Introduction
Craniofacial development in mammals is the result of
a series of complex environmental and genetic factors (1), and cleft palate (CP) is one of the
most common types of craniofacial malformation in humans (2). This congenital deformity is due to
the disruption of palatal genesis, which involves multiple cellular
processes, including the proliferation of mesenchymal cells and
growth of bilateral palate shelves (3). Embryonic palate mesenchymal (EPM)
cells derive from cranial neural crest cells, which are composed of
pluripotent stem cells that can proliferate and differentiate into
distinct craniofacial cells during palatal morphogenesis (4). Any perturbations during EPM cell
proliferation, differentiation and apoptosis can result in CP
(3).
Maternal conditions during gestation have a direct
impact on fetal growth; they affect the fetus through the nutrients
that reach the fetus via the placenta (5). Nutrients beneficial for growth, as
well as toxic substances, can thus be transported from the mother
to the fetus (6). There is ample
evidence demonstrating that smoking, intake of various medications,
presence of fever or flu, and a lack of adequate folic acid and
other vitamins during pregnancy are risk factors for CP (7–10).
Several studies have demonstrated that various metabolites can be
transferred across the placenta during pregnancy (11–16).
Dubé et al (14) revealed
that maternal obesity is associated with placental weight and
increased levels of cholesterol and lipoprotein in the newborn.
Paolini et al (16)
collected blood samples from fetuses after administering stable
isotopes to their mothers. The results demonstrated that
isotope-labeled amino acid levels, including leucine and
phenylalanine, are significantly lower in the intrauterine
growth-restricted group compared with the control group.
Dexamethasone (DXM) is a glucocorticoid that is
often used to treat a range of diseases, including asthma,
allergies, rheumatoid arthritis and inflammatory bowel disease
(https://www.chemicalbook.com).
Corticosteroids administered early in pregnancy have been reported
to be associated with a fetal orofacial cleft (17). Accordingly, corticosteroids are
well-established experimental teratogens in animal CP models
(18–20). The mechanisms by which
corticosteroids may induce CP remain uncertain. However, previous
studies have revealed that DXM serves a role in the progress of
palatal fusion and restrains EPM cells from proliferation and
apoptosis, while also affecting the differentiation of medial edge
epithelial cells (21,22).
High-spatial-resolution 1H magnetic
resonance spectroscopy (MRS) is a non-invasive method to profile
metabolites (23). Compared with
lower magnetic field strengths, ultra-high field strengths,
including 7-Tesla (T) and 9.4T, provide a higher spectral
resolution and signal-to-noise ratio, which significantly enhances
the information obtained from these spectra. In the last few years,
this approach has mainly been used to study biochemical alterations
in neurology, including neurodegeneration and brain tumors
(24–28). Since the proton is one of the most
sensitive nuclei for MRS and nearly all metabolites contain
hydrogen atoms, investigation by 1H MRS provides an
opportunity to test tissue metabolites non-invasively. Therefore,
researchers have utilized high-spatial-resolution 1H MRS
to investigate metabolic alterations in the uterus leading to
congenital diseases.
Song et al (29) used 1H MRS to calculate
placental metabolism in relation to intrauterine growth
restriction; N-acetyl aspartate and choline levels decreased in
comparison with normal placental levels, whereas lipids appeared to
increase. Song et al (29)
therefore concluded that choline and lipids could serve as
potential biomarkers to predict pregnancy outcomes. Zhou et
al (30) concluded that an
1H MRS-based metabolomics approach could be an efficient
and convenient technique to differentiate individuals with oral
squamous cell carcinoma from healthy controls. Additionally,
researchers have attempted to identify the irregular metabolic
profile of CP mouse models using in vivo 7T 1H
MRS, and revealed that elevated lipid and choline levels in CP
tissues may have the potential to serve as biomarkers of
craniofacial congenital malformations (31).
Based on the aforementioned findings, the present
study used 9.4T MRS to identify the alterations in metabolites in
maternal organs and fetal palatal tissues. The present study
demonstrated the effects of small molecular metabolites in multiple
organs that interact to promote the occurrence of CP in
embryos.
Materials and methods
Animal experiments
A total of 40 female and 20 male 8-week-aged Kunming
mice, weighing 30 g, were raised in a standardized laboratory
animal room (22–25°C; 70% humidity; 12-h light/dark cycle; food and
water ad libitum). All experimental animals were provided by
the Center for Laboratory Animal Sciences of Shantou Medical
University. Female mice were mated with fertile males overnight (2
females: 1 male). The day on which vaginal plugs were observed was
defined as embryonic gestation day 0.5 (E0.5). A total of 24
gestational females were randomly divided into two groups. Between
E8.5 and E13.5, the treatment group was administered DXM (Jiangsu
Chengxin Pharmaceutical Co., Ltd.) as 8 mg/kg twice per day by
subcutaneous injection. At the same time, the control group was
injected with the same volume normal saline. The present study was
approved by the Animal Ethics Committee of Shantou University
Medical College (Shantou, China).
Preparation of samples
Cesarean sections were performed on pregnant mice on
day E14.5, and the fetuses were explanted. Samples, including the
serum of maternal mice, amniotic fluid and placenta tissues, and
palatal tissues of fetal mice (only mice with anatomical CP were
chosen for the experimental group), were collected for 9.4T MRS
analyses.
A total of 30–40 mg placental and palatal tissues of
each female mouse were divided evenly into two samples. Amniotic
fluid and serum were centrifuged at 352 × g for 10 min at 4°C to
remove particulate contaminants. Following cryopreservation in
liquid nitrogen, the samples were stored at −80°C.
Tissue samples were homogenized by ultrasound, and
1.5 ml sonicate was extracted using a mixture of methanol and
chloroform (2:1 v:v). This was followed by a centrifugation step
(0°C, 1,891 × g, 20 min) in order to separate the water- and
fat-soluble layers. Hydrophilic and lipophilic metabolites,
respectively, were acquired when the solvents were removed by
lyophilization and evaporated with nitrogen gas. The water-soluble
metabolites were reconstituted in 600 µl D2O containing
0.5 mM 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt
(TSP), while the lipid-soluble metabolites were reconstituted in
600 µl deuterated chloroform (CDCl3; 0.03% v/v
trimethylsilyl).
Each bio-fluid sample (300 µl) was mixed with 250 µl
D2O and 200 µl PBS (0.2 M
Na2HPO4/NaH2PO4; pH
7.4; 99.9% D2O) to reduce the variations in pH across
samples. Additionally, 0.3 mM TSP was used as an internal reference
standard.
