, which is considered to be the most
vulnerable region of the brain to ischemia and hypoxia. The slides
were stained with hematoxylin and eosin: The sections were placed
in 0.5% hematoxylin staining solution for 10 min at room
temperature; then immersed in 0.5% eosin aqueous solution for 5 min
at room temperature. Sections were rinsed with running water for 20
min after each step. Dead neurons were determined by the presence
of hypereosinophilic cytoplasm and pyknotic nuclei. Nonviable
neurons were counted in 3 random microscopic fields per section,
and the percentage of nonviable neurons in 3 sections per animal
was averaged (high-power fields of view, ×200 magnification) using
a light microscope (Olympus BX51; Olympus Corporation).
Immunofluorescence staining
The hippocampal slides were deparaffinized,
rehydrated in a descending ethanol series (100, 95, 85 and 75%, and
ddH2O) and immersed in 3%
H2O2/methanol for 10 min at room temperature
to inactivate endogenous peroxidase activity. Antigens were
heat-retrieved in sodium citrate buffer (10 mM sodium citrate;
0.05% Tween-20; pH 6.0) at 100°C for 10 min. Following blocking in
3% bovine serum albumin for 20 min at room temperature, the tissues
were incubated overnight at room temperature with the following
primary antibodies: α7nAChR antibody (1:200; cat. no. sc-58607;
Santa Cruz Biotechnology, Inc.) and anti-RNA binding protein fox-1
homolog 3 (NeuN) rabbit polyclonal antibody (1:1,000; cat. no.
GB11138; Servicebio Biotechnology, Inc.). Following washing 3 times
with PBS, sections were incubated for 1 h at room temperature with
Cy3-labeled goat anti-rat IgG (1:200; cat. no. GB22302; Servicebio
Biotechnology, Inc.) and fluorescein isothiocyanate-labeled
goat-anti rabbit IgG (1:200; cat. no. GB22303; Servicebio
Biotechnology, Inc.). The sections were subsequently mounted in
Mowiol mounting medium (cat. no. 81381, Sigma-Aldrich; Merck KGaA)
containing 1 µg/ml DAPI for DNA staining. Finally, images were
captured using a laser scanning confocal microscope (Olympus
FV3000; Olympus Corporation) at magnification, ×200.
Western blot analysis
The hippocampal tissues were homogenized in a lysis
buffer (Beyotime Institute of Biotechnology Co., Ltd.) and were
placed on ice and incubated for 30 min. The homogenates were
centrifuged at 12,000 × g at 4°C for 10 min to separate the
supernatant. The proteins were quantified by using the BCA kit
(Servicebio Biotechnology, Inc.). A total of 40 µg protein per lane
was separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes. The membranes were blocked for 2 h at room
temperature with blocking buffer and then incubated with primary
antibodies targeting α7nAChR (1:500; cat. no. sc-58607; Santa Cruz
Biotechnology, Inc.) or GAPDH (1:1,000; cat. no. AS1039; Aspen,
Wuhan, China) at 4°C overnight. The membranes were washed with TBS
containing 0.05% Tween-20, and membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies against
α7nAChR (cat. no. GB23302; 1:3,000; Servicebio Biotechnology, Inc.)
and GADPH (AS1107; 1:5,000; Aspen) for 1 h at room temperature. The
bands were developed using enhanced chemiluminescence (Beyotime
Institute of Biotechnology, Co., Ltd.) and images were obtained
using a Bio-Image Analysis System (Bio-Rad Laboratories, Inc.).
Densitometric analysis was performed with ImageJ v1.49 (National
Institutes of Health).
Statistical analysis
Continuous variables are presented as mean ±
standard deviation when data was normally distributed, or as a
median (with 25 and 75th percentiles) when data was not normally
distributed. Normal distribution was confirmed with the
Kolmogorov-Smirnov test. Variables were compared with one-way
analysis of variance, or the Kruskal-Wallis test for nonparametric
data. If there was a significant difference in the overall
comparison of the groups, comparisons between groups were made
using the Bonferroni post-hoc test. Survival data for Kaplan-Meier
curves were examined using the log-rank test. P<0.05 was
considered to be statistically significant.
