Introduction
Breast cancer (BC) is the most common malignancy in
women, and one of the three most common cancer types worldwide
(1). Early-stage breast cancer
without detectable distant metastases is a potentially curable
disease (1). The current in
vivo diagnostic tools for the detection of early-stage BC
include mammography and ultrasound (2). Due to the limited sensitivity of
traditional diagnostic methods, certain micro-molecules such as
microRNAs (miRNAs) have been considered as potential biomarkers for
early-stage BC diagnosis (3,4).
miRNAs are a class of small, evolutionarily
conserved, non-coding RNAs that are 18–25 nucleotides in length
(5). miRNAs are capable of
inducing translational repression or degradation of target mRNAs by
binding to their 3′ untranslated regions (3′UTRs), and participate
in almost all key cellular processes (5). Extracellular vesicle-packaged miRNAs
are a class of circulating miRNAs that are packaged into
extracellular vesicle and can be detected in the serum (6,7).
Extracellular vesicle-packaged miRNAs have systemic effects in
primary breast cancer and contribute to processes within the blood
circulation (8,9).
Extracellular vesicle-packaged miRNAs have the
potential to serve as biomarkers for evaluating breast cancer, and
numerous extracellular vesicle-packaged miRNAs indicative of breast
cancer have been identified (10,11).
For example, a systematic review and meta-analysis suggested that
miRNA-21 was a potential biomarker for the early diagnosis of
breast cancer, with high sensitivity and specificity (12). In addition, the combination of five
serum miRNAs, including miRNA-1248, miRNA-1307-3p, miRNA-4634,
miRNA-6861-5p and miRNA-6875-5p, has been reported to be able to
detect early stage breast cancer with a sensitivity as high as 98%
(13). In addition, serum
extracellular vesicle-packaged miRNA-373 was reported to be
associated with more aggressive breast cancer (14).
As a potential biomarker for early-stage breast
cancer diagnosis, plasma extracellular vesicle-packaged miRNA
detection is a non-invasive procedure when compared with diagnostic
procedures involving tissue biomarkers.
In order to screen potential extracellular
vesicle-packaged miRNAs for early-stage breast cancer diagnosis,
the present study attempted to analysis the extracellular
vesicle-packaged miRNA expression profile in blood and tissue
clinical samples. Specifically, the profiles of extracellular
vesicle-packaged miRNAs extracted from the plasma of patients with
early-stage breast cancer were compared with those of the control
group. The profiles of miRNAs extracted from cancer tissues of
patients with early-stage BC were also compared with the normal
breast tissues of the control group. The four sets of data were
analyzed in order to identify the extracellular vesicle-packaged
miRNAs that can be used as diagnosis biomarkers of early-stage
breast cancer.
Materials and methods
Patient cohorts
In the BC group, plasma samples were collected from
patients with Stage I breast cancer (T1N0M0) (1), who did not have other systemic
diseases or cancer at the time of their initial diagnosis, prior to
receiving any treatment. BC tissues from these patients were
collected at the time of surgery. All the patients involved were
women. The age range of patients was 20–63 years.
In the control group, plasma samples were collected
from patients with benign breast disease, including breast
fibroadenoma and mammary adenosis; these patients also did not have
other systemic diseases or cancer at the time of collection. The
plasma samples were collected at the time of diagnosis prior to
surgery, and normal breast tissue from the same patient was
collected during surgery, which served as the benign lesion sample.
The diagnoses of the patients were confirmed by clinical and
pathological findings. A summary of the clinicopathological
characteristics of control and BC groups is presented in Table I. All experiments were performed in
accordance with the approved guidelines and regulations of the
Shenzhen People's Hospital. All experimental protocols were
approved by the Ethics Committees of Shenzhen People's Hospital.
Written informed consent was obtained from all participants, in
accordance with the Declaration of Helsinki.
 | Table I.Characteristics of the study
participants. |
Table I.
Characteristics of the study
participants.
| Factor | Breast cancer
group, n=12 | Control group,
n=10 |
|---|
| No. of blood
samples | 12 | 10 |
| No. of tissue
samples | 5 | 7 |
| Age, years | 46.83±7.70 | 35.80±12.45 |
| Tumor size
(cm) | 1.29±0.37 | – |
| Estrogen receptor
status |
|
|
|
Positive | 10 (83%) | – |
|
Negative | 2 (17%) | – |
| Progesterone
receptor status |
|
|
|
Positive | 8 (67%) | – |
|
Negative | 4 (33%) | – |
| HER2 status |
|
|
|
Positive | 3 (25%) | – |
|
Negative | 9 (75%) | – |
| Ki-67 index
(%) |
|
|
|
<10% | 2 (16%) | – |
|
10–20% | 5 (45%) | – |
|
>20% | 5 (42%) | – |
| Molecular
subtype |
|
|
| Luminal
A | 5 (42%) | – |
| HER2
positive | – | – |
|
Triple-negative breast
cancer | – | – |
| Luminal
B | 7 (58%) | – |
Sample preparation
Venous blood samples (6 ml/patient) were collected
in tubes containing 10.8 mg EDTA as the anticoagulant (BD
Biosciences). Plasma was separated from blood cells by
centrifugation at 1,000 × g for 10 min at 4°C. The plasma layer was
further centrifuged at 16,000 × g for 10 min at 4°C prior to
extracellular vesicle extraction. Tissue samples were identified by
a clinical pathologist and cut into 1 cm3 sections for
storage at −80°C until further processing.
Extraction of plasma extracellular
vesicles
Extracellular vesicles were isolated from the plasma
using ExoQuick precipitation (System Biosciences, LLC), according
to the manufacturer's protocol. Briefly, the extracellular vesicles
were precipitated by incubation; the ExoQuick extracellular vesicle
precipitation reagent was added at 4°C for 60 min. The
extracellular vesicle pellet was collected by centrifugation at
1,500 × g for 10 min at 4°C and resuspended in 10 mM PBS to four
times the original plasma volume.
Transmission electron microscopy
A copper mesh was placed on a clean wax plate and
100 µl of the extracellular vesicle suspension was added. After 4
min, the copper mesh was removed and placed in 2% phosphotungstic
acid for 5 min at room temperature. The mesh was dried on a filter
paper. The mesh was examined under a transmission electron
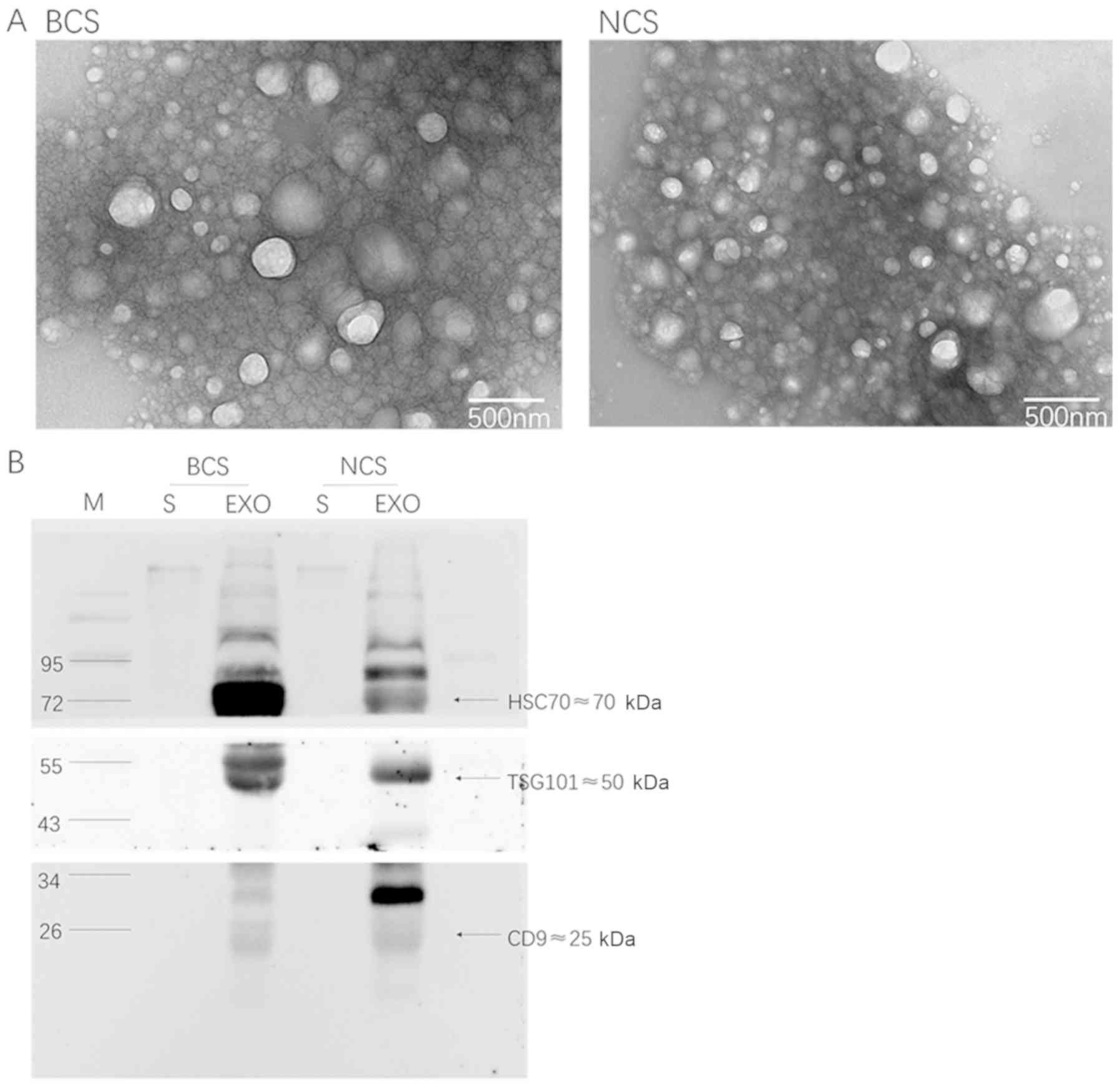
microscope (HT7700; Hitachi, Ltd.) and images were captured.