Metabolomic measurements by 9.4T
nuclear magnetic resonance (NMR)
The samples were analyzed by a 9.4T NMR spectrometer
(Bruker Avance). After autoshimming, a ZGPR pulse sequence was used
(number of scans: 64; mixing time: 100 µsec; spectral width: 13.9
ppm; time domain:16K; delay 11.5 sec). Weak irradiation on the
water signal was applied to suppress solvents. Chemical shifts in
all samples were referenced to TSP. All spectra detected by the
9.4T NMR spectrometer were processed using MestReNova version 9.0.1
software (Mestrelab Research, S.L.). The spectral range between 0.5
and 9.0 ppm was segmented into buckets with equal widths of 0.002
ppm each. Each bucket was internally normalized to the total sum of
the spectral integrals prior to pattern recognition analysis to
compensate for differences in sample concentration. For the data
analyses, metabolites in all 1H-NMR spectra were
assigned with reference to published data and Chenomx NMR suite
version 7.1 (Chenomx, Inc.).
Statistical analysis
Experimental data were processed using SIMCA-P
version 14.1 software (Umetrics; Sartorius Stedim Biotech) for
multivariate statistical analysis. First, principal component
analysis (PCA) classing was used for an overview of the data. Next,
the data were further analyzed via orthogonal partial least squares
discriminant analysis (OPLS-DA). The model quality was evaluated
using the R2Y and Q2 values, reflecting the
explained model analytical ability and predictability,
respectively. An R2Y score of 1 demonstrated that the
model explained 100% of the variance, and the closer the
Q2 score was to 1 the higher the reliability of
prediction. To validate the quality of the OPLS-DA model,
permutation tests with 200 iterations were performed. The criteria
for validity were as follows: All blue Q2-values to the
left are lower than the original points to the right, or the blue
regression line of the Q2-points intersects the vertical
axis (on the left) at ≤0.
Data of 12 DXM-treated samples and 12 control
samples were used for multivariate statistical analysis. VIP
(variable importance in projection) predictive values that had
passed the permutation tests were obtained based on the OPLS-DA
model. A Student's unpaired t-test was performed automatically
during OPLS analysis. Data are presented as the mean ± standard
deviation. Those metabolites corresponding to different ppm with
P-values ≤0.05 and VIP values ≥1 were defined as significantly
different.
Histological sections
Palates of the embryos were harvested at E14.5 and
processed in paraffin wax. Histological sections of embryonic
palates were cut and stained with hematoxylin and eosin.
Palate of the embryos were harvested at E14.5 and
fixed in 4% paraformaldehyde for at ≥24 h at room temperature, then
they were dehydrated through an ethanol series and embedded in
paraffin for sectioning by routine procedures. Deparaffinized
sections (3 µm) were stained with 0.25% hematoxylin and 0.5% eosin
for 3 min respectively at room temperature. For general morphology,
stained sections were viewed by light microscope.
Results
Histological appearance of clefts
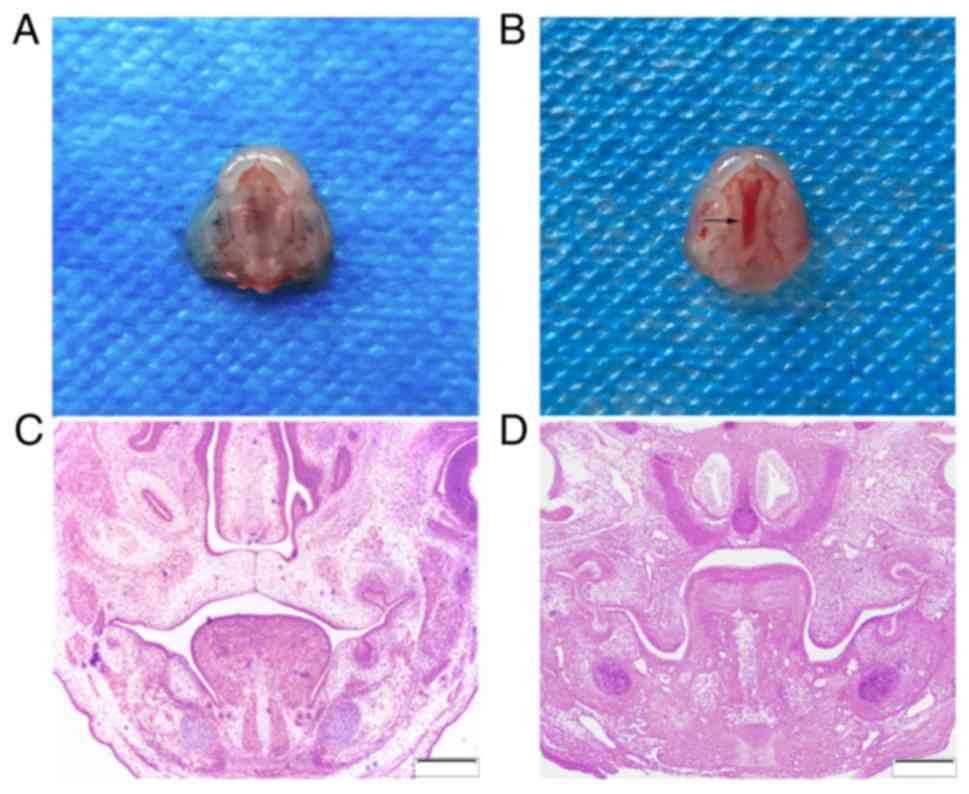
A total of 289 embryo mice were harvested with a
100% live birth rate. Among the 137 embryos exposed to DXM, 72
developed CPs, giving a CP-induction rate of 52.6% (Fig. 1A and B). None of the 152 murine
embryos from the 12 pregnant mice treated with normal saline
developed a CP.
The E14.5 embryos in the control group exhibited
bilaterally elevated shelves. These touched at an epithelial line
between them. The tongue was located between the bilateral lower
mandibles and palate shelves (Fig.
1C). The majority of the DXM-exposed embryos, however,
exhibited vertically oriented shelves. The tongues were much higher
in the experimental group compared with in the control group
(Fig. 1D).