Results
US preconditioning decreases the
number of defibrillations required and shortens the duration of
CPR
The present study initially used a total of 45 rats;
however, 5 were excluded due to instrumental or technical failure
during animal preparation. Therefore, 40 rats were randomly
assigned to one of five groups (n=8/group). No differences in basic
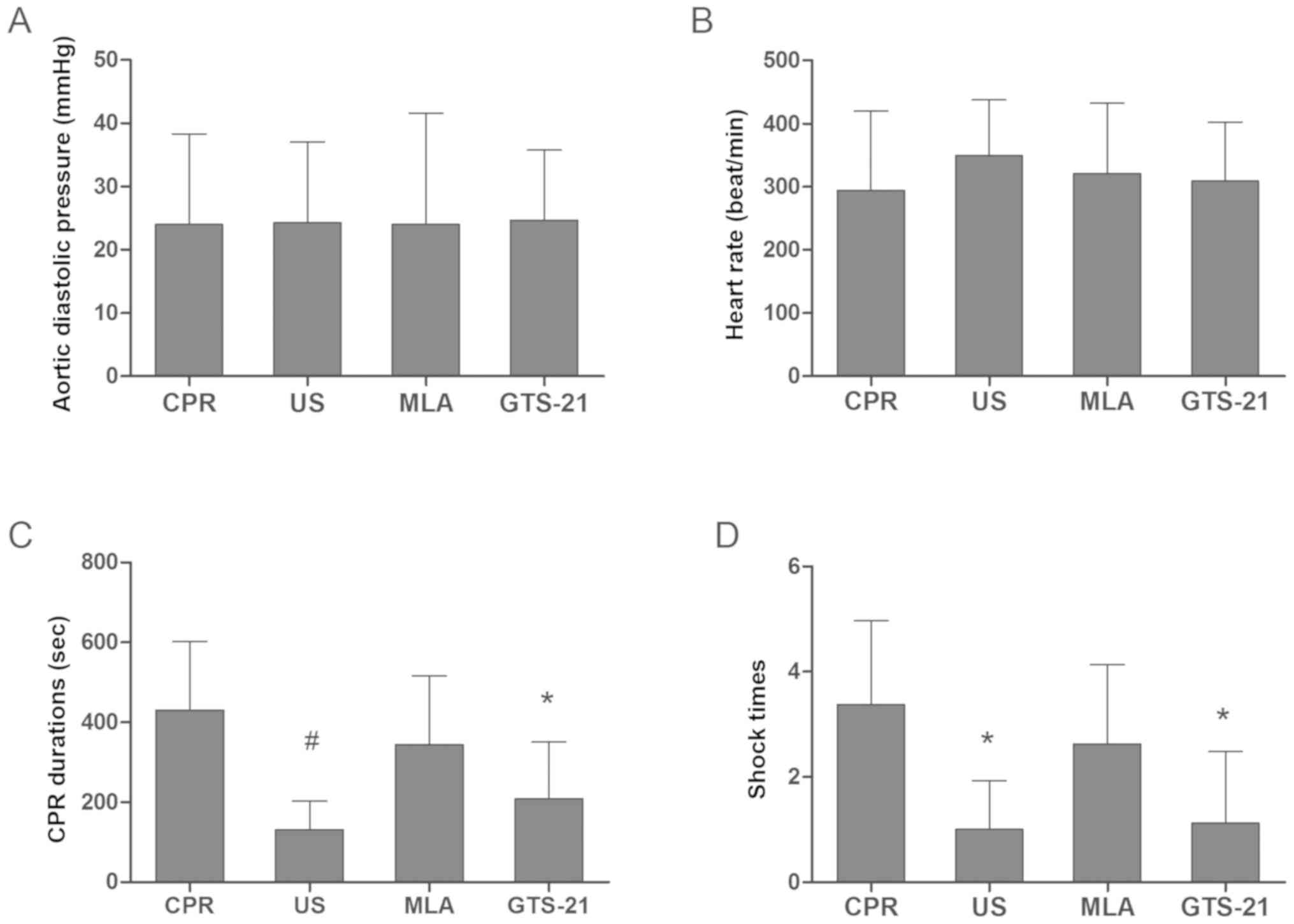
parameters among the 5 groups were detected at baseline (Table I). During CPR, the aortic diastolic
pressure was maintained at an even level, and no significant
difference was observed among the resuscitation groups (Fig. 2A). US also did not alter the heart
rates of rats when compared with those in CPR, MLA and GTS groups
during resuscitation (Fig. 2B).
However, US markedly decreased the number of defibrillations
required and shortened the duration of CPR when compared with the
CPR group (Fig. 2C and D).
However, inhibition of α7nAChR by MLA decreased the protective
effects of US, as there was no significant difference between the
CPR and MLA groups. Compared with the US group, GTS-21 treatment
had a similar effect in decreasing the number of defibrillations
required and the duration of CPR.
 | Table I.Baseline characteristics. |
Table I.
Baseline characteristics.
|
| Groups |
|---|
|
|
|
|---|
| Variables | Sham | CPR | US | US+MLA | GTS-21 |
|---|
| Weight, g | 375.2±15.4 | 378.3±16.8 | 371.0±16.6 | 367.0±15.9 | 374.1±14.6 |
| HR at baseline,