Western blot analysis
The extracellular vesicle pellet was dissolved in
RIPA protein lysis buffer (Sangon Biotech, Co., Ltd.), and the
protein concentration was determined using the bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.). The proteins
were separated on an SDS-PAGE gel (the concentrations of separating
gel and stacking gel were 12 and 5% respectively). A total of 30 µg
of protein was loaded per lane, while the protein ladder (Thermo
Fisher Scientific, Inc.; 26616) was loaded 3 µl per lane. The
proteins were electrotransferred onto a PVDF membrane. Membranes
were blocked with 5% skimmed milk in TBST at room temperature for 1
h. The membrane was then incubated with the following primary
antibodies at 4°C overnight: Tumor susceptibility gene 101 protein
(TSG101; Abcam; ab83, dilution 1:1,000), CD9 (Abcam; ab92762,
dilution 1:1,000) and heat shock cognate 70 protein (HSC70; Santa
Cruz; sc7298, dilution 1:300). The membrane was then incubated with
the HRP-conjugated corresponding secondary antibodies (Proteintech;
SA00001-1, dilution 1:1,000 and CST; 7074P2, dilution 1:10,000) at
room temperature for 1 h and treated with enhanced
chemiluminescence detection reagents (4A Biotech; 4AW012-100). The
specific protein bands were visualized and recorded on film using a
chemiluminescence imaging system (2000; Tanon).
RNA preparation and miRNA
sequencing
Total RNA was extracted from the extracellular
vesicles and tissues using TRIzol® reagent (Life
Technologies; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The purity of the isolated RNA was
determined according to the ratio of radiation absorbance at 260 nm
to that at 280 nm, using a Nanodrop ND-1000 system (Thermo Fisher
Scientific, Inc.); 200 ng total RNA was used for the construction
of the miRNA library using the NEBNext Multiplex Small RNA Library
Prep Set for Illumina kit (New England BioLabs, Inc.). Each RNA
sample underwent adaptor ligation, cDNA synthesis and polymerase
chain reaction amplification. The quality of the RNA library was
verified by Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.).
The library was sequenced on the Hiseq 2500 (Illumina Inc.,).
RNA-seq datasets were generated with 250 bp paired-end reads with a
10 million-read sequencing depth of the multiplexed samples.
miRNA data processing
Fastx_toolkit (http://hannonlab.cshl.edu/fastx_toolkit/) was used to
remove low quality reads from the sequencing data. A quality
statistic was performed using FastQC software (version 0.13.0)
(https://www.bioinformatics.babraham.ac.uk/projects/fastqc/)
as a quality control tool for the high-throughput sequencing data.
All reads were compared with the reference sequence, human mature
miRNA from miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php),
using the FastQC high precision sequence alignment algorithm
(15). Gene expression was
quantified in reads per kilobase of transcript per million mapped
reads (RPKM) (16,17).
Bioinformatics analysis
Differential miRNA expression analysis was
performed. EdgeR was used to determine the differentially expressed
miRNAs (18). P<0.05 and the
absolute value of log2 (FoldChange) <1 (|log2
(FoldChange)|>1) were considered to indicate a statistically
significant difference. Clustering analysis was performed using a
hierarchical clustering method based on the expression level of
differentially expressed miRNAs [log10(RPKM+1)]. miRTarBase
(http://mirtarbase.mbc.nctu.edu.tw/php/index.php) was
used to identify potential human miRNA target genes. Gene Ontology
(GO) analysis was performed using topGO (version 2.18.0)
(https://bioconductor.org/packages/release/bioc/html/topGO.html)
to investigate the biological processes, cellular components and
specific molecular functions of the identified differentially
expressed coding genes. Pathway analysis was performed using KOBAS
(kobas2.0-20150126) (18) to
determine the involvement of co-expressed genes in different
biological pathways according to the Kyoto Encyclopedia of Genes
and Genomes (KEGG). The hypergeometric test was used for
statistical analysis. miRNA-target gene-network analysis was
performed using mirWalk (http://mirwalk.umm.uni-heidelberg.de/) (19). A receiver operating characteristic
(ROC) curve was generated to calculate the relationship between
sensitivity and specificity for the disease group compared with the
healthy controls. Data analysis and ROC curve analysis was
performed using SPSS (version 19.0; IBM Corp.) and GraphPad Prism
(version 7; GraphPad Software, Inc.).
The Kaplan-Meier method was used to compute the
survival analyses using OncoLnc (http://oncolnc.org/) and Oncomir (http://www.oncomir.org). OncoLnc is a tool for
interactively exploring survival correlations, and for downloading
clinical data coupled to expression data for mRNAs, miRNAs or long
noncoding RNAs (lncRNAs). OncoLnc contains survival data for 8,647
patients from 21 cancer studies performed by The Cancer Genome
Atlas (TCGA; http://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga),
along with miRNAs from TCGA. OncomiR is an online resource for
exploring miRNA dysregulation in cancer. Using combined miRNA-seq,
RNA-seq and clinical data from TCGA, it is able systematically
perform statistical analyses to identify dysregulated miRNAs that
are associated with tumor development and progression in the
majority of cancer types (20).
Statistical analysis
The analysis of differentially expressed miRNAs was
evaluated using R studio software (version 3.4.2) with the edgeR
package (21,22). Differential expression was assessed
for each gene using an exact test analogous to Fisher's exact test,
but adapted for over-dispersed data (22). P<0.05 was considered to indicate
a statistically significant difference. Data are expressed as the
median with range, and were analyzed using GraphPad Prism software
(version 7.0). Pearson correlation analysis was performed to assess
the correlation between age and miRNA expression profile using SPSS
(version 19). The scatter plot was drawn using SPSS (version
19).
Results
Extracellular vesicles isolation and
validation
The present study initially assessed the
extracellular vesicles that were isolated from the plasma samples.
Transmission electron microscopy revealed spherical vesicles of
30–100 nm in diameter in all samples (Fig. 1A), suggesting that the present
study had successfully purified extracellular vesicles from the
plasma. According to the standard curve, the mean protein
concentration of extracellular vesicles in the breast cancer group
was 24.16 µg/µl, and was 23.84 µg/µl in the control group. Western
blotting was performed to detect three conventional extracellular
vesicle protein markers, namely TSG101, CD9 and HSC70 (15). With an equal amount of protein
loaded in each lane, these three extracellular vesicle protein
markers were all highly enriched in the isolated extracellular
vesicles relative to the plasma (Fig.
1B). These results confirmed the successful purification of
intact extracellular vesicles from all plasma samples.
Extracellular vesicle-packaged miRNA
expression profiles in breast cancer and control groups
The optical density (OD)260/OD280 value range of
total RNA extracted from extracellular vesicles in each samples
ranged between 1.70 and 2.00, which was indicative of high RNA
purity. Differential miRNA expression analysis was performed to
identify the candidate extracellular vesicle-packaged miRNAs. A
series of bioinformatics analyses were performed to reveal the
relationship between extracellular vesicle-packaged miRNAs and the
pathogenesis and progression of early-stage breast cancer. The
edgeR method, based on negative binomial distribution, was adopted.