Hydrophilic metabolic alterations
observed in multiple organs
Palatal tissue
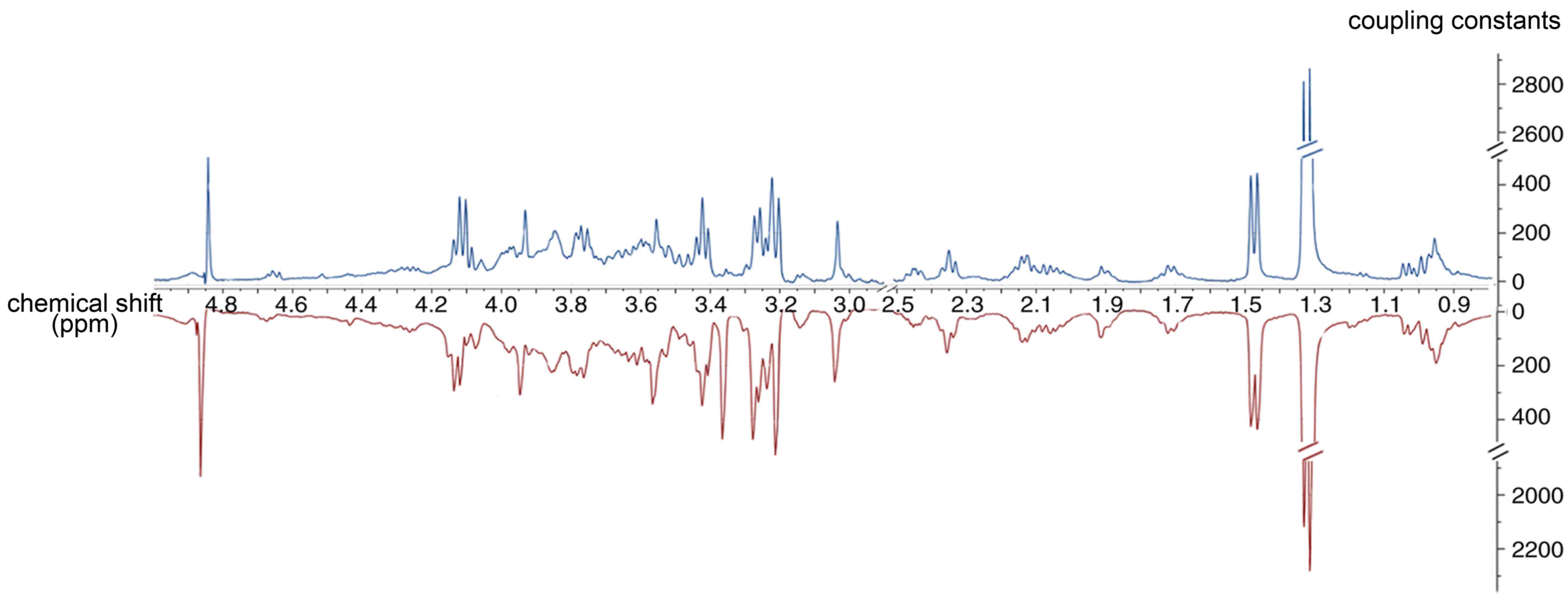
Representative 1H NMR spectra of the
palatal tissue obtained from one of the maternal mice at E14.5 are
shown in Fig. 2. The palatal
tissue spectrum contained peaks mainly representing amino acids,
glutamate-containing metabolites and carboxylic acids, including
citrate. The metabolic resonances were assigned according to a
previous study (32) and the
Chenomx NMR suite (Chenomx NMR suite version 7.1 (Chenomx, Inc.).
The identification of metabolites is shown in Table I.
 | Table I.Metabolite identification. |
Table I.
Metabolite identification.
| Metabolite | Chemical shift |
|---|
| Valine | 0.99(d),3.72(t),
1.96(m), 0.91(d) |
| Lactate | 4.11(q),
1.32(d) |
| Alanine | 3.77(q),
1.48(d) |
| Acetate | 1.91(s) |
| Glutamate | 2.08(m), 2.34(m),
3.75(m) |
| Glutamine | 2.15(m), 2.44(m),
3.77(m) |
| Citrate | 2.55(d),
2.65(d) |
| Creatinine | 3.03(s),
3.92(s) |
| Choline | 3.2(s), 4.05(t),
3.51(t) |
| Phosphocholine | 3.22(s), 4.21(t),
3.61(t) |
| TMAO | 3.27(s) |
| β-glucose | 4.66(d) |
| α-glucose | 5.23(d) |
| Succinate | 2.41(s) |
| Taurine | 3.26(t),
3.40(t) |
| Betaine | 3.26(s),
3.93(s) |
| Hippurate | 3.98(d), 7.56(t),
7.65(t), 7.84(d) |
| Leucine | 3.65(d), 1.95(m),
0.94(t), 1.02(d) |
| Glycine | 3.57(s) |
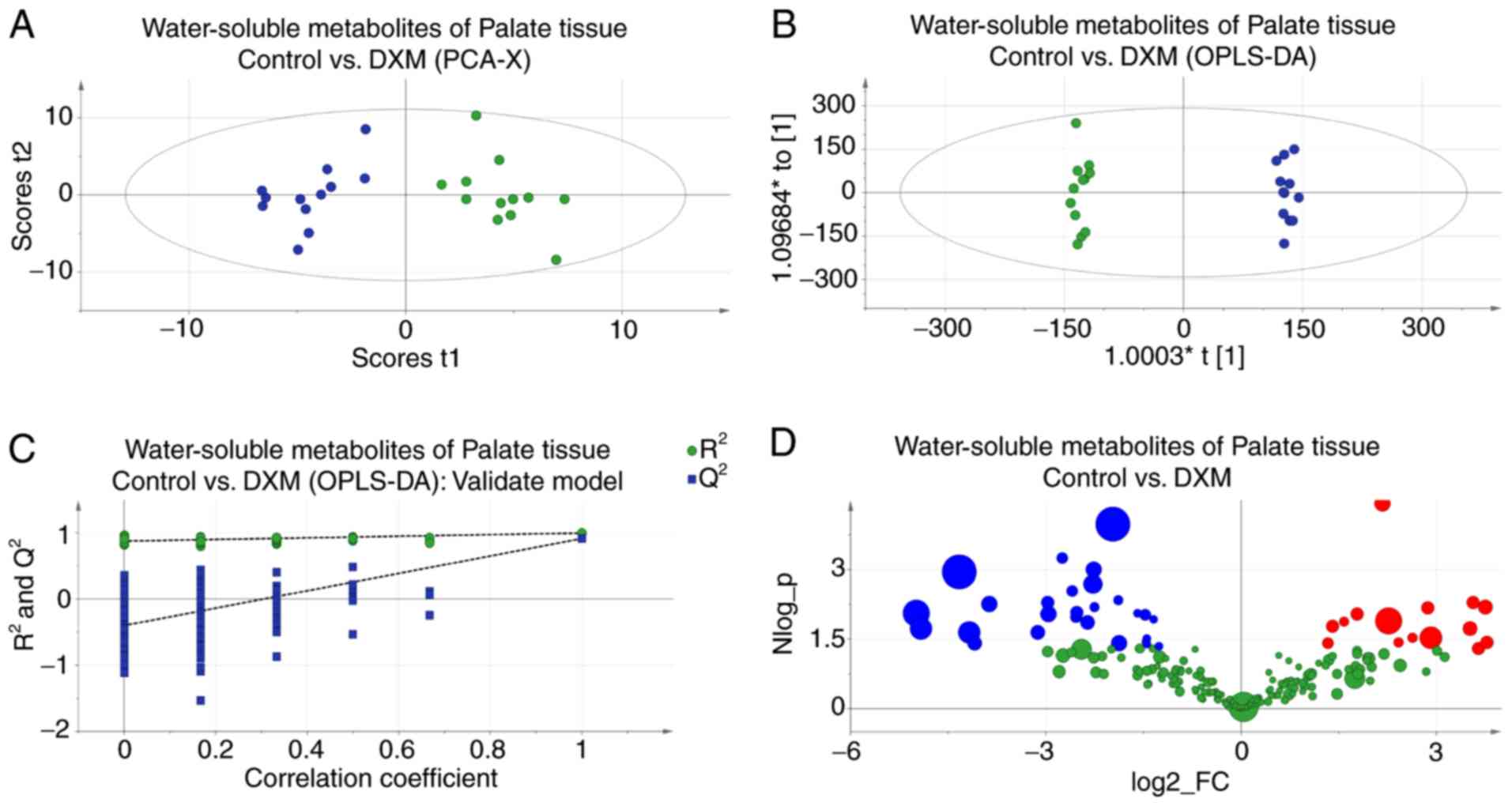
PCA score plots revealed the inherent clustering of
groups based on their similarity and dissimilarity, indicating a
clear distinction between the control and experimental groups
(Fig. 3A). The significance of the
differences between the two groups was further confirmed by an
OPLS-DA test (R2Y=0.997; Q2=0.913; Fig. 3B). The results of 200 permutation
tests (Fig. 3C) demonstrated that
this model was reliable and consistent. The metabolites responsible
for the significant separations between the two groups were
identified using volcano plots (Fig.
3D). The present study identified lower levels of creatinine +
creatine, leucine, valine, acetate and citrate, and higher levels
of lactate, alanine, proline + inositol and glutamate-containing
metabolites in the CP embryos. Detailed statistical descriptions of
all alterations are shown in Table
II.
 | Table II.Statistical descriptions of
water-soluble metabolites in palate and placenta tissues. |
Table II.
Statistical descriptions of
water-soluble metabolites in palate and placenta tissues.
|
| Palate tissue | Placenta
tissue |
|---|
|
|
|
|
|---|
| Metabolites | P-value | VIP value | Mean ± SD
(control) | Mean ± SD
(DXM) | Fold-change | P-value | VIP value | Mean ± SD
(control) | Mean ± SD
(DXM) | Fold-change |
|---|
| Leucine | 0.00 | 4.89 |
7649.21±3895.92 |
1957.82±1510.09 | 0.26 | – | – | – | – | – |
| Valine | 0.00 | 1.78 |
1050.90±2738.71 | 218.99±170.16 | 0.21 | 0.00 | 1.39 | 25.58±12.27 | 4.24±8.41 | 0.17 |