bpm | 367±19 | 371±22 | 366 ±23 | 359±18 | 366±21 |
| SBP, mmHg | 138.4±13.4 | 139.8±14.2 | 135.5±11.1 | 133.4±9.1 | 135.5±11.7 |
| DBP, mmHg | 117.6±11.0 | 124.4±11.8 | 125.3±8.3 | 117.9±9.8 | 123.9±11.3 |
| MAP at baseline,
mmHg | 124.8±10.9 | 129.5±11.9 | 129.0±8.6 | 123.0±8.8 | 127.9±10.7 |
| Preparation time,
min | 44.0±1.1 | 43.0±0.8 | 43.2±0.8 | 43.2±1.0 | 44.0±1.0 |
| Temperature at
baseline,°C | 37.0±0.1 | 36.9±0.0 | 36.9±0.1 | 37.0±0.1 | 37.0±0.0 |
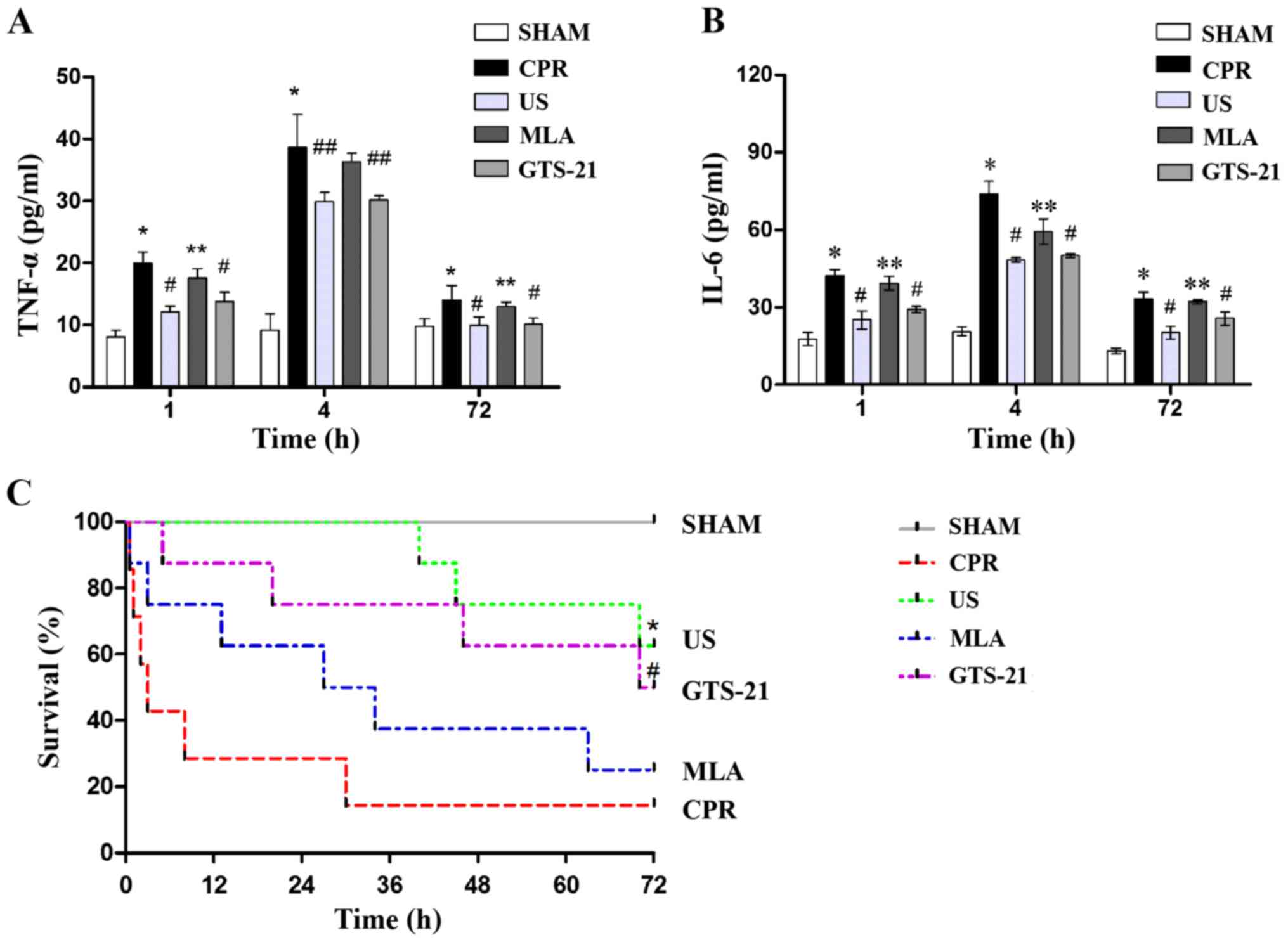
US suppresses the serum levels of
TNF-α and IL-6 and improves the 72 h survival rate following
ROSC
An increase in circulating inflammatory cytokines
was observed in all animals following resuscitation when compared
with the SHAM group. The levels of TNF-α and IL-6 in the serum
reached their peak at 4 h following the ROSC. There was a
significant decrease in the levels of TNF-α and IL-6 in the US
group when compared with the CPR group, indicating that US may
suppress the inflammatory response following resuscitation
(Fig. 3A and B). In addition, an
improved 72 h survival was observed in the US group when compared
with the CPR group (Fig. 3C). The
inhibition of α7nAChR resulted in increased levels of TNF-α and
IL-6 in MLA-treated animals. In the GTS group, the levels of
inflammatory cytokines were similar to that of the US group,
thereby demonstrating that the nicotinic receptor was associated
with the protective effect of USA.