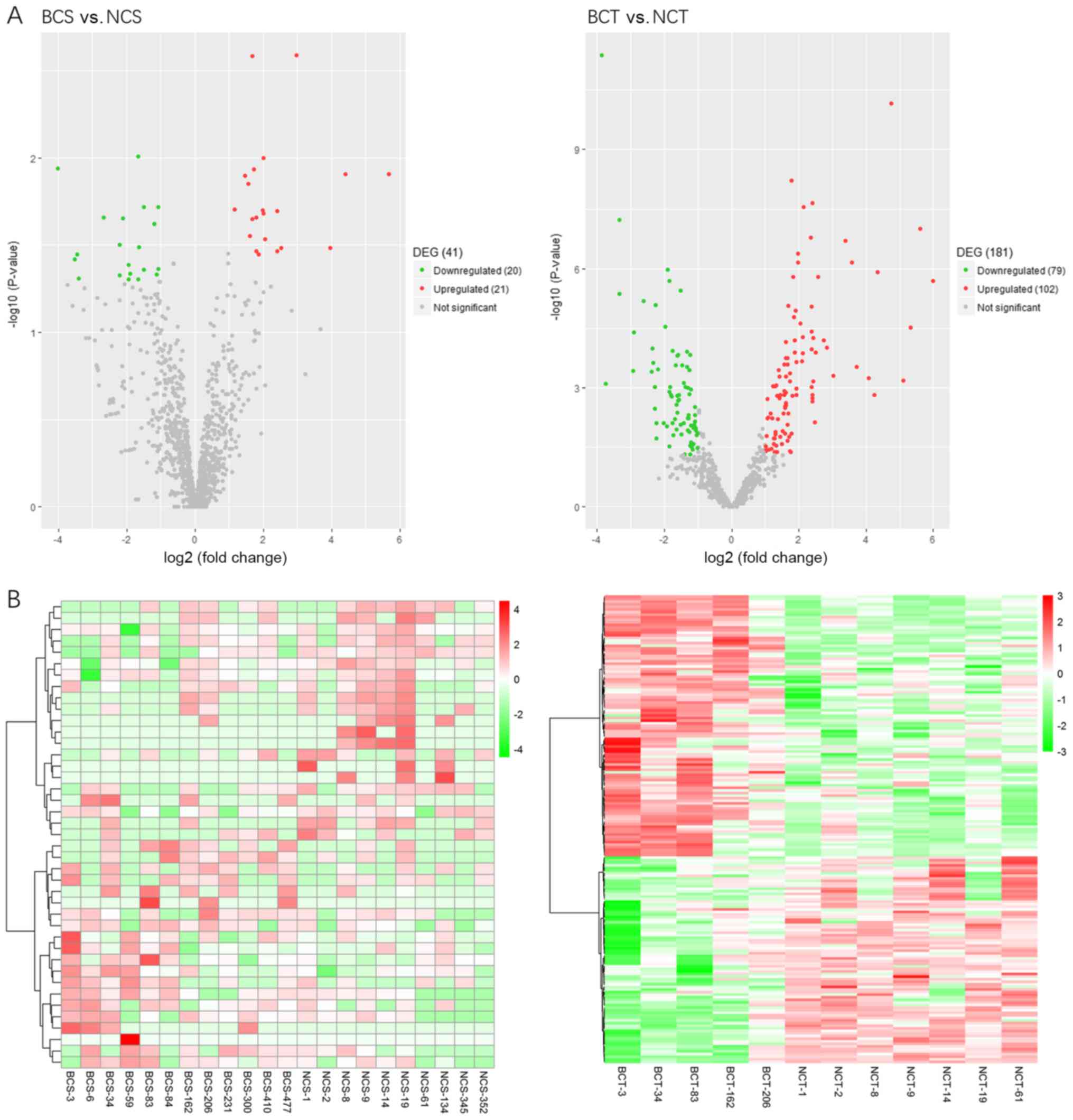
When compared with the control group, there were 41 differentially
expressed miRNAs out of 1,111 miRNAs in the blood samples of the BC
group, of which 21 were significantly upregulated, while 20 were
significantly downregulated miRNAs (P<0.05). In addition, when
compared with the control group, there were 181 differentially
expressed miRNAs in the tissue samples of the BC group, of which
102 were significantly upregulated, while 79 were significantly
downregulated (P<0.05) (Table
II). A volcano plot illustrating the distribution of
differential miRNA between breast cancer and control group is
presented in Fig. 2A.
 | Table II.List of differentially expressed
extracellular particle miRNAs between the two groups. |
Table II.
List of differentially expressed
extracellular particle miRNAs between the two groups.
| Differential
expression | Blood samples,
early-stage BC vs. control | Tissue samples,
early-stage BC vs. control |
|---|
| Significantly
upregulated miRNA (P<0.05) | 21 | 102 |
| Significantly
downregulated miRNA (P<0.05) | 20 | 79 |
Hierarchical clustering was performed using R
software based on the RPKM values of differentially expressed
miRNAs. Hierarchical clustering analysis revealed differences in
the extracellular vesicle-packaged miRNA expression in the blood
samples between the breast cancer and control groups (Fig. 2B).
GO and pathway analysis
miRTarBase was used to identify potential human
miRNA target genes of differentially expressed miRNAs. GO was used
to classify the functions of the target genes from three structured
networks, including biological processes, cellular components and
molecular function.
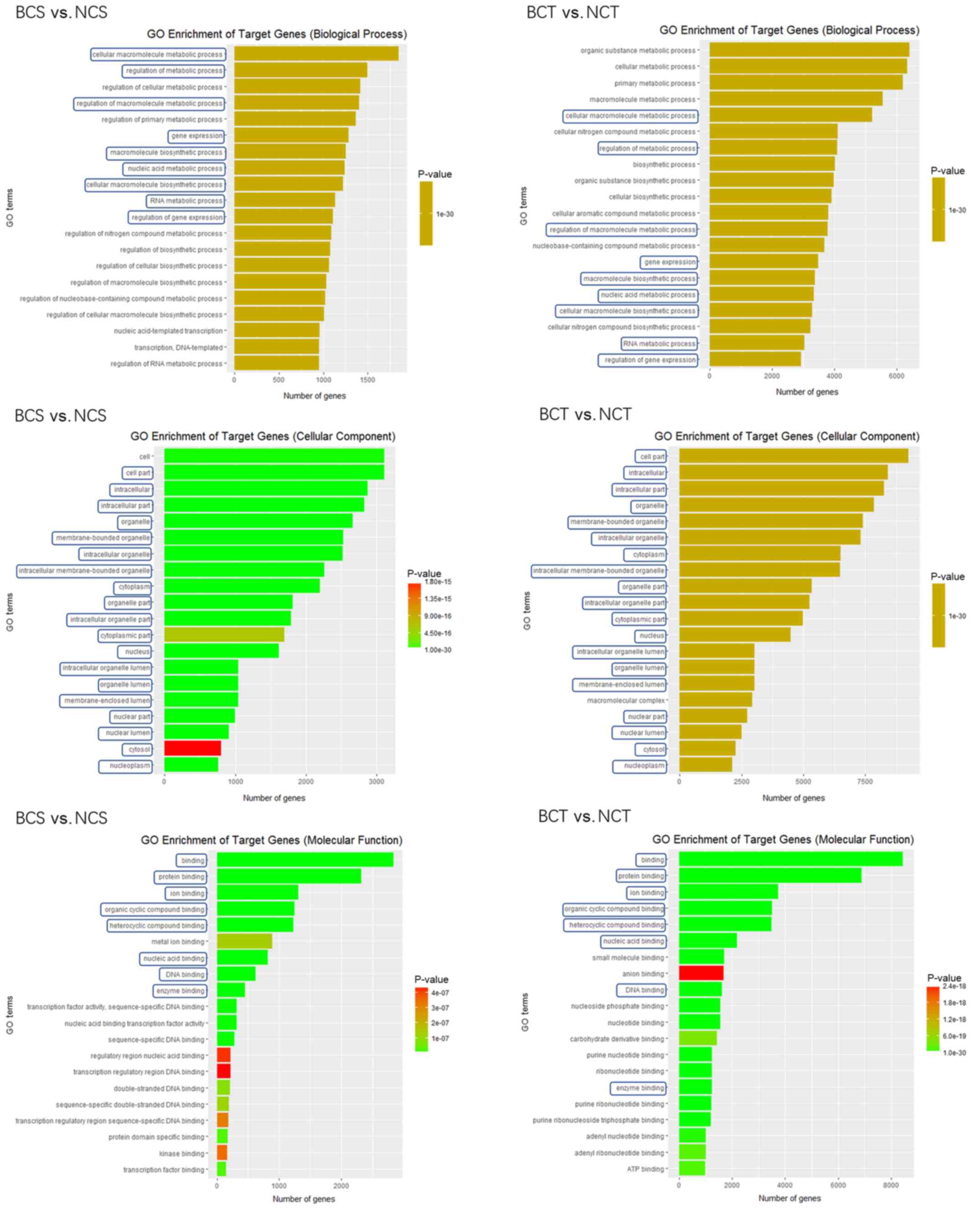
In the present study, GO enrichment was performed in
blood and tissue samples comparing the early-stage breast cancer
group with the control group (Fig.
