|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui XB, Zhang SM, Xu YX, Dang HW, Liu CX,
Wang LH, Yang L, Hu JM, Liang WH, Jiang JF, et al: PFN2, a novel
marker of unfavorable prognosis, is a potential therapeutic target
involved in esophageal squamous cell carcinoma. J Transl Med.
14:1372016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma S, Bao JY, Kwan PS, Chan YP, Tong CM,
Fu L, Zhang N, Tong AH, Qin YR, Tsao SW, et al: Identification of
PTK6, via RNA sequencing analysis, as a suppressor of esophageal
squamous cell carcinoma. Gastroenterology. 143:675–686 e612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
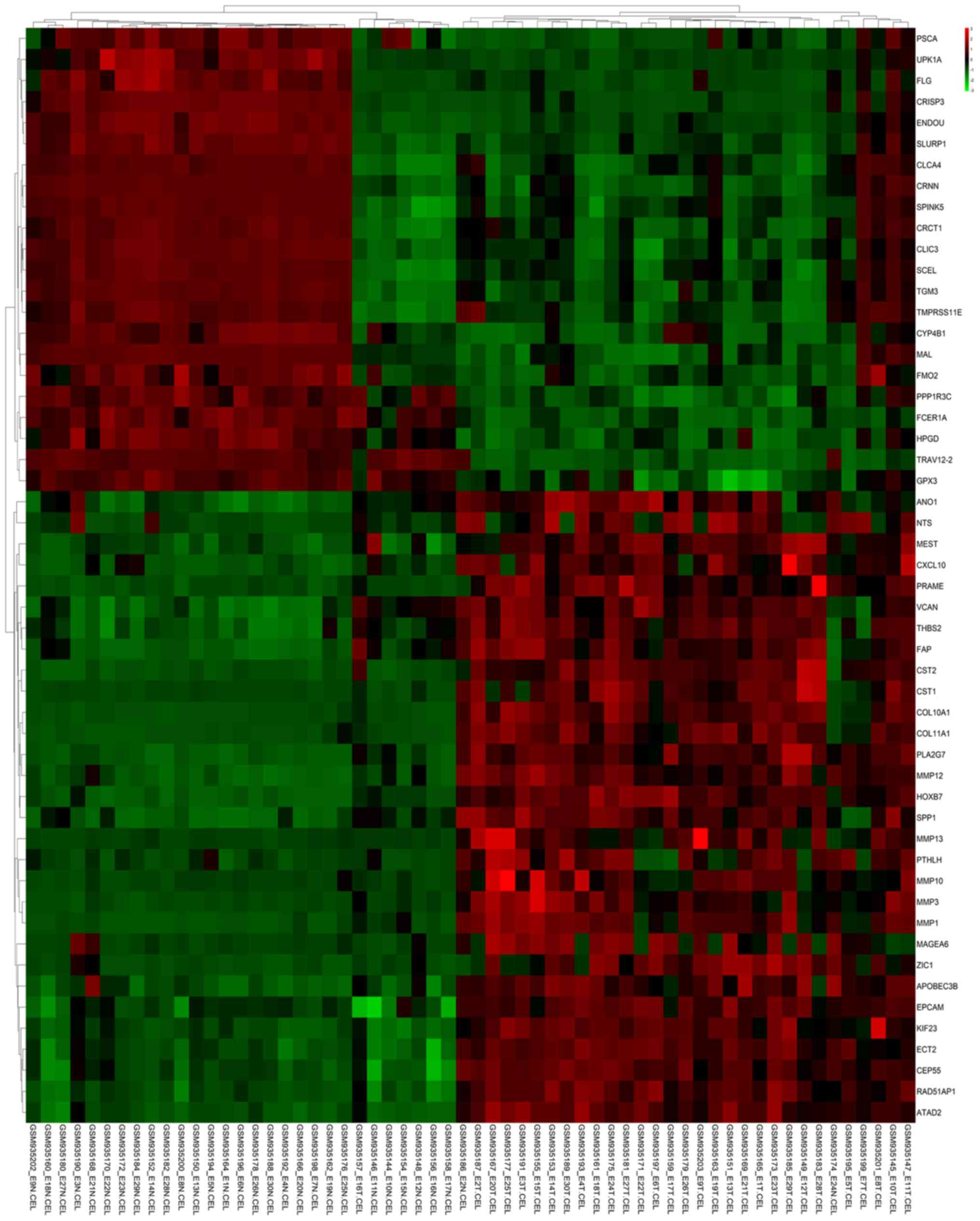
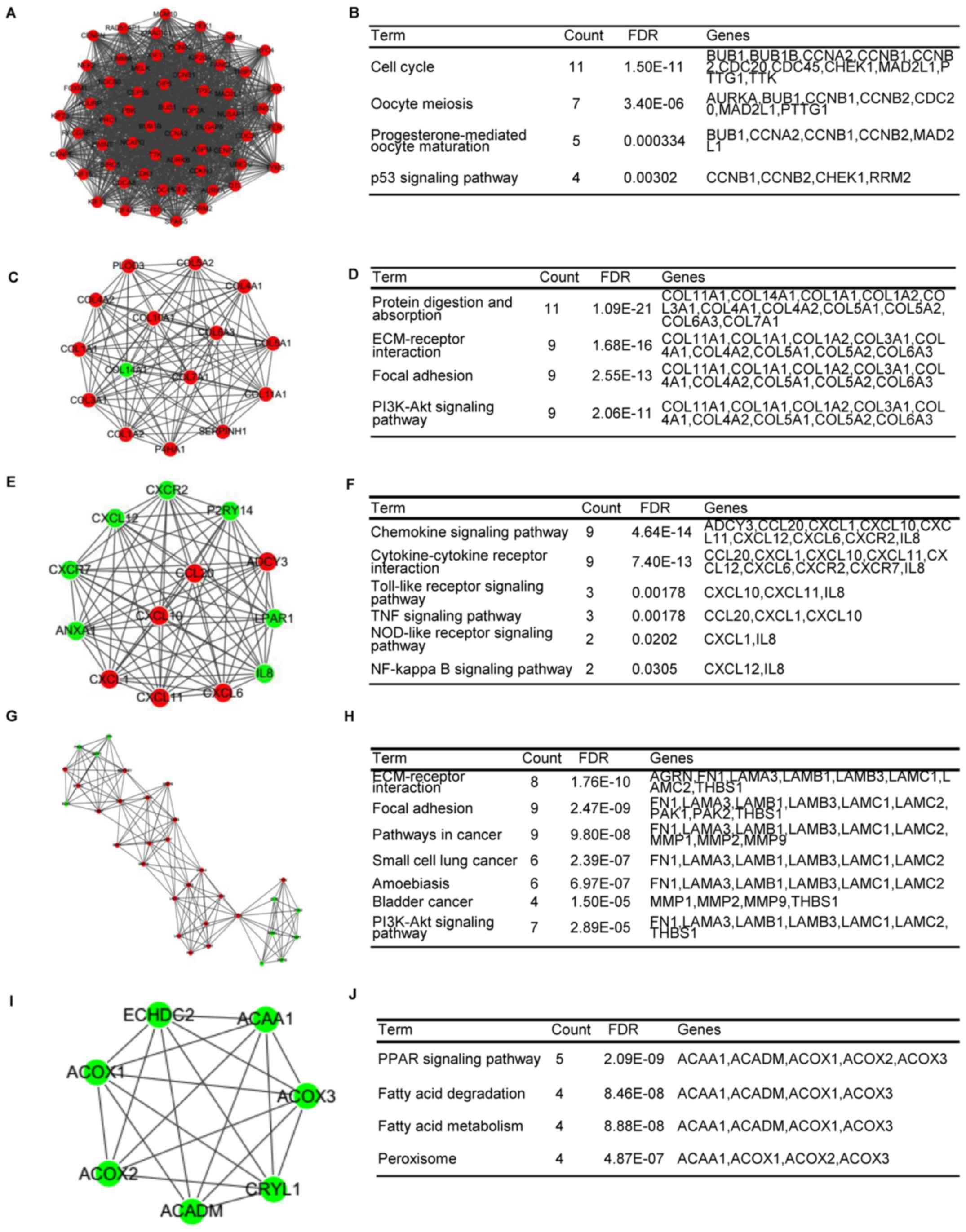
4
|
Zhang J, Li S, Shang Z, Lin S, Gao P,
Zhang Y, Hou S, Mo S, Cao W, Dong Z, et al: Targeting the
overexpressed ROC1 induces G2 cell cycle arrest and apoptosis in
esophageal cancer cells. Oncotarget. 8:29125–29137. 2017.PubMed/NCBI
|
|
5
|
Hers I, Vincent EE and Tavare JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durán-Prado M, Gahete MD, Hergueta-Redondo
M, Martínez-Fuentes AJ, Cordóba-Chacón J, Palacios J,
Gracia-Navarro F, Moreno-Bueno G, Malagón MM, Luque RM and Castaño
JP: The new truncated somatostatin receptor variant sst5TMD4 is
associated to poor prognosis in breast cancer and increases
malignancy in MCF-7 cells. Oncogene. 31:2049–2061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Lu Y, Wang J, Koch AE, Zhang J and
Taichman RS: CXCR6 induces prostate cancer progression by the
AKT/mammalian target of rapamycin signaling pathway. Cancer Res.