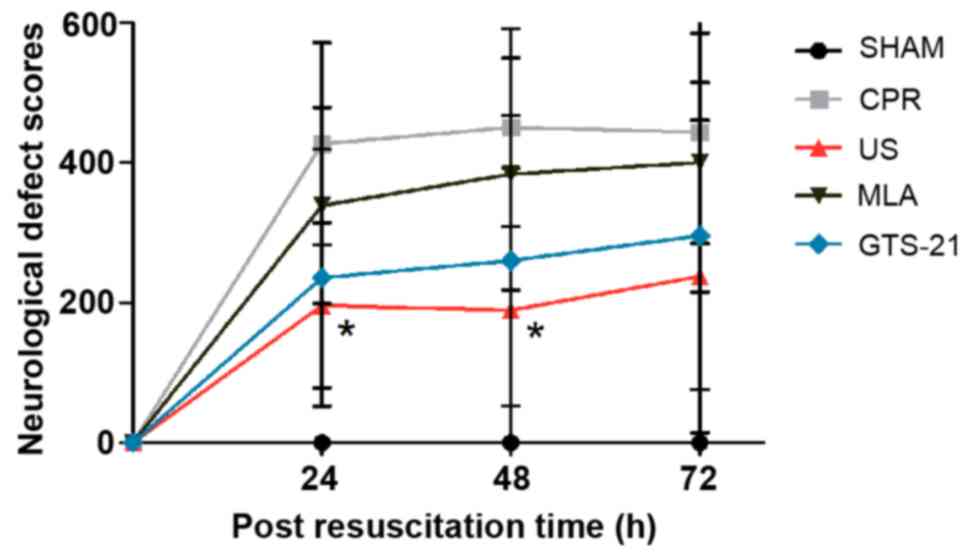
US improves NDS following
resuscitation
Daily evaluations of the NDS revealed that less
neurofunctional impairment occurred in animals in the US group when
compared with those in the CPR group at 24 and 48 h post-ROSC
(Fig. 3). At 72 h following
resuscitation there was still a trend towards improved neurological
function in the US group, yet the differences between the US and
CPR groups were not statistically significant. There was no
significant difference between the CPR and GTS groups, although the
NDS mean value of the former group was larger compared with the
latter group (Fig. 4). The
inhibition of α7nAChR by MLA abrogated the neuroprotective effect
of US treatment, as evidenced by the larger NDS in the MLA group
following ROSC.
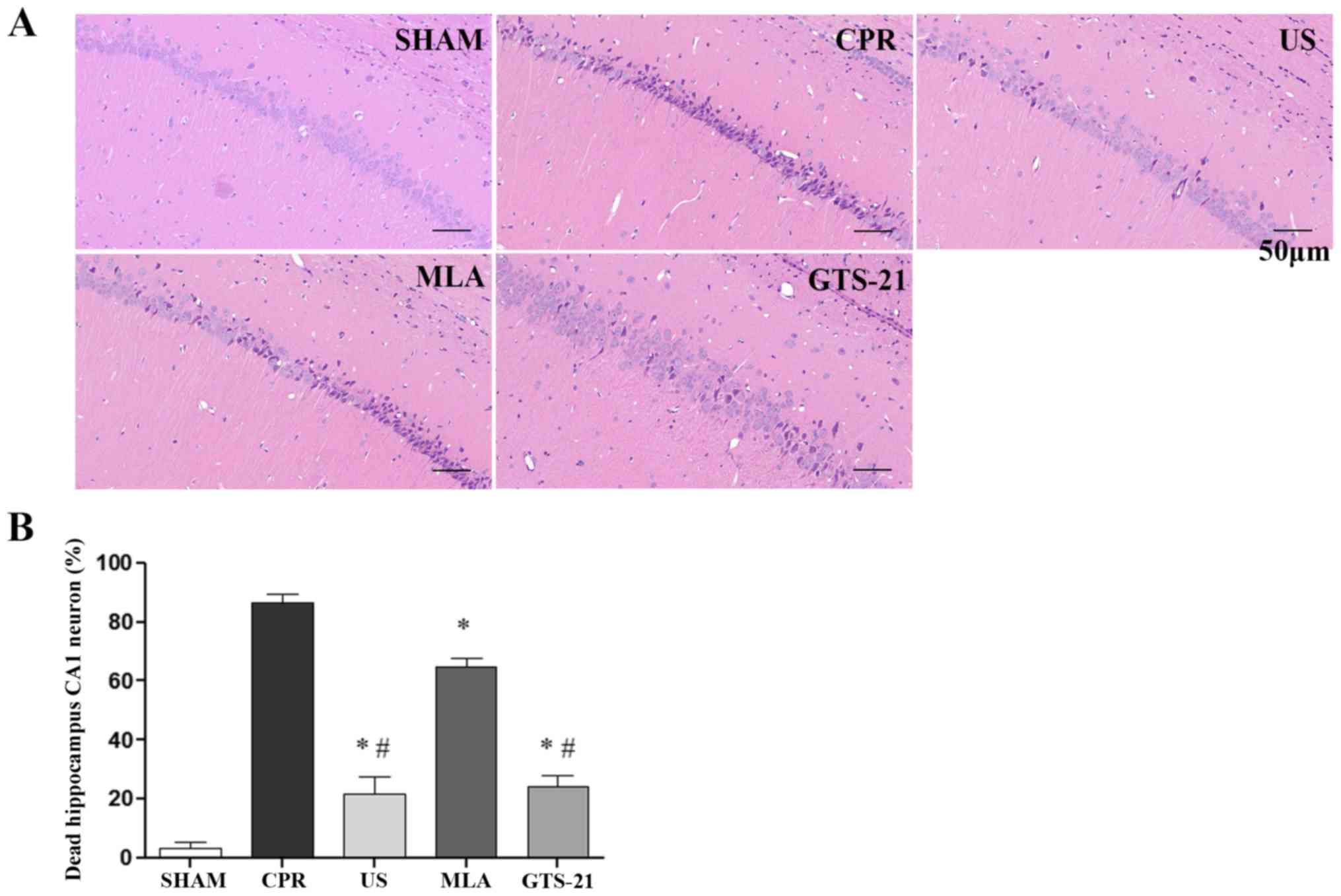
US attenuates CPR-induced injury to
hippocampal neurons
The photomicrographs of hippocampal neurons revealed
that CPR resulted in an increase in the amount of darkly stained
smaller nuclei and neurons with vacuolization of the cytoplasm in
the hippocampal CA1 region at 72 h after ROSC, which suggested that
a large amount of neuronal mortality had occurred in the
hippocampal CA1 region (Fig. 5A).