3). In the biological process analysis, the 20 most prominent
terms in the blood and tissue samples were compared, and 9 out of
20 terms were identical, including ‘cellular macromolecule
metabolic process’, ‘regulation of gene expression’ and ‘RNA
metabolic process’. In the cellular components analysis, the
present study contrasted the 20 most prominent terms in the blood
and tissue samples, and 19 out of 20 terms were identical,
including ‘nucleoplasm’, ‘cytosol’ and ‘nuclear lumen’. In the
molecular function analysis, the present study contrasted the 20
most prominent terms in the blood and tissue samples, and 8 out of
20 terms were observed to be identical, including ‘enzyme binding’,
‘DNA binding’ and ‘nucleic acid binding’.
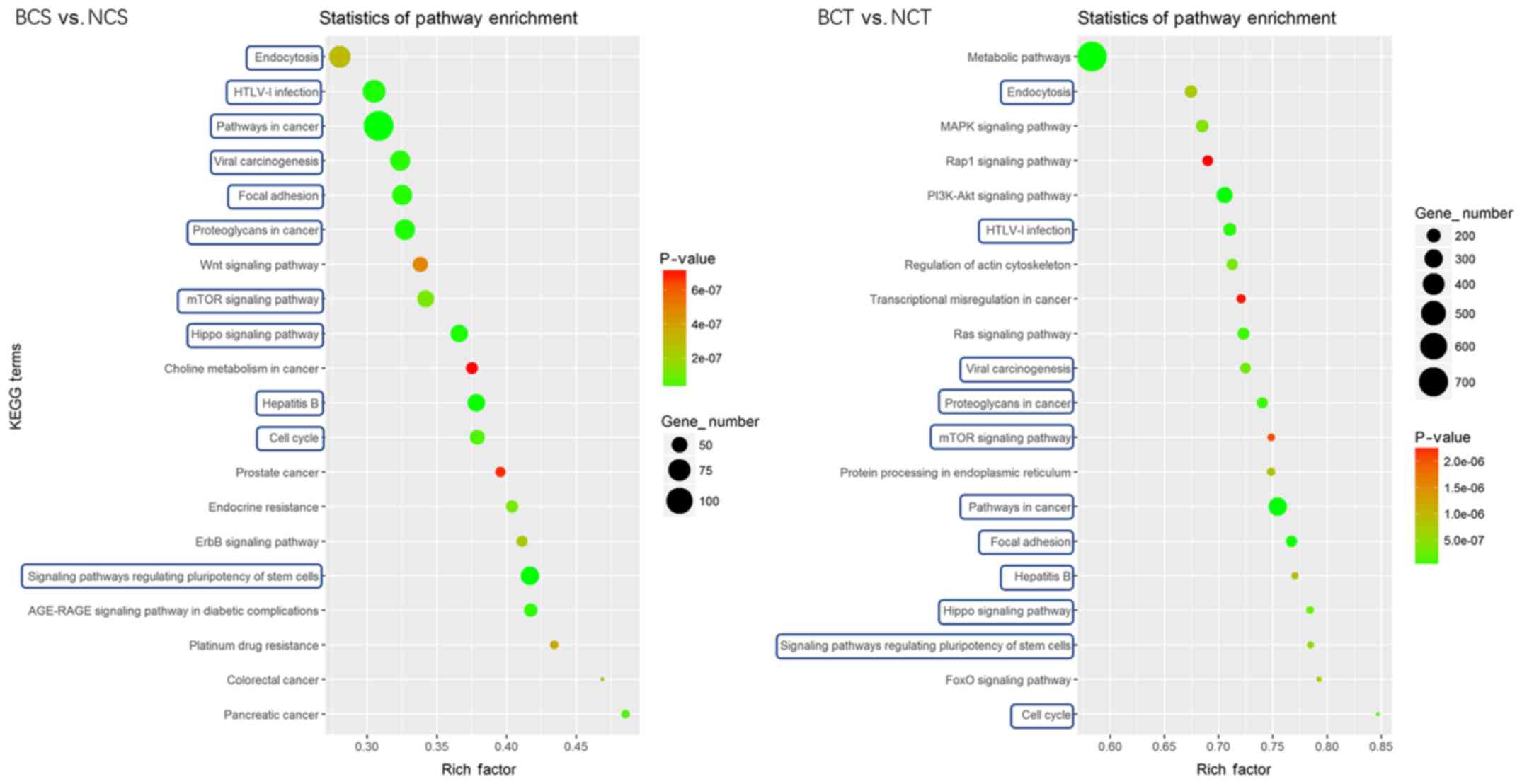
KEGG pathway analysis was performed to identify
significantly affected pathways. The results indicated that in the
blood samples, the target genes of differentially expressed miRNAs
were enriched in ‘ErbB signaling pathway’, ‘cell cycle’, ‘signaling
pathways regulating pluripotency of stem cells’ and ‘pathways in
cancer’. In the tissue samples, the target genes of differentially
expressed miRNAs were enriched in ‘PI3K-Akt signaling pathway’,
‘MAPK signaling pathway’, ‘mTOR signaling pathway’ and ‘pathways in
cancer’. Comparison of the 20 most prominent terms of the KEGG
pathway analysis in the blood and tissue samples revealed that 11
out of 20 terms were the same, including ‘signaling pathways
regulating pluripotency of stem cells’, ‘pathways in cancer’, ‘cell
cycle’, ‘proteoglycans in cancer’ and the ‘mTOR signaling pathway’
(Fig. 4).
Cross-referencing of the blood and
tissue analyses
Comparison of the early BC group with the control
group revealed that six miRNAs were significantly altered in the
blood and tissue analyses (Table
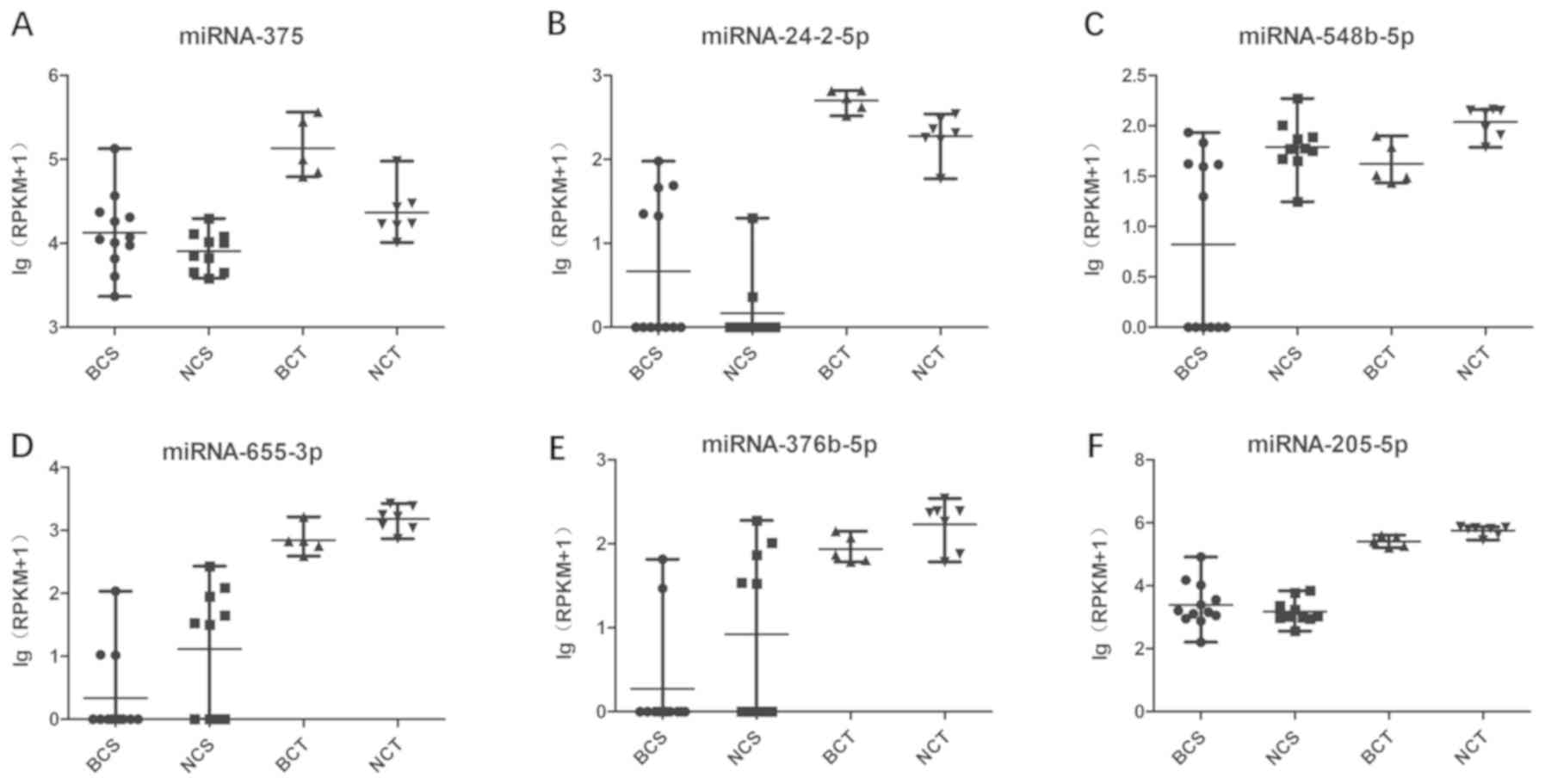
III and Fig. 5). miRNA-205-5p
was significantly upregulated in the blood samples (P=0.011650),
yet was significantly downregulated in the tissue samples
(P=0.000304). Excluding miRNA-205-5p, a total of five miRNAs
exhibited identical changing trends in the blood and tissue
analyses. These miRNAs included miRNA-375, miRNA-24-2-5p,
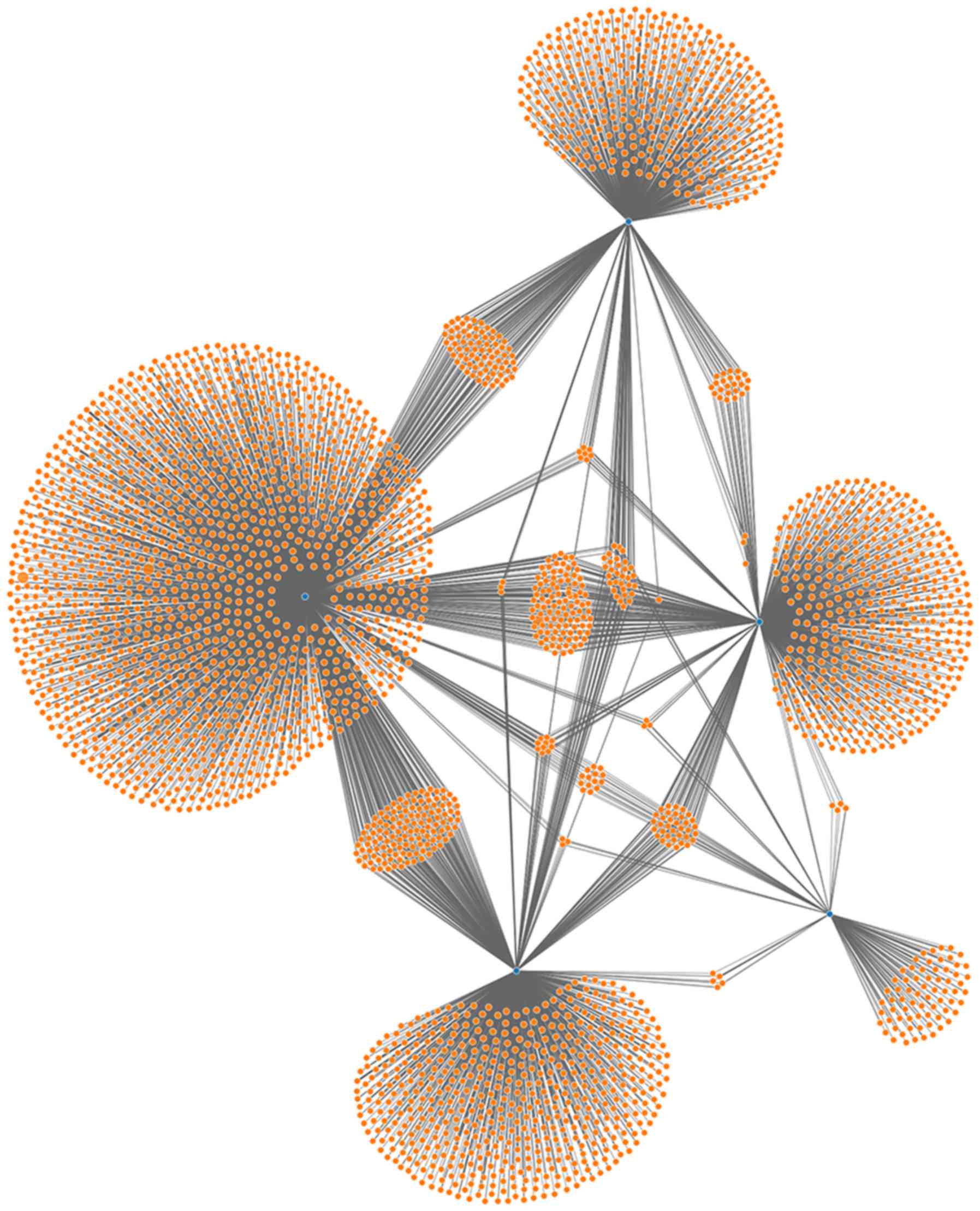
miRNA-548b-5p, miRNA-655-3P and miRNA-376b-5p. Network analysis of