68:10367–10376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuoka T and Yashiro M: The role of
PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers (Basel).
6:1441–1463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okawa T, Michaylira CZ, Kalabis J, Stairs
DB, Nakagawa H, Andl CD, Johnstone CN, Klein-Szanto AJ, El-Deiry
WS, Cukierman E, et al: The functional interplay between EGFR
overexpression, hTERT activation, and p53 mutation in esophageal
epithelial cells with activation of stromal fibroblasts induces
tumor development, invasion, and differentiation. Genes Dev.
21:2788–2803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu N, Wang C, Clifford RJ, Yang HH, Su H,
Wang L, Wang Y, Xu Y, Tang ZZ, Ding T, et al: Integrative genomics
analysis of genes with biallelic loss and its relation to the
expression of mRNA and micro-RNA in esophageal squamous cell
carcinoma. BMC Genomics. 16:7322015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Altermann E and Klaenhammer TR:
Pathwayvoyager: Pathway mapping using the kyoto encyclopedia of
genes and genomes (KEGG) database. BMC Genomics. 6:602005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC and Lempicki RA:
DAVID bioinformatics resources: Expanded annotation database and
novel algorithms to better extract biology from large gene lists.
Nucleic Acids Res. 35:W169–W175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui XB, Pang XL, Li S, Jin J, Hu JM, Yang
L, Liu CX, Li L, Wen SJ, Liang WH, et al: Elevated expression
patterns and tight correlation of the PLCE1 and NF-κB signaling in
Kazakh patients with esophageal carcinoma. Med Oncol. 31:7912014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holzman LB, Marks RM and Dixit VM: A novel
immediate-early response gene of endothelium is induced by
cytokines and encodes a secreted protein. Mol Cell Biol.
10:5830–5838. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abraham S, Knapp DW, Cheng L, Snyder PW,
Mittal SK, Bangari DS, Kinch M, Wu L, Dhariwal J and Mohammed SI:
Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary
bladder. Clin Cancer Res. 12:353–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura R, Kataoka H, Sato N, Kanamori M,
Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T, et
al: EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci.
96:42–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto H, Tei M, Uemura M, Takemasa I,
Uemura Y, Murata K, Fukunaga M, Ohue M, Ohnishi T, Ikeda K, et al:
Ephrin-A1 mRNA is associated with poor prognosis of colorectal
cancer. Int J Oncol. 42:549–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wada H, Yamamoto H, Kim C, Uemura M, Akita
H, Tomimaru Y, Hama N, Kawamoto K, Kobayashi S, Eguchi H, et al:
Association between ephrin-A1 mRNA expression and poor prognosis
after hepatectomy to treat hepatocellular carcinoma. Int J Oncol.
45:1051–1058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Désert R, Mebarki S, Desille M, Sicard M,
Lavergne E, Renaud S, Bergeat D, Sulpice L, Perret C, Turlin B, et
al: ‘Fibrous nests’ in human hepatocellular carcinoma express a
Wnt-induced gene signature associated with poor clinical outcome.