However, the aforementioned neuronal changes were less severe in
the US and GTS groups in comparison with CPR groups, indicating
that US treatment and GTS-21 exerted similar neuroprotective
effects. The inhibition of α7nAChR by MLA abrogated the
neuroprotective effect of US treatment, as evidenced by an increase
in the amount of neuronal death in the CA1 region (Fig. 5B).
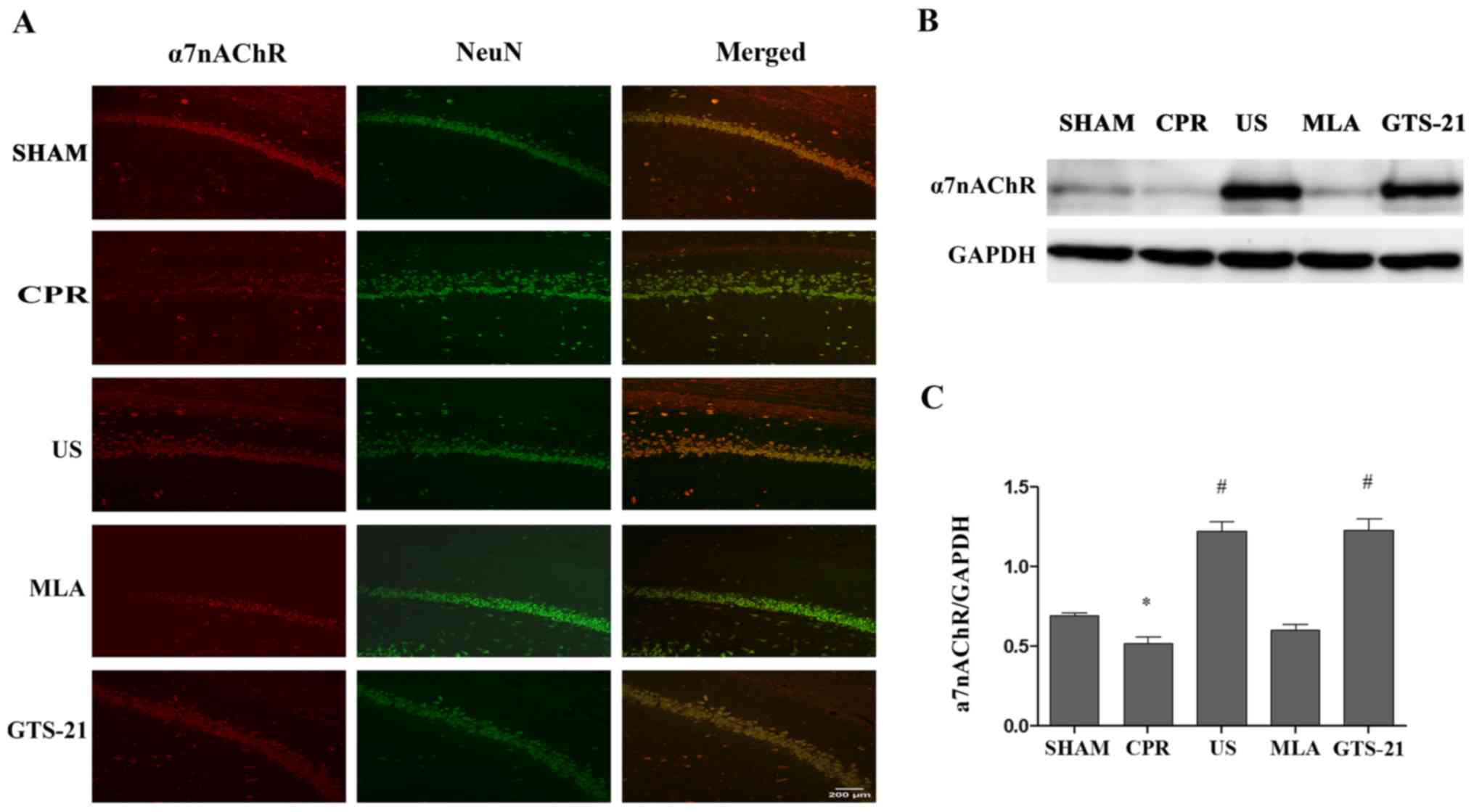
US increases the expression of α7nAChR
in hippocampal neurons following CPR
As demonstrated in Fig.
6, a marked decrease in the number of α7nAChR-positive cells
co-located in the neuron was observed visually in the CA1 region of
the hippocampus in the CPR group compared with the SHAM group.
However, US pretreatment reversed the decrease in α7nAChR levels
caused by CPR, suggesting that the central cholinergic signaling
may be repaired by US following resuscitation (Fig. 6A). In addition, no significant
difference was identified between the MLA and CPR groups. However,
GTS-21 had a similar effect as US treatment. The western blot
analysis results of α7nAChR protein expression were consistent with
the immunofluorescence analysis data (Fig. 6B and C).