| Lactate | 0.00 | 1.67 | 68.35±26.84 | 918.34±977.56 | 13.44 | 0.00 | 5.55 | 516.03±276.06 | 120.48±255.53 | 0.23 |
| Alanine | 0.02 | 1.12 | 282.29±197.32 | 750.48±596.57 | 2.66 | 0.00 | 1.98 | 60.24±33.65 | 12.06±23.24 | 0.20 |
| Acetate | 0.00 | 1.70 |
1014.00±1056.01 | 130.01±156.89 | 0.13 | – | – | – | – | – |
|
N-acetyl-glycoproteins | 0.00 | 1.28 | 559.06±540.29 | 71.13±58.40 | 0.13 | – | – | – | – | – |
|
Glutamate/glutathione | 0.00 | 1.31 | 68.73±35.95 | 501.48±499.33 | 7.30 | – | – | – | – | – |
|
Glutamate/glutamine | 0.00 | 1.02 | 44.30±16.26 | 524.68±534.09 | 11.84 | 0.00 | 1.41 | 31.09±20.42 | 5.60±13.07 | 0.18 |
| Glutamine | 0.02 | 1.25 | 69.47±41.33 | 793.78±988.57 | 11.43 | – | – | – | – |
|
| Glutathione | 0.00 | 1.10 | 36.97±19.61 |
3864.80±4236.00 | 104.54 | – | – | – | – | – |
| Citrate | 0.02 | 1.20 |
2183.87±2904.82 | 120.63±144.93 | 0.06 | – | – | – | – |
|
| Creatinine +
creatine | 0.04 | 1.61 |
1081.13±1605.99 | 63.43±102.37 | 0.06 | 0.01 | 1.19 | 26.46±11.11 | 4.89±12.10 | 0.18 |
| Succinate | – | – | – | – | – | 0.00 | 1.11 | 19.27±23.77 | 2.92±5.08 | 0.15 |
| Choline | – | – | – | – | – | 0.01 | 2.15 | 115.54±54.83 | 49.35±61.16 | 0.43 |
| Phosphocholine | 0.05 | 1.17 | 47.36±35.40 | 590.87±905.90 | 12.48 | 0.00 | 4.50 | 321.47±190.61 | 50.34±142.88 | 0.16 |
| TMAO | – | – | – | – | – | 0.02 | 3.51 | 340.12±275.78 | 86.09±214.74 | 0.25 |
| Proline +
inositol | 0.03 | 2.44 | 338.04±342.57 |
2559.04±3299.32 | 7.57 | 0.01 | 1.41 | 3.26±6.07 | 35.69±38.47 | 10.93 |
|
Phenylacetylglycine | – | – | – | – | – | 0.00 | 1.21 | 24.95±2.61 | 2.61±6.44 | 0.10 |
| D-Glucose | – | – | – | – | – | 0.01 | 1.04 | 25.01±16.76 | 6.99±14.33 | 0.28 |
| Betaine | – | – | – | – | – | 0.00 | 1.98 | 53.45±29.63 | 8.81±21.99 | 0.16 |
| Glycine | 0.00 | 1.17 | 556.24±527.30 | 97.10±157.17 | 0.17 | – | – |
| – | – |
Placental tissue
The data from placental tissues also revealed a
significant intergroup distinction, which was demonstrated by the
PCA and OPLS-DA score plots (Fig. 4A
and B). The R2Y and Q2 values of the
placental tissue OPLS-DA model were 0.930 and 0.524, respectively;
the reliability of this model also passed a permutation test
(Fig. 4C). According to the
volcano plot (Fig. 4D) and the
resolved results of NMR spectra (Table II), the alteration in valine for
the experimental group was similar to that found in the palatal
tissue, but the levels of glutamate-containing metabolites were
decreased, which was the opposite of what was observed in the
palatal tissues. Certain metabolite levels were abnormal in
placental tissues but normal in palatal tissues. In particular,
succinate levels were lower in the experimental group. Notably,
choline and its primary and secondary metabolites, including
phosphorylcholine, betaine and trimethylamine N-oxide (TMAO), were
also found at reduced levels in the experimental group.