miRNAs and target genes was performed based on the five miRNAs, and
it was revealed that these five miRNAs were associated with the
same target genes and regulated each other, thereby affecting
biological processes (Fig. 6). At
the same time, the statistical analysis of patient's age showed
that the mean age between the breast cancer group and control group
was significantly different (P=0.019). However, bivariate
correlation analysis revealed that there was no significant
correlation between these 5 miRNAs (miR-375, miR-24-2-5p,
miR-548b-5p, miR-655-3p and miR-376b-5p) and age in the blood
groups (Table SI and Fig. S1).
 | Table III.Overexpressed and downregulated
miRNAs in blood and tissues from BC patients as compared to the
control group. |
Table III.
Overexpressed and downregulated
miRNAs in blood and tissues from BC patients as compared to the
control group.
| miRNA | P-value | FC
(log2) |
Upregulated/downregulated |
|---|
| miRNA-375 |
|
|
|
| BCS vs.
NCS | 0.019806 | 1.169516 | Upregulated |
| BCT vs.
NCT |
8.92×10−6 | 2.378307 | Upregulated |
| miRNA-24-2-5p |
|
|
|
| BCS vs.
NCS | 0.020269 | 2.415220 | Upregulated |
| BCT vs.
NCT | 0.016187 | 1.026315 | Upregulated |
| miRNA-548b-5p |
|
|
|
| BCS vs.
NCS | 0.023904 | −1.197655 | Downregulated |
| BCT vs.
NCT | 0.007955 | −1.418292 | Downregulated |
| miRNA-655-3p |
|
|
|
| BCS vs.
NCS | 0.041027 | −1.954611 | Downregulated |
| BCT vs.
NCT | 0.008961 | −1.211824 | Downregulated |
| miRNA-376b-5p |
|
|
|
| BCS vs.
NCS | 0.049708 | −1.948349 | Downregulated |
| BCT vs.
NCT | 0.024284 | −1.217745 | Downregulated |
| miRNA-205-5p |
|
|
|
| BCS vs.
NCS | 0.011650 | 1.726517 | Upregulated |
| BCT vs.
NCT | 0.000304 | −1.333712 | Downregulated |
Evaluation of extracellular
vesicle-packaged miRNAs as early-stage breast cancer diagnostic
biomarkers
To evaluate the utility of extracellular
vesicle-packaged miRNA levels for discriminating cases of
early-stage BC from controls, ROC curve analysis was performed. A
total of five miRNAs identified in the primary analyses were highly
differentially expressed between patients with early-stage BC and
controls (Fig. 7). The highest
area under the curve (AUC) for a single miRNA was achieved with
miRNA-548b-5p [AUC=0.785; 95% confidence interval (CI)=0.585–0.984;
P=0.022].