Discussion
The results of the present study clearly
demonstrated that the survival rate was significantly improved when
rats were exposed to a single dose of US at 24 h prior to CPR. In
addition, US treatment decreased the number of electrical shocks
required for ROSC, shortened the duration of CPR and attenuated
neurological dysfunction. It was also observed that the levels of
circulating inflammatory cytokines were suppressed following
resuscitation and the expression of α7nAChR was promoted in
hippocampal neurons by US treatment. It was also observed that the
inhibition of α7nAChR by MLA abrogated the protective effect of US,
suggesting that ultrasonic protection against IRI resulted from CPR
was strongly dependent on the activation of CAP via α7nAChR.
It has previously been demonstrated that mice that
had been subjected to US 24 h prior to IRI were protected against
acute kidney injury (AKI) (13).
Although the role of US in kidney IRI has been investigated, the
protective effects of US during global IRI resulting from CA/CPR
have yet to be completely elucidated. Therefore, the aim of the
present study was to observe if US prior to VF would improve
patient outcomes following CPR.
Inflammatory cytokines have been implicated in the
pathophysiology of post-cardiac arrest syndrome, which include
myocardial dysfunction and hypotension, often leading to
multi-organ system dysfunction and mortality. The results of the
present study indicated that receiving US 24 h prior to CPR
prevented the increase in TNF-α and IL-6 following resuscitation.
TNF-α has been reported to be inversely correlated with myocardial
function (14), and the
administration of a monoclonal antibody directed against TNF-α
attenuates myocardial dysfunction and improves short-term survival
in the early post cardiac arrest period (15). IL-6 in cerebrospinal fluid and
blood have been indicated to be correlated with neurological
outcome following CA (16). It has
also been demonstrated that IL-6 values on intensive care unit
admission are associated with increased mortality in out-hospital
patients with CA (17). Therefore,
it is reasonable to conclude that the inhibition of TNF-α and IL-6
by US could contribute to the improvement in survival following
CPR. In addition, the anti-inflammatory effect of US treatment may
depend on α7nAChR as it was abolished by MLA and was imitated by
GTS-21, suggesting that the protection provided by US prior to CPR
is dependent on the CAP.
The present study also revealed that US may decrease
the number of defibrillations required during CPR and shorten the
CPR duration. The data demonstrated there was no difference in
myocardial reperfusion during CPR, as evidenced by consistent
aortic diastolic pressure among the CPR, US, MLA and GTS groups. A
potential explanation for this result is the alterations in gap
junction intercellular communication during resuscitation. Gap
junctions are composed of connexins, predominantly connexin 43, in
the ventricle, and provide aqueous conduits between cells to allow
electrical current flow (18). It
has been demonstrated that the concentration of TNF-α is rapidly
increased in rats following VF (19), and TNF-α may decrease the
expression of connexin 43 in the heart (20), which is associated with gap
junction uncoupling and results in increasing cardiac arrhythmias
and impaired defibrillation. Regional gap junction uncoupling
markedly increases the threshold values of biphasic defibrillations
(21). Therefore, a potential
explanation for the anti-arrhythmic effects of US may be due to its
potential to inhibit gap junction uncoupling by suppressing the
inflammatory cytokines during CPR. In the present study, MLA was
observed to reverse the anti-arrhythmic effects of US treatment,
whereas GTS-21 exerted anti-arrhythmic effects similar to US
treatment, suggesting that the anti-arrhythmic effects of US was
driven through the CAP via α7nAChR.
In addition to the aforementioned cardioprotective
effects of US during resuscitation, the present study also
demonstrated the neuroprotective effects of US. Brain injury is a
common reason for morbidity and mortality following resuscitation,
and the results of the present study supported the hypothesis that
US may alleviate cerebral IRI following CPR, as evidenced by the
improvement in neurological deficits and the decreases in the
severity of injury to hippocampal neurons following US treatment.
Although MLA aggravated neurological dysfunction in US-treated
animals, GTS-21 failed to attenuate neurological impairment
following ROSC when compared with control animals, despite the
presence of a trend toward improved neurological function in
GTS-treated animals. A potential explanation for this result may be
associated with the usage of GTS-21 in the present study. As GTS-21
has a biological half-life of 12–24 h (22), only one dose was delivered 30 min
prior to VF. The improved neurological outcomes may be observed if
GTS-21 is administered twice a day over 3 consecutive days. In
addition, it is well known that cerebral IRI largely depends on the
duration of ischemia. As US treatment shortened the duration of
CPR, these cardiac effects may also serve a role in the
neuro-protection observed.
Furthermore, the present study examined whether the
hippocampal neurons expressed α7nAChR on their surface following
treatment with US, which may suggest a potential involvement of the
CAP in cerebral reperfusion injury. It has previously been
demonstrated that CA and CPR dysregulates central cholinergic
signaling and engenders large increases in neuroinflammation and
neuronal damage (23). The results
of the present study also demonstrated that the expression of
α7nAChR in the hippocampal CA1 region was decreased at 72 h
following ROSC in the CPR group. However, in the US group, the
hippocampal neurons exhibited high levels of α7nAChR on their
surface, indicating that US may restore the central cholinergic
signaling against IRI induced by CPR. However, the contribution of
US to the brain response against IRI via the CAP requires
additional verification in future studies. US has been demonstrated
to markedly increase the spike frequency of primary hippocampal
neurons in culture (24). The
increase in excitability is largely mediated by the mechanical
effects of US, which may result in a mechanical distortion of cell
membranes, and these mechanical deformations may cause the
activation of mechanosensitive ion channels (25), which may alter the polarization
state of neurons and cause upregulation or suppression in activity.