Amniotic fluid
Inherent clustering of groups and the model tests
are shown in Fig. 5. A trend
similar to that in the choline-containing metabolites of the
placental tissue was found in the amniotic fluid. There was a
decline in the experimental group, but the TMAO levels did not show
any significant alterations. Additionally, alanine, creatinine,
taurine, α-glucose and hippurate were present at lower levels in
the experimental group, whereas lactate levels were elevated
(Table III, table only shows
significantly changed metabolites).
 | Table III.Statistical descriptions of
water-soluble metabolites in serum and amniotic fluid. |
Table III.
Statistical descriptions of
water-soluble metabolites in serum and amniotic fluid.
|
| Amniotic fluid | Serum |
|---|
|
|
|
|
|---|
| Metabolites | P-value | VIP value | Mean ± SD
(control) | Mean ± SD
(DXM) | Fold-change | P-value | VIP value | Mean ± SD
(control) | Mean ± SD
(DXM) | Fold-change |
|---|
| Lactate | 0.03 | 4.13 | 156.34±365.95 | 672.69±671.50 | 4.30 | – | – | – | – | – |
| Alanine | 0.05 | 1.01 | 53.13±43.71 | 19.19±33.97 | 0.36 | – | – | – | – | – |
| Glutamine |
|
|
|
|
| 0.01 | 1.48 | 28.56±34.67 | 0.87±2.74 | 0.03 |
| Citrate |
|
|
|
|
| 0.05 | 1.04 | 5.61±8.06 | 23.65±28.33 | 4.21 |
| Creatinine | 0.00 | 1.10 | 25.15±18.93 | 0.63±1.45 | 0.03 | 0.03 | 7.32 | 1.73±3.98 | 714.85±1029.54 | 412.54 |
| Phosphocholine | 0.00 | 1.35 | 34.42±19.91 | 2.57±4.04 | 0.07 | – | – | – | – | – |
|
Taurine/betaine | 0.00 | 1.21 | 34.80±24.25 | 4.37±5.31 | 0.13 | – | – | – | – | – |
| Choline | 0.00 | 1.35 | 55.77±50.54 | 9.08±11.23 | 0.16 | – | – | – |
| – |
| Glycine | 0.00 | 1.40 | 39.26±26.78 | 1.72±4.81 | 0.04 | – | – | – | – | – |
| Hippurate | 0.00 | 1.20 | 52.22±34.88 | 15.26±25.45 | 0.29 | – | – | – | – | – |
| α-glucose | 0.00 | 1.04 | 27.78±18.74 | 4.46±6.17 | 0.16 | – | – | – | – | – |
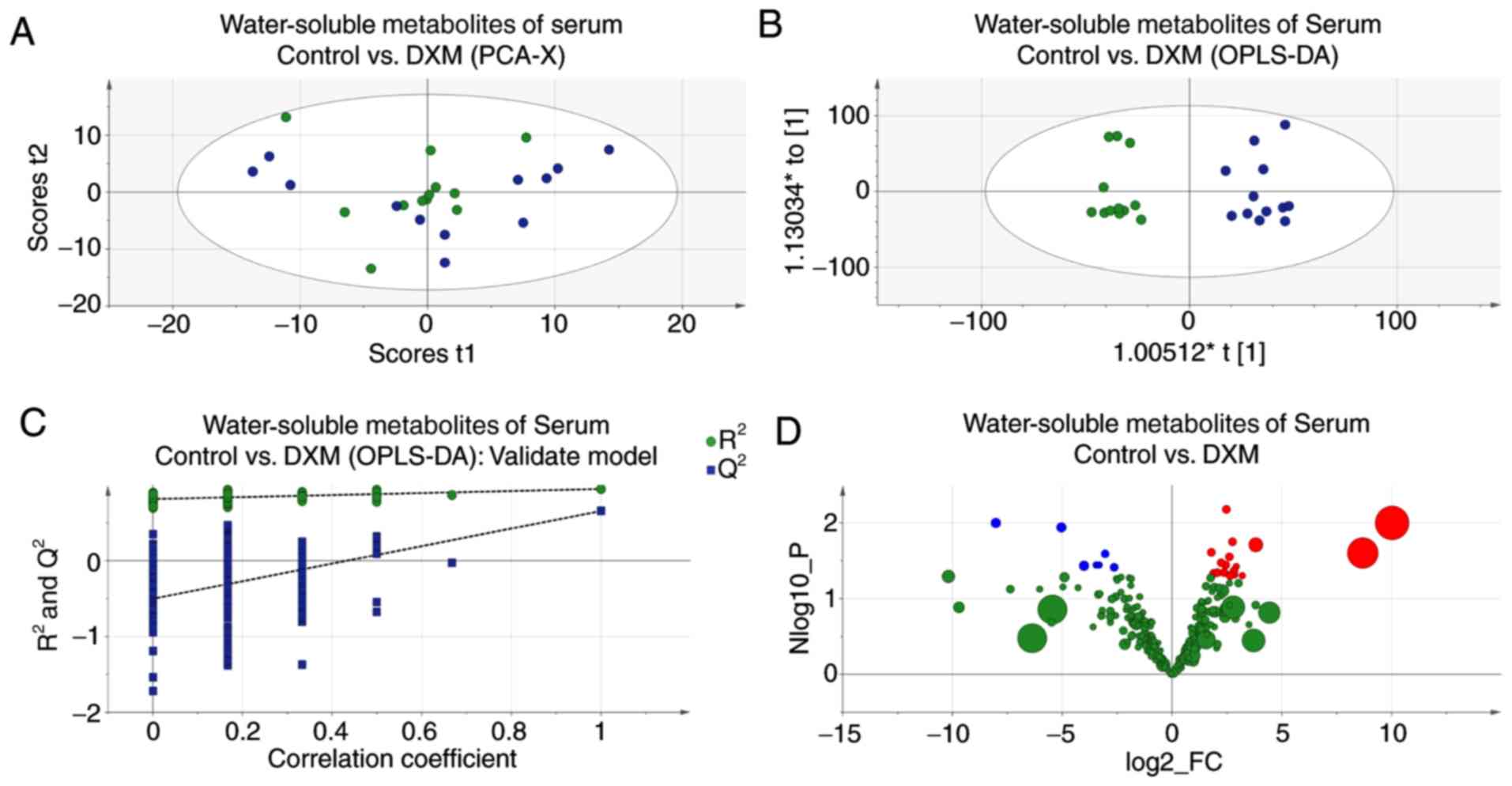
Serum of maternal mice
Although the score plot of PCA showed less
distinction, the model of OPLS-DA was still with satisfactory
credibility and predictability (Fig.