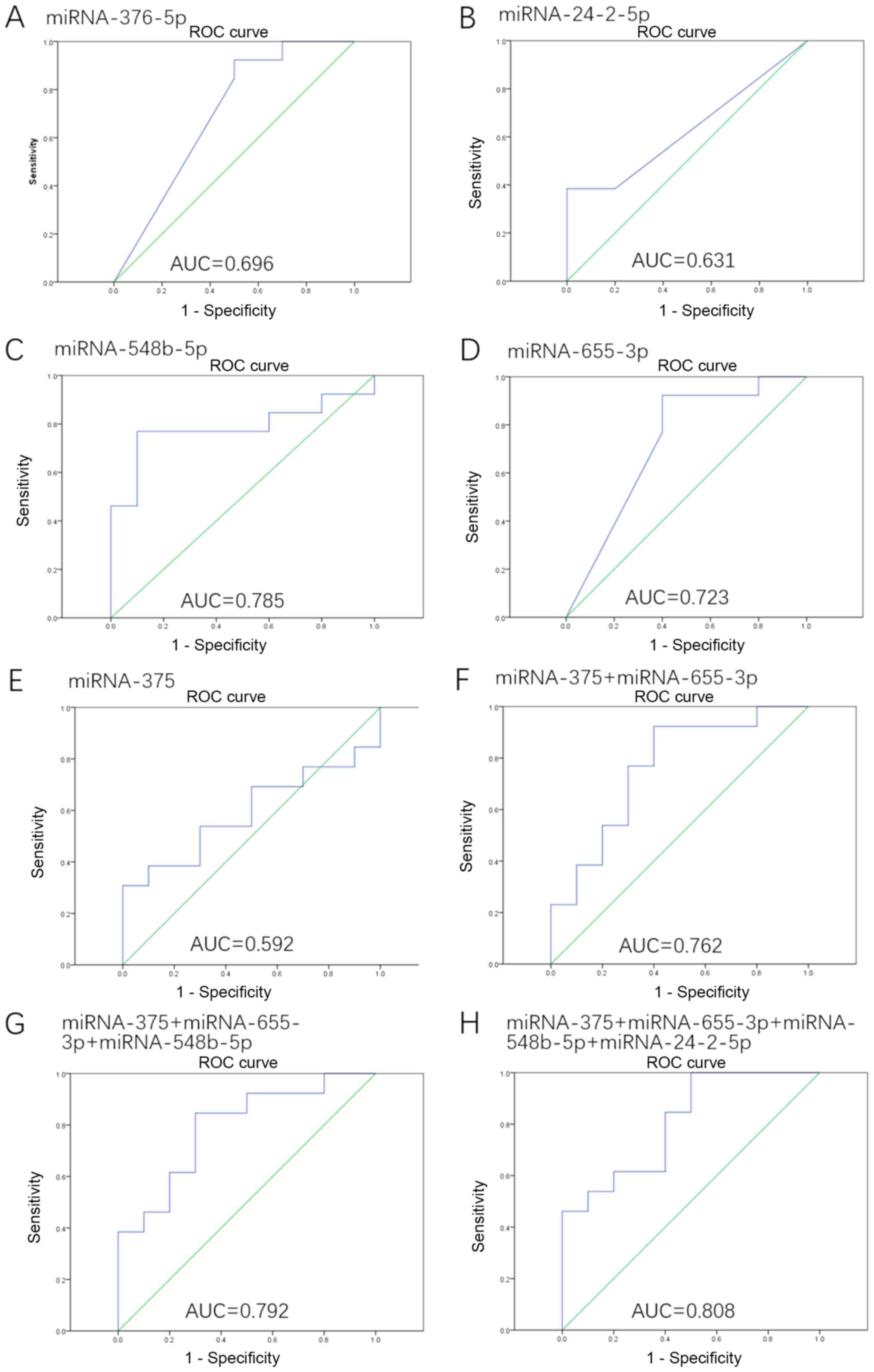 | Figure 7.ROC curves for the miRNAs that were
significantly different in patients with early-stage BC when
compared with controls. ROC curve with AUC for (A) miRNA-376b-5p,
(B) miRNA-24-2-5p, (C) miRNA-548b-5p, (D) miRNA-655-3p, (E)
miRNA-375, (F) the combination of miRNA-375 and miRNA-655-3p, (G)
the combination of miRNA-375, miRNA-655-3p and miRNA-548b-5p, and
(H) the combination of miRNA-375, miRNA-655-3p, miRNA-548b-5p and
miRNA-24-2-5p. miRNA, microRNA; ROC, receiver operating
characteristic; AUC, area under the curve. |
In addition, the different combinations of these
miRNAs were investigated (these results are presented in Table SI), and the highest AUC was
achieved via the combination of miRNA-375, miRNA-24-2-5p,
miRNA-548b-5p and miRNA-655-3p (AUC=0.808; 95% CI=0.629–0.986;
P=0.013). An increase in the AUC was observed when miRNA-375 was
combined with other miRNAs (Fig.
7), such as miRNA-375 + miRNA-655-3p (AUC=0.762; 95%
CI=0.557–0.966; P=0.035), miRNA-375 + miRNA-655-3p + miRNA-548b-5p
(AUC=0.792; 95% CI=0.605–0.979; P=0.018).
Because the highest AUC was achieved via the
combination of miRNA-375, miRNA-24-2-5p, miRNA-548b-5p and
miRNA-655-3p, KEGG pathway analysis of these four miRNAs was
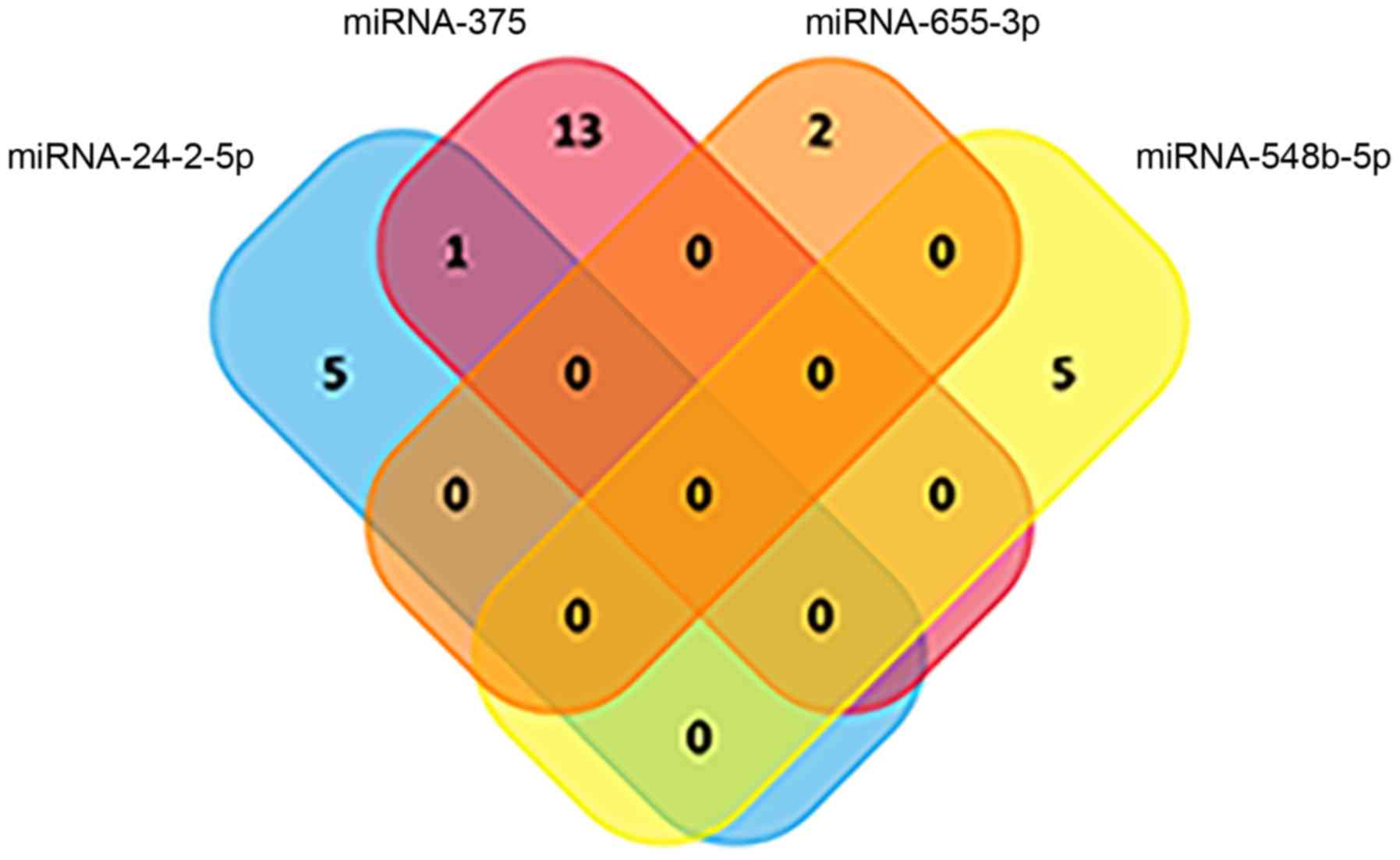
subsequently performed. As illustrated in Fig. 8, the Venn diagram had little
overlap, indicating that the four miRNAs were involved in
independent pathways. Taken together, these data demonstrated that
extracellular vesicle-packaged miRNAs exhibit potential as
diagnostic markers for early-stage breast cancer.