US application to the left kidney activates the adrenergic splenic
nerve, as the left kidney is closed to the spleen. We hypothesized
that, in the present study, US activated the adrenergic splenic
nerve resulting in the release of norepinephrine, which binds to
adrenergic receptors on nearby CD4+ T cells. This stimulates the
production of acetylcholine, which binds to α7nAChRs on splenic
myeloid cells and results in decreased inflammation and IRI
(8). However, the mechanism of the
contribution of US treatment to the brain response against IRI via
the CAP remains to be determined.
There were several limitations to the present study:
Firstly, it is difficult to argue that the protective effects
against whole-body IRI were as a result of US pretreatment in such
a short time period (24 h). However, it has been demonstrated that
mice that had been subjected to US for 5 min 1 day prior to IRI
were protected against AKI (8).
Therefore, it is reasonable to interpret the results of the present
study, as the same US application protocol described by Gigliotti
et al (8) was used. In
addition, US treatment should also be applied following CA, in
order to investigate whether this also exerts a beneficial effect.
The spleen should also be investigated, as the spleen is the tissue
target for US-mediated tissue protection (13), and therefore may be more effective.
Additional studies are required in order to investigate the
different conditions of US treatment. Secondly, in a clinical
setting, patients treated with CPR typically have a pre-existing
clinical disease; however, the animals used in the present study
were healthy. Therefore, the outcome of this study in a rat model
of CPR requires further verification in large animal and clinical
studies.
In summary, the present study demonstrated that US
exerted a remarkable ability to improve the clinical outcomes
following CPR, and the mechanism underlying the protection of US
was associated with the activation of CAP via the a7nAChR.
Importantly, the noninvasive US regimen relies on US settings
within approved United States of America Food and Drug
Administration guidelines, and has been used for the treatment of
solid tumors, uterine fibroids, glaucoma, kidney stones, deep
venous thrombosis and musculoskeletal injuries (26–28).
In addition, the US method used in the present study was simple and
may be administered by a routine clinical imaging system, which is
suitable for emergency operation. Therefore, this
non-pharmacological approach, involving therapeutic US within the
spectrum of use currently approved for humans, provides an
attractive alternative therapy for post-resuscitation syndrome.
Acknowledgements
Professor Wei Peng in the University of Utah School
of Medicine contributed to the editing of this manuscript. The
abstract was presented at Resuscitation Science Symposium 2018 Nov
10–11 in Chicago, IL and published as abstract no. 254 in
Circulation 138 (Suppl 2): 2018.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 81571866) and Health
Commission of Hubei Province (grant no. WJ2019M160).
Availability of data and materials
The datasets used and analyzed in the present study
are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, YS, LG and PS contributed to the conception and
design of research. YZ, YS, TS, LL and WS performed the
experiments. YZ, YS, TS, LL, WS and PS analyzed data. YZ, YS, TS,
LL, WS and PS interpreted results of the experiments. YZ, YS and TS
prepared figures/ YZ, YS, TS, LL, WS and PS the drafted manuscript.
YZ, YS, TS, LG and PS edited and revised manuscript. PS approved
the final version of manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All protocols in the animal model were conducted in
strict accordance with the guidelines of the Ministry of Science
and Technology of the People's Republic of China for Animal Care
and Use, which conformed to the Guide for the Care and Use of
Laboratory Animals published by the United States of America
National Institutes of Health (9).
The study was approved by the Medical Ethics Committee of Huazhong
University of Science and Technology (approval no. 2017-S2191).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adrie C, Adib-Conquy M, Laurent I, Monchi
M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P,
Spaulding C, et al: Successful cardiopulmonary resuscitation after
cardiac arrest as a ‘sepsis-like’ syndrome. Circulation.
106:562–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borovikova LV, Ivanova S, Zhang M, Yang H,
Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW and Tracey
KJ: Vagus nerve stimulation attenuates the systemic inflammatory
response to endotoxin. Nature. 405:458–462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao M, He X, Bi XY, Yu XJ, Gil Wier W and
Zang WJ: Vagal stimulation triggers peripheral vascular protection
through the cholinergic anti-inflammatory pathway in a rat model of
myocardial ischemia/reperfusion. Basic Res Cardiol. 108:3452013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Z, Baker W, Hiraki T and Greenberg JH:
The effect of right vagus nerve stimulation on focal cerebral
ischemia: An experimental study in the rat. Brain Stimul. 5:1–10.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue T, Abe C, Sung SS, Moscalu S,
Jankowski J, Huang L, Ye H, Rosin DL, Guyenet PG and Okusa MD:
Vagus nerve stimulation mediates protection from kidney
ischemia-reperfusion injury through α7nAChR+ plenocytes.
J Clin Invest. 126:1939–1952. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun P, Wang J, Zhao S, Yang Z, Tang Z,
Ravindra N, Bradley J, Ornato JP, Peberdy MA and Tang W: Improved
outcomes of cardiopulmonary resuscitation in rats treated with
vagus nerve stimulation and its potential mechanism. Shock.