6); three varying metabolites were found in the serum of
maternal mice. Glutamine levels were lower in the experimental
mice, whereas citrate and creatine levels were increased. These
alterations are shown in Table
III.
Lipophilic metabolic alterations in
multiple organs
The results of PCA, OPLS-DA and permutation tests,
and differentiated metabolites of the corresponding tissues are
shown in Fig. 7. In palatal and
placental tissues, cholesterol and lipid levels were significantly
different. Generally, the relative concentrations of cholesterol
and lipids in palatal tissues of the DXM group were higher compared
with those of the control group, except for (CH2)n lipid
concentrations, which were lower. In the placental tissues, the
alterations in cholesterol levels exhibited the opposite trend.
Nevertheless, lipid levels for the different lipid forms varied and
most of them were unsaturated lipids (Table IV).
 | Table IV.Statistical descriptions of
lipid-soluble metabolites in palate and placenta tissues. |
Table IV.
Statistical descriptions of
lipid-soluble metabolites in palate and placenta tissues.
|
|
| Placenta
tissue | Palate tissue |
|---|
|
|
|
|
|
|---|
| Metabolites | Moieties | P-value | VIP value | Mean ± SD
(Control) | Mean ± SD
(DXM) | Fold-change | P-value | VIP value | Mean ± SD
(Control) | Mean ± SD
(DXM) | Fold-change |
|---|
| Cholesterol |
26,27-CH3 | 0.00 | 2.58 | 195.36±145.18 | 23.36±51.59 | 0.74 | 0.00 | 1.01 | 67.47±232.96 | 493.15±203.86 | 7.30 |
|
|
21-CH3 | 0.03 | 1.85 | 160.08±127.68 | 21.26±43.23 | 0.80 | 0.00 | 1.38 | 72.22±249.19 | 640.01±103.68 | 8.86 |
|
|
19-CH3 | 0.00 | 1.03 | 25.61±17.37 | 3.30±8.92 | 0.80 | – | – | – | – | – |
|
|
18-CH3 | 0.00 | 3.18 | 273.41±200.89 | 26.39±70.91 | 0.73 | 0.00 | 1.91 | 175.53±606.38 | 1319.61±169.66 | 7.51 |
| Ω3 | CH3 | 0.00 | 4.73 | 249.26±122.32 | 24.19±85.85 | 0.50 | – | – | – | – | – |
| Lipids | CH3 | 0.00 | 2.83 | 3.24±58.20 | 0.58±53.55 | 17.93 | 0.02 | 1.32 | 977.91±1588.02 | 2140.05±238.69 | 2.19 |
|
|
(CH2)n | – | – | – | – | 0.28 | 0.00 | 2.11 | 1659.69±210.56 | 456.80±237.02 | 0.28 |
|
|
CH2CH=C | 0.00 | 1.78 | 3.01±23.81 | 1.22±19.29 | 7.90 | – | – | – | – | – |
|
|
=CHCH2CH= | 0.02 | 1.16 | 30.63±19.02 | 4.86±14.59 | 0.62 | – | – | – | – | – |
|
| CH=C | 0.03 | 1.28 | 24.58±40.30 | 14.04±18.79 | 1.64 | – | – | – | – | – |
Discussion
The present study identified differences in the
metabolites of DXM-induced CP tissues and other organs using
ultra-high-field strength MRS at 9.4T, which uncovered an
association between maternal metabolic conditions and fetal CP.
Based on these findings, it was hypothesized that a diagnostic
method may be developed, which, using 9.4T MRS, could detect the
levels of metabolites in maternal body fluids.
DXM is commonly used as an anti-inflammatory and
immunosuppressive medication that may occasionally be administered
to pregnant women. Clinical studies have shown that it can cross
the placenta intact and may therefore affect the developing fetus
(21,33–35).
Antenatal pharmacotherapy with corticosteroids can reduce the
morbidity and mortality due to neonatal respiratory distress
syndrome by improving lung maturation (35–37).
As a side effect, corticosteroid administration to pregnant women
can cause their fetuses to develop craniofacial deformities. There
is evidence that systemic administration of DXM during the early
stages of pregnancy is associated with the development of CP in
humans (6,21,34,38,39).
Generally, autoimmune diseases do not affect fertility (40). Treatments with DXM, however, may
have an impact on birth outcomes.
Metabolomics has the ability to detect early
dysregulation of metabolism associated with disease. The present
study investigated these alterations via in vitro testing
using 9.4T MRS involving DXM-induced CP mice, and indicated that
their palatal tissue contained significantly increased levels of
alanine, glutamate and glutamate-containing metabolites compared
with the tissue of mice in the control group. However, the
concentrations of valine tended to be lower compared with those of
normal controls.
Increases in glutamate may be an indication of the
reduced role of amino acids in the formation of the neural system.
Glutamate has been reported to be associated with brain metabolites
and can be measured with a 9.4T MRS imager (41,42).
Glutamate is known to be a major excitatory neurotransmitter in the
central nervous system and is closely associated with a number of
nervous system diseases (42,43).
γ-aminobutyric acid (GABA), the principal inhibitory
neurotransmitter in the nervous system, is a product of glutamate
(44). GABA is also considered to
serve a role in the formation of the nervous system (45,46).
Studies have reported that the 67-kDa isoform of glutamic acid
decarboxylase (GAD67) and GAD67-derived GABA are involved in the
formation of the palate (47,48).
Phosphocholine has been studied in regard to its association with
cell membrane phospholipid metabolism (31,48).