Kaplan-Meier analysis
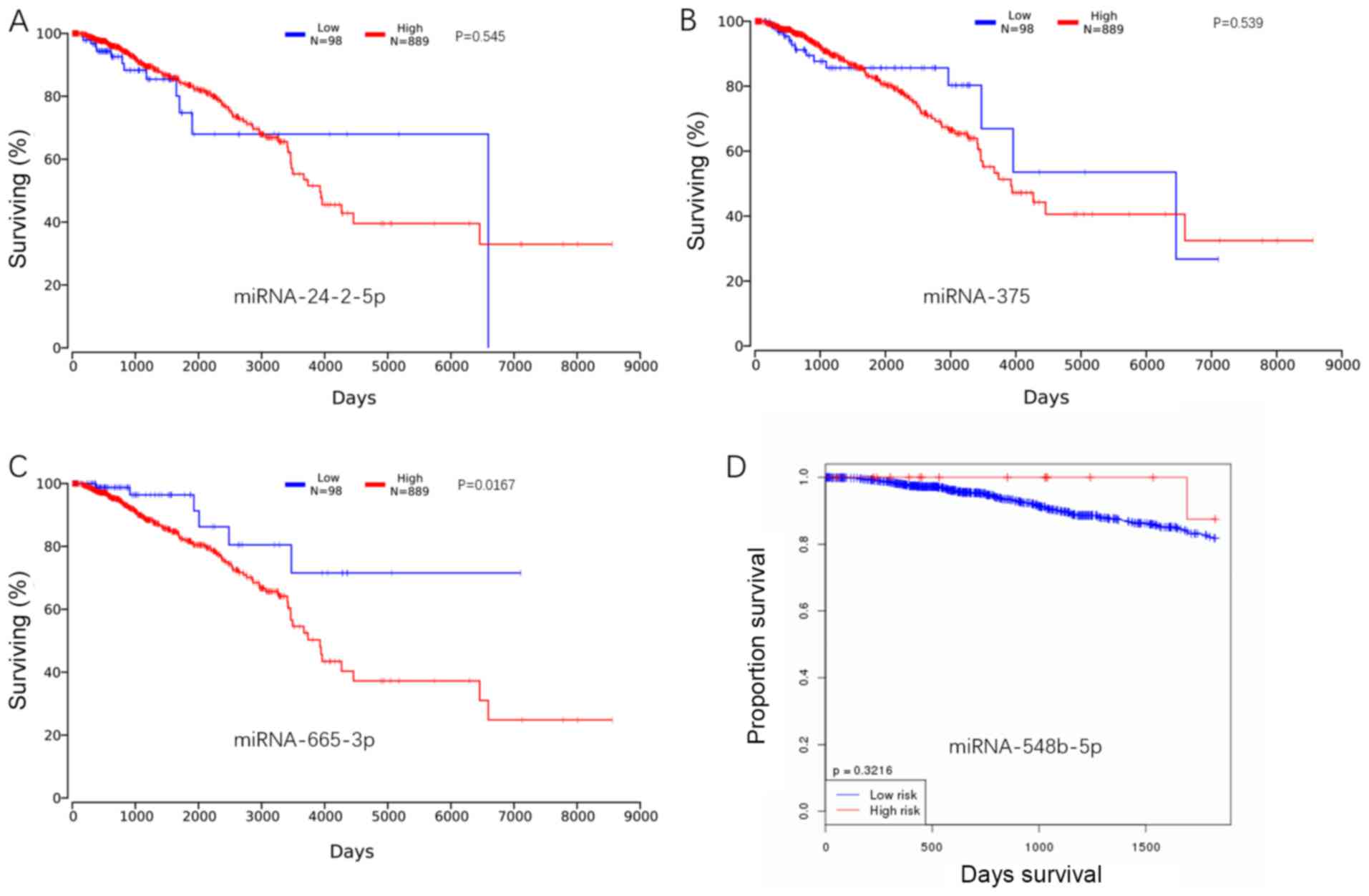
Based on online data from TCGA, the Kaplan-Meier
curves were constructed, and log-rank test analysis was performed.
Low expression of miRNA-24-2-5p was associated with a high survival
rate over the period between 3,000 and 6,000 days. A similar trend
was also observed in miRNA-375; low expression of miRNA-375 was
associated with high survival over the period between 2,000 and
6,000 days. For miRNA-655-3p, a decrease in this miRNA in breast
cancer was identified, although its lower expression was also
associated with a higher survival rate. miRNA-548b-5p was decreased
in the breast cancer group, and the high expression of
miRNA-548b-5p was associated with a high survival rate (Fig. 9). However, it was not possible to
include miRNA-376b-5p in the Kaplan-Meier analysis, as there were
no expression data for miRNA-376b-5p in breast cancer within TCGA
database, but the Kaplan-Meier curve and log-rank test analysis of
miRNA-376b-5p was available based on cancer of the bladder, colon,
pancreas, rectum and stomach. Apart from bladder cancer, the
results demonstrated that the increased expression of miRNA-376b-5p
was associated with a higher survival rate in the other cancer
types examined (Fig. S2).
Discussion
The aim of the present study was to identify the
plasma extracellular vesicle-packaged miRNAs that had the potential
to be biomarkers of early-stage BC, and that served an important
role in the tumorigenesis of BC. The present study identified 35
differentially expressed extracellular vesicle-packaged miRNAs in
the plasma and the 175 differentially expressed miRNAs in the
tissues, and the differentially expressed miRNAs in the plasma and
tissue groups were compared. A total of 1,111 miRNAs in all blood
and tissue samples were sequenced. miRNA-375 and miRNA-24-2-5p were
upregulated in both blood and tissue samples from the breast cancer
group, when compared with the control. However, the remaining
miRNAs (miRNA-548b-5p, miRNA-655-3p and miRNA-376-5p) were
downregulated in both blood and tissue sample of the breast cancer
group, when compared with the control. The cause of this phenomenon
may be associated with the selective loading of extracellular
vesicles. Taken together, the results suggested that these five
miRNAs may exert pivotal functions in the tumorigenesis of breast
cancer. There are conflicting reports about miRNA-375. It has
previously been reported to be a key driver of the proliferation of
ERα-positive breast cells (23);
conversely, it has also been reported that microRNA-375 may inhibit
the viability, migration and invasion of human BC cells by
targeting paired box 6 (24). An
additional report stated that miRNA-375 could inhibit the cancer
stem cell phenotype and tamoxifen resistance by degrading homeobox
B3 in human estrogen receptor (ER)-positive BC (25). However, the present study suggested
that miRNA-375 functions as a tumor promoter, as it was upregulated
in both plasma and tissue samples from the BC group. In contrast
with the present findings, another study demonstrated that miRNA-24
was downregulated in patients with BC; however, that study focused
on circulating miRNAs, as opposed to extracellular vesicle-packaged
miRNAs in the blood (26). In
addition, miRNA-24 was reported to participate in the acquisition
of drug-resistance in BC cells (27). Coincidentally, it was reported that
miR-24-2 could control the expression of H2A histone family member
X and induce apoptosis by targeting the anti-apoptotic gene BCL-2
in BC (28). miR-548 has been
reported to be upregulated in ER-positive BC cells when compared
with ER-negative BC cells (29).
miRNA-548 decreases nuclear paraspeckle assembly transcript 1
expression and promotes the apoptosis of BC cells (30), in accordance with the present
findings. In the present study, miRNA-548-5p was downregulated in
both plasma and tissue samples from the BC group, and it has been
reported that miRNA-548-3p inhibits the proliferation of BC cells
by regulating the expression of enoyl-CoA hydratase, short chain 1
(31). miRNA-655 suppresses
epithelial-to-mesenchymal transition by targeting paired related
homeobox 1 in triple-negative BC (32). miRNA-655 can inhibit pituitary
tumor cell tumorigenesis and is involved in a p53/PTTG1 regulator
of sister chromatid separation, securin regulation feedback loop
(33). In the present study,
miRNA-655-3p was downregulated in the plasma and tissue samples of
the BC group, thereby functioning as a tumor suppressor, similar to
the above reports. miRNA-376b has been reported to promote BC
metastasis by directly targeting homeobox D10 (34). miRNA-376b-5p represses angiogenesis
both in vivo and in vitro (35), and thus miRNA-376b-5p was suggested
to be a potential tumor suppressor. In the present study,
miRNA-376b-5p was downregulated in the plasma and tissue samples
from the BC group. Overall, the current study observed the same
changes in these five miRNAs between early BC and control groups in
clinical samples. The bioinformatics analysis revealed that there
is a common target between the miRNAs, and they may form a network
of mutual regulation.