49:698–703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hougardy JM, Sadis C and Le Moine A:
Ultrasonic stimulation of the cholinergic anti-inflammatory pathway
for renal protection. J Am Soc Nephrol. 24:1340–1342. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gigliotti JC, Huang L, Ye H, Bajwa A,
Chattrabhuti K, Lee S, Klibanov AL, Kalantari K, Rosin DL and Okusa
MD: Ultrasound prevents renal ischemia-reperfusion injury by
stimulating the splenic cholinergic anti-inflammatory pathway. J Am
Soc Nephrol. 24:1451–1460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Research NRCU: Guide for the Care and Use
of Laboratory Animals. National Academies Press; Washington, DC:
1996
|
|
10
|
Lin JY, Liao XX, Li H, Wei HY, Liu R, Hu
CL, Huang GQ, Dai G and Li X: Model of cardiac arrest in rats by
transcutaneous electrical epicardium stimulation. Resuscitation.
81:1197–1204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong P, Zhao S, Wang J, Yang Z, Qian J, Wu
X, Cahoon J and Tang W: Mild hypothermia preserves cerebral cortex
microcirculation after resuscitation in a rat model of cardiac
arrest. Resuscitation. 97:109–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye S, Weng Y, Sun S, Chen W, Wu X, Li Z,
Weil MH and Tang W: Comparison of the durations of mild therapeutic
hypothermia on outcome after cardiopulmonary resuscitation in the
rat. Circulation. 125:123–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gigliotti JC, Huang L, Bajwa A, Ye H, Mace
EH, Hossack JA, Kalantari K, Inoue T, Rosin DL and Okusa MD:
Ultrasound modulates the splenic neuroimmune axis in attenuating
AKI. J Am Soc Nephrol. 26:2470–2481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niemann JT, Garner D and Lewis RJ: Tumor
necrosis factor-alpha is associated with early postresuscitation
myocardial dysfunction. Crit Care Med. 32:1753–1758. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niemann JT, Youngquist ST, Shah AP, Thomas
JL and Rosborough JP: TNF-α blockade improves early
post-resuscitation survival and hemodynamics in a swine model of
ischemic ventricular fibrillation. Resuscitation. 84:103–107. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oda Y, Tsuruta R, Kasaoka S, Inoue T and
Maekawa T: The cutoff values of intrathecal interleukin 8 and 6 for
predicting the neurological outcome in cardiac arrest victims.
Resuscitation. 80:189–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaahersalo J, Skrifvars MB, Pulkki K,
Stridsberg M, Røsjø H, Hovilehto S, Tiainen M, Varpula T, Pettilä V
and Ruokonen E; FINNRESUSCI Laboratory Study Group, : Admission
interleukin-6 is associated with post resuscitation organ
dysfunction and predicts long-term neurological outcome after
out-of-hospital ventricular fibrillation. Resuscitation.
85:1573–1579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van Kempen MJ, Fromaget C, Gros D, Moorman
AF and Lamers WH: Spatial distribution of connexin 43, the major
cardiac gap junction protein, in the developing and adult rat
heart. Circ Res. 68:1638–1651. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niemann JT, Rosborough J, Youngquist S,
Lewis RJ, Phan QT and Filler S: The proinflammatory cytokine
response following resuscitation in the swine model depends on the
method of ventricular fibrillation induction. Acad Emerg Med.
15:939–944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fernandez-Cobo M, Gingalewski C, Drujan D
and De Maio A: Downregulation of connexin 43 gene expression in rat
heart during inflammation. The role of tumour necrosis factor.
Cytokine. 11:216–224. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sims JJ, Schoff KL, Loeb JM and Wiegert
NA: Regional gap junction inhibition increases defibrillation
thresholds. Am J Physiol Heart Circ Physiol. 285:H10–H16. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pavlov VA, Ochani M, Yang LH,
Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR,
Rosas-Ballina M, Czura CJ, et al: Selective a7-nicotinic
acetylcholine receptor agonist GTS-21 improves survival in murine
endotoxemia and severe sepsis. Crit Care Med. 35:1139–1144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Norman GJ, Morris JS, Karelina K, Weil ZM,
Zhang N, Al-Abed Y, Brothers HM, Wenk GL, Pavlov VA, Tracey KJ and
Devries AC: Cardiopulmonary arrest and resuscitation disrupts
cholinergic anti-inflammatory processes: A role for cholinergic a7
nicotinic receptors. J Neurosci. 31:3446–3452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khraiche ML, Phillips WB, Jackson N and
Muthuswamy J: Ultrasound induced increase in excitability of single
neurons. Conf Proc IEEE Eng Med Biol Soc. 2008:4246–4249.
2008.PubMed/NCBI
|
|
25
|
Mihran RT, Lineaweaver S, Barnes FS and
Wachtel H: Effects of pulsed acoustic and mechanical stimuli on the
excitability of isolated neuronal and cardiac cells. Appl Occup
Environ. Hyg. 11:271–274. 1996.
|
|
26
|
Lu T, Loh TM, El-Sayed HF and Davies MG:
Single-center retrospective review of ultrasound-accelerated versus
traditional catheter-directed thrombolysis for acute lower
extremity deep venous thrombosis. Vascular. 25:525–532. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nazer B, Gerstenfeld EP, Hata A, Crum LA
and Matula TJ: Cardiovascular applications of therapeutic
ultrasound. J Interv Card Electrophysiol. 39:287–294. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miller DL, Smith NB, Bailey MR, Czarnota
GJ, Hynynen K and Makin IR; Bioeffects Committee of the American
Institute of Ultrasound in Medicine, : Overview of therapeutic
ultrasound applications and safety considerations. J Ultrasound
Med. 31:623–634. 2012. View Article : Google Scholar : PubMed/NCBI
|