The MRS data presented in the current study demonstrated that the
choline and phosphorylcholine levels in the placentas of the
experimental models were decreased significantly compared with
those of the normal controls. This could be interpreted as
reflecting an alteration in the cell membrane metabolism of
subjects. Therefore, the data suggested that a quantitative test of
amino acids, choline and phosphocholine lipids conducted by 9.4T
MRS may serve as a prenatal test to determine potential
craniofacial deformities.
Although they are limited, the results of the
present study are informative and provide a basis for further
studies. More research is required to clarify the interactions of
these metabolites and their effects on multiple organs. The present
study revealed that there is an association between maternal
metabolites and the development of CP, but more studies are
required to address the functions of these amino acids,
specifically phosphocholine and lipids. Furthermore, since the
results of the present study cannot suffice to prove the
feasibility of a prenatal diagnostic method, several additional
studies will be conducted in the future to address this question.
Additionally, the findings of abnormal data were based on a
comparison of experimental models and normal controls. In order to
apply these findings clinically and enable prenatal examinations in
human subjects, the reference values of maternal metabolites in
humans will have to be determined.
In conclusion, the present study demonstrated that
there is an association between the state of maternal metabolites
and the subsequent formation of fetal CP. This finding provides a
foundation for a possible diagnostic method using ultra-high
field-strength MRS to test maternal metabolites, and thus, to
identify fetuses with CP.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81571920), the Natural
Science Foundation of Guangdong Province (grant nos. 2015A030313436
and 2016A030313061) and The Science and Technology Project of
Shantou Shanfuke (grant no. [2012] 165).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YX and WZ developed the study concept, designed the
experiments and conducted the experiment of MRS. ZS and HZ
performed software analysis and conducted the experiment of MRS. JZ
and LS interpreted the data. WL, XZ and JC performed investigations
and conducted the experiment of MRS. ST developed the study
concept, designed the experiments, and agreed to be accountable for
all aspects of this study.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Shantou University Medical College (Shantou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mossey PA, Little J, Munger RG, Dixon MJ
and Shaw WC: Cleft lip and palate. Lancet. 374:1773–1785. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watkins SE, Meyer RE, Strauss RP and
Aylsworth AS: Classification, epidemiology, and genetics of
orofacial clefts. Clin Plast Surg. 41:149–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang Q, Li L, Jin C, Lee JM and Jung HS:
Role of region-distinctive expression of Rac1 in regulating
fibronectin arrangement during palatal shelf elevation. Cell Tissue
Res. 361:857–868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bush JO and Jiang R: Palatogenesis:
Morphogenetic and molecular mechanisms of secondary palate
development. Development. 139:231–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozmen A, Unek G and Korgun ET: Effect of
glucocorticoids on mechanisms of placental angiogenesis. Placenta.
52:41–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bandoli G, Palmsten K, Forbess Smith CJ
and Chambers CD: A review of systemic corticosteroid use in
pregnancy and the risk of select pregnancy and birth outcomes.
Rheum Dis Clin North Am. 43:489–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bauer MK, Harding JE, Bassett NS, Breier
BH, Oliver MH, Gallaher BH, Evans PC, Woodall SM and Gluckman PD:
Fetal growth and placental function. Mol Cell Endocrinol.
140:115–120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jahanbin A, Shadkam E, Miri HH, Shirazi AS
and Abtahi M: Maternal folic acid supplementation and the risk of
oral clefts in offspring. J Craniofac Surg. 29:e534–e541. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suhl J, Romitti PA, Cao Y, Rocheleau CM,
Burns TL, Conway K, Rajaraman P, Agopian AJ and Stewart P; National
Birth Defects Prevention Study, : Maternal occupational cadmium
exposure and nonsyndromic orofacial clefts. Birth Defects Res.
110:603–609. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waller DK, Hashmi SS, Hoyt AT, Duong HT,
Tinker SC, Gallaway MS, Olney RS, Finnell RH, Hecht JT and Canfield
MA; National Birth Defects Prevention Study, : Maternal report of
fever from cold or flu during early pregnancy and the risk for
noncardiac birth defects, National Birth Defects Prevention Study,
1997–2011. Birth Defects Res. 110:342–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bax BE and Bloxam DL: Energy metabolism
and glycolysis in human placental trophoblast cells during
differentiation. Biochim Biophys Acta. 1319:283–292. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baardman ME, Kerstjens-Frederikse WS,
Berger RM, Bakker MK, Hofstra RM and Plösch T: The role of
maternal-fetal cholesterol transport in early fetal life: Current
insights. Biol Reprod. 88:242013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cetin I, Ronzoni S, Marconi AM, Perugino
G, Corbetta C, Battaglia FC and Pardi G: Maternal concentrations
and fetal-maternal concentration differences of plasma amino acids
in normal and intrauterine growth-restricted pregnancies. Am J
Obstet Gynecol. 174:1575–1583. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dubé E, Gravel A, Martin C, Desparois G,
Moussa I, Ethier-Chiasson M, Forest JC, Giguère Y, Masse A and
Lafond J: Modulation of fatty acid transport and metabolism by
maternal obesity in the human full-term placenta. Biol Reprod.
87:14, 1–11. 2012. View Article : Google Scholar
|
|
15
|
Gil-Sánchez A, Koletzko B and Larqué E:
Current understanding of placental fatty acid transport. Curr Opin
Clin Nutr Metab Care. 15:265–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paolini CL, Marconi AM, Ronzoni S, Di Noio
M, Fennessey PV, Pardi G and Battaglia FC: Placental transport of
leucine, phenylalanine, glycine, and proline in intrauterine
growth-restricted pregnancies. J Clin Endocrinol Metab.
86:5427–5432. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skuladottir H, Wilcox AJ, Ma C, Lammer EJ,
Rasmussen SA, Werler MM, Shaw GM and Carmichael SL: Corticosteroid
use and risk of orofacial clefts. Birth Defects Res A Clin Mol
Teratol. 100:499–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinsky L and Digeorge AM: Cleft palate in
the mouse: A teratogenic index of gluocorticoid potency. Science.
147:402–403. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu X, Gao JH, Liao YJ, Tang SJ and Lu F:
Dexamethasone alters epithelium proliferation and survival and
suppresses Wnt/β-catenin signaling in developing cleft palate. Food
Chem Toxicol. 56:67–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lan SJ, Yang XG, Chen Z, Yang TY, Xiang
CH, Zhang D, Li YX and Rong L: Role of GATA-6 and bone
morphogenetic protein-2 in dexamethasone-induced cleft palate
formation in institute of cancer research mice. J Craniofac Surg.