Int J Biochem Cell Biol. 81:195–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salem O, Erdem N, Jung J, Münstermann E,
Wörner A, Wilhelm H, Wiemann S and Körner C: The highly expressed
5′isomiR of hsa-miR-140-3p contributes to the tumor-suppressive
effects of miR-140 by reducing breast cancer proliferation and
migration. BMC Genomics. 17:5662016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alabsi AM, Lim KL, Paterson IC, Ali-Saeed
R and Muharram BA: Cell cycle arrest and apoptosis induction via
modulation of mitochondrial integrity by Bcl-2 family members and
caspase dependence in Dracaena cinnabari-treated H400 human oral
squamous cell carcinoma. Biomed Res Int. 2016:49040162016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He Y, Liu J, Zhao Z and Zhao H:
Bioinformatics analysis of gene expression profiles of esophageal
squamous cell carcinoma. Dis Esophagus. 30:1–8. 2017. View Article : Google Scholar
|
|
27
|
Chan TF, Poon A, Basu A, Addleman NR, Chen
J, Phong A, Byers PH, Klein TE and Kwok PY: Natural variation in
four human collagen genes across an ethnically diverse population.
Genomics. 91:307–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yue Y, Song M, Qiao Y, Li P, Yuan Y, Lian
J, Wang S and Zhang Y: Gene function analysis and underlying
mechanism of esophagus cancer based on microarray gene expression
profiling. Oncotarget. 8:105222–105237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abdel-Fatah TM, Middleton FK, Arora A,
Agarwal D, Chen T, Moseley PM, Perry C, Doherty R, Chan S, Green
AR, et al: Untangling the ATR-CHEK1 network for prognostication,
prediction and therapeutic target validation in breast cancer. Mol
Oncol. 9:569–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim MK, James J and Annunziata CM:
Topotecan synergizes with CHEK1 (CHK1) inhibitor to induce
apoptosis in ovarian cancer cells. BMC Cancer. 15:1962015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parikh RA, Appleman LJ, Bauman JE,
Sankunny M, Lewis DW, Vlad A and Gollin SM: Upregulation of the
ATR-CHEK1 pathway in oral squamous cell carcinomas. Genes
Chromosomes Cancer. 53:25–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiang W, Wu X, Huang C, Wang M, Zhao X,
Luo G, Li Y, Jiang G, Xiao X and Zeng F: PTTG1 regulated by
miR-146-3p promotes bladder cancer migration, invasion, metastasis
and growth. Oncotarget. 8:664–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng W, Xiaoyan X, Shenglei L, Hongtao L
and Guozhong J: PTTG1 cooperated with GLI1 leads to
epithelial-mesenchymal transition in esophageal squamous cell
cancer. Oncotarget. 8:92388–92400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaistha BP, Honstein T, Müller V, Bielak
S, Sauer M, Kreider R, Fassan M, Scarpa A, Schmees C, Volkmer H, et
al: Key role of dual specificity kinase TTK in proliferation and
survival of pancreatic cancer cells. Br J Cancer. 111:1780–1787.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miao R, Wu Y, Zhang H, Zhou H, Sun X,
Csizmadia E, He L, Zhao Y, Jiang C, Miksad RA, et al: Utility of
the dual-specificity protein kinase TTK as a therapeutic target for
intrahepatic spread of liver cancer. Sci Rep. 6:331212016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fu X, Chen G, Cai ZD, Wang C, Liu ZZ, Lin
ZY, Wu YD, Liang YX, Han ZD, Liu JC and Zhong WD: Overexpression of
BUB1B contributes to progression of prostate cancer and predicts
poor outcome in patients with prostate cancer. Onco Targets Ther.
9:2211–2220. 2016.PubMed/NCBI
|
|
37
|
Chen H, Lee J, Kljavin NM, Haley B, Daemen
A, Johnson L and Liang Y: Requirement for BUB1B/BUBR1 in tumor
progression of lung adenocarcinoma. Genes Cancer. 6:106–118.
2015.PubMed/NCBI
|
|
38
|
Zhang Y, Xu Y, Li Z, Zhu Y, Wen S, Wang M,
Lv H, Zhang F and Tian Z: Identification of the key transcription
factors in esophageal squamous cell carcinoma. J Thoracic Dis.
10:148–161. 2018. View Article : Google Scholar
|