27:1600–1605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carmichael SL, Shaw GM, Ma C, Werler MM,
Rasmussen SA and Lammer EJ; National Birth Defects Prevention
Study, : Maternal corticosteroid use and orofacial clefts. Am J
Obstet Gynecol. 197:585.e1–7. 2007. View Article : Google Scholar
|
|
22
|
Pratt RM: Receptor-dependent mechanisms of
glucocorticoid and dioxin-induced cleft palate. Environ Health
Perspect. 61:35–40. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laura H, Wilson SF, Lunte CE and Larive
CK: Concentration profiling in rat tissue by high-resolution
magic-angle spinning NMR spectroscopy: Investigation of a model
drug. Anal Chem. 77:2978–2984. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holmes E, Nicholls AW, Lindon JC, Connor
SC, Connelly JC, Haselden JN, Damment SJ, Spraul M, Neidig P and
Nicholson JK: Chemometric models for toxicity classification based
on NMR spectra of biofluids. Chem Res Toxicol. 13:471–478. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bogner W, Gruber S, Trattnig S and Chmelik
M: High-resolution mapping of human brain metabolites by free
induction decay (1)H MRSI at 7 T. NMR Biomed. 25:873–882. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin L, Cao B, Xu Z, Sui Y, Chen J, Luan Q,
Yang R, Li S and Li KF: In vivo HMRS and lipidomic profiling
reveals comprehensive changes of hippocampal metabolism during
aging in mice. Biochem Biophys Res Commun. 470:9–14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nassirpour S, Chang P and Henning A: High
and ultra-high resolution metabolite mapping of the human brain
using 1H FID MRSI at 9.4T. Neuroimage. 168:211–221.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao R, Tkac I, Schmidt AT and Georgieff
MK: Fetal and neonatal iron deficiency causes volume loss and
alters the neurochemical profile of the adult rat hippocampus. Nutr
Neurosci. 14:59–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song F, Wu W, Qian Z, Zhang G and Cheng Y:
Assessment of the placenta in intrauterine growth restriction by
diffusion-weighted imaging and proton magnetic resonance
spectroscopy. Reprod Sci. 24:575–581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou J, Xu B, Huang J, Jia X, Xue J, Shi
X, Xiao L and Li W: 1H NMR-based metabonomic and pattern
recognition analysis for detection of oral squamous cell carcinoma.
Clin Chim Acta. 401:8–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin F, Shen Z, Peng L, Wu R, Hu X, Zhang G
and Tang S: Metabolic characterization of all-trans-retinoic acid
(ATRA)-induced craniofacial development of murine embryos using in
vivo proton magnetic resonance spectroscopy. PLoS One.
9:e960102014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Czeizel AE and Rockenbauer M:
Population-based case-control study of teratogenic potential of
corticosteroids. Teratology. 56:335–340. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Runnard Heimel PJ, Franx A, Schobben
AF, Huisjes AJ, Derks JB and Bruinse HW: Corticosteroids,
pregnancy, and hellp syndrome: A review. Obstet Gynecol Surv.
60:57–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pradat P, Robert-Gnansia E, Di Tanna GL,
Rosano A, Lisi A and Mastroiacovo P; Contributors to the MADRE
database, : First trimester exposure to corticosteroids and oral
clefts. Birth Defects Res A Clin Mol Teratol. 67:968–970. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Regazzi FM, Silva LCG, Lúcio CF, Veiga
GAL, Angrimani DSR, Kishi D, Barbosa MMM and Vannucchi CI:
Influence of prenatal maternal corticosteroid therapy on clinical
and metabolic features and pulmonary function of preterm newborn
puppies. Theriogenology. 97:179–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmidt AF, Kemp MW, Rittenschober-Böhm J,
Kannan PS, Usuda H, Saito M, Furfaro L, Watanabe S, Stock S, Kramer
BW, et al: Low-dose betamethasone-acetate for fetal lung maturation
in preterm sheep. Am J Obstet Gynecol. 218:132.e1–132.e9. 2018.
View Article : Google Scholar
|
|
37
|
Travers CP, Carlo WA, McDonald SA, Das A,
Bell EF, Ambalavanan N, Jobe AH, Goldberg RN, D'Angio CT, Stoll BJ,
et al: Mortality and pulmonary outcomes of extremely preterm
infants exposed to antenatal corticosteroids. Am J Obstet Gynecol.
218:130.e1–130 e13. 2018. View Article : Google Scholar
|
|
38
|
Xiao WL, Liu XY, Liu YS, Zhang DZ and Xue
LF: The relationship between maternal corticosteroid use and
orofacial clefts-a meta-analysis. Reprod Toxicol. 69:99–105. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitchell K, Kaul M and Clowse ME: The
management of rheumatic diseases in pregnancy. Scand J Rheumatol.
39:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dobberthien BJ, Tessier AG and Yahya A:
Improved resolution of glutamate, glutamine and γ-aminobutyric acid
with optimized point-resolved spectroscopy sequence timings for
their simultaneous quantification at 9.4 T. NMR Biomed. 31:2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ramadan S Lin A and Stanwell P: Glutamate
and glutamine: A review of in vivo MRS in the human brain. NMR
Biomed. 26:1630–1646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meldrum BS: Glutamate as a
neurotransmitter in the brain- review of physiology and pathology.
J Nutr 130 (4S Suppl). 1007S–1015S. 2000.
|
|
43
|
Patel AB, de Graaf RA, Mason GF, Rothman
DL, Shulman RG and Behar KL: The contribution of GABA to glutamate
glutamine cycling and energy metabolism in the rat cortex in vivo.
Proc Natl Acad Sci USA. 102:5588–5593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mi D, Li Z, Lim L, Li M, Moissidis M, Yang
Y, Gao T, Hu TX, Pratt T, Price DJ, et al: Early emergence of
cortical interneuron diversity in the mouse embryo. Science.
360:81–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li K and Xu E: The role and the mechanism
of gamma-aminobutyric acid during central nervous system
development. Neurosci Bull. 24:195–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Asada H, Kawamura Y, Maruyama K, Kume H,
Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T and Obata K: Cleft
palate and decreased brain gamma-aminobutyric acid in mice lacking
the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad
Sci USA. 94:6496–6499. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kakizaki T, Oriuchi N and Yanagawa Y:
GAD65/GAD67 double knockout mice exhibit intermediate severity in
both cleft palate and omphalocele compared with GAD67 knockout and
VGAT knockout mice. Neuroscience. 288:86–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Begley JK, Redpath TW, Bolan PJ and
Gilbert FJ: In vivo proton magnetic resonance spectroscopy of
breast cancer: A review of the literature. Breast Cancer Res.
14:2072012. View Article : Google Scholar : PubMed/NCBI
|