The present study performed pathway analysis and GO
analysis based on the differentially expressed extracellular
vesicle-packaged miRNAs in the plasma and tissue samples. There was
a large difference in the differentially expressed miRNAs between
the plasma and tissue samples. However, a comparison of the 20 most
prominent terms of KEGG pathway and GO analysis revealed that some
of the terms were identical: 45% (9/20) in biological process, 95%
(19/20) in cellular component, 40% (8/20) in molecular function and
55% (11/20) of the KEGG pathways. This indicated that the
differentially expressed miRNAs had similar effects in the blood
and tissue microenvironments. The ROC curve analysis revealed that
miRNA-548b-5p had a greater distinguishing ability among the five
miRNAs. However, the combination of miRNA-375, miRNA-655-3p,
miRNA-548b-5p and miRNA-24-2-5p had the highest distinguishing
ability. Furthermore, this analysis revealed that there was a
gradual increase in the AUC when miRNA-375 was combined with other
miRNAs. The KEGG pathways of these four miRNAs was subsequently
analyzed, and there was almost no overlap. Therefore, it can be
assumed that these four miRNAs have independent functions.
Based on online data from TCGA, Kaplan-Meier curve
and log-rank test analysis were performed. Consistent with the
previous results, miRNA-24-2-5p and miRNA-375, which were increased
in the breast cancer group, were partially negatively associated
with patient survival. However, the Kaplan-Meier curve and log-rank
test analysis result for miRNA-655-3p was the opposite, as a
decrease in the expression of this miRNA in breast cancer was
associated with a higher survival rate. It was hypothesized that
the reason for this discrepancy was the limited sample size in the
study cohort. However, miRNA-548b-5p, an miRNA that was decreased
in the breast cancer group, was positively associated with patient
survival. It is notable that no expression data for miRNA-376b-5p
in breast cancer were identified in the TCGA database. The
Kaplan-Meier curve and log-rank test analysis of miRNA-376b-5p was
available based on cancer of the bladder, colon, pancreas, rectum
and stomach. Apart from bladder cancer, the results demonstrated
that the increased expression of miRNA-376b-5p was associated with
a higher survival rate in the other examined cancer types.
In conclusion, the present study provided a set of
plasma extracellular vesicle-packaged miRNA-based biomarkers for
the diagnosis of early-stage BC and indicated the complexity of
miRNA regulation in the microenvironments of the blood and tissue.
These findings may enrich the presently available knowledge on
early-stage breast cancer diagnosis biomarkers, thus improving the
curability of breast cancer in the future. However, the expression
of the identified miRNAs was not verified by quantitative PCR
analysis; there are plans to use a larger cohort of patients and
compare the expression of the identified miRNAs among breast cancer
patients with different disease stages in a future study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Sanming Project
of Medicine in Shenzhen (grant no. SZSM201512015).
Availability of data and materials
The datasets generated and analyzed during the
current study are not publicly available due to the ‘Interim
measures for the management of human genetic resources’ promulgated
by the Chinese government; however, they are available from
corresponding author on reasonable request.
Authors' contributions
NX and MM conceived and designed the study. CY and
JH performed the experiments and wrote the manuscript. MM and DZ
performed the data analysis and revised the manuscript critically.
YY and HH collected the patient's samples. All authors contributed
to the preparation of the final manuscript and lent shape to the
final paper.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of Shenzhen People's Hospital (no. LL-KT-201801099) and written
informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Opstal-van Winden AW, Rodenburg W,
Pennings JL, van Oostrom CT, Beijnen JH, Peeters PH, van Gils CH
and de Vries A: A bead-based multiplexed immunoassay to evaluate
breast cancer biomarkers for early detection in pre-diagnostic
serum. Int J Mol Sci. 13:13587–13604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sinn P, Aulmann S, Wirtz R, Schott S,
Marmé F, Varga Z, Lebeau A, Kreipe H and Schneeweiss A: Multigene
assays for classification, prognosis, and prediction in breast
cancer: A critical review on the background and clinical utility.
Geburtshilfe Frauenheilkd. 73:932–940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herranz H and Cohen SM: MicroRNAs and gene
regulatory networks: Managing the impact of noise in biological
systems. Genes Dev. 24:1339–1344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashby J, Flack K, Jimenez LA, Duan Y,
Khatib AK, Somlo G, Wang SE, Cui X and Zhong W: Distribution
profiling of circulating microRNAs in serum. Anal Chem.
86:9343–9349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK,
Pritchard CC, Gibson DF, Mitchell PS, Bennett CF,
Pogosova-Agadjanyan EL, Stirewalt DL, et al: Argonaute2 complexes
carry a population of circulating microRNAs independent of vesicles
in human plasma. Proc Natl Acad Sci USA. 108:5003–5008. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Desrochers LM, Antonyak MA and Cerione RA:
Extracellular vesicles: Satellites of information transfer in
cancer and stem cell biology. Dev Cell. 37:301–309. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomasetti M, Lee W, Santarelli L and
Neuzil J: Exosome-derived microRNAs in cancer metabolism: Possible
implications in cancer diagnostics and therapy. Exp Mol Med.
49:e2852017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Graveel CR, Calderone HM, Westerhuis JJ,
Winn ME and Sempere LF: Critical analysis of the potential for
microRNA biomarkers in breast cancer management. Breast Cancer
(Dove Med Press). 7:59–79. 2015.PubMed/NCBI
|
|
11
|
Hannafon BN, Trigoso YD, Calloway CL, Zhao
YD, Lum DH, Welm AL, Zhao ZJ, Blick KE, Dooley WC and Ding WQ:
Plasma exosome microRNAs are indicative of breast cancer. Breast
Cancer Res. 18:902016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Cai Q, Huang Y, Li S, Yang H, Sun
L, Chen K and Wang Y: MicroRNA-21 as a potential diagnostic
biomarker for breast cancer patients: A pooled analysis of
individual studies. Oncotarget. 7:34498–34506. 2016.PubMed/NCBI
|
|
13
|
Shimomura A, Shiino S, Kawauchi J,
Takizawa S, Sakamoto H, Matsuzaki J, Ono M, Takeshita F, Niida S,
Shimizu C, et al: Novel combination of serum microRNA for detecting
breast cancer in the early stage. Cancer Sci. 107:326–334. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eichelser C, Stuckrath I, Muller V,
Milde-Langosch K, Wikman H, Pantel K and Schwarzenbach H: Increased
serum levels of circulating exosomal microRNA-373 in
receptor-negative breast cancer patients. Oncotarget. 5:9650–9663.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao CL, Mai ZB, Lian XL, Zhong JY, Jin
JJ, He QY and Zhang G: FANSe2: A robust and cost-efficient
alignment tool for quantitative next-generation sequencing
applications. PLoS One. 9:e942502014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mortazavi A, Williams BA, McCue K,
Schaeffer L and Wold B: Mapping and quantifying mammalian
transcriptomes by RNA-Seq. Nat Methods. 5:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bloom JS, Khan Z, Kruglyak L, Singh M and
Caudy AA: Measuring differential gene expression by short read
sequencing: Quantitative comparison to 2-channel gene expression
microarrays. BMC Genomics. 10:2212009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong
S, Kong L, Gao G, Li CY and Wei L: KOBAS 2.0: A web server for
annotation and identification of enriched pathways and diseases.
Nucleic Acids Res. (39): (Web Server Issue). W316–W322. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herrera-Perez Z, Gretz N and Dweep H: A
comprehensive review on the genetic regulation of cisplatin-induced
nephrotoxicity. Curr Genomics. 17:279–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong NW, Chen Y, Chen S and Wang X:
OncomiR: An online resource for exploring pan-cancer microRNA
dysregulation. Bioinformatics. 34:713–715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rajkumar AP, Qvist P, Lazarus R, Lescai F,
Ju J, Nyegaard M, Mors O, Børglum AD, Li Q and Christensen JH:
Experimental validation of methods for differential gene expression
analysis and sample pooling in RNA-seq. BMC Genomics. 16:5482015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Souza Rocha Simonini P, Breiling A,
Gupta N, Malekpour M, Youns M, Omranipour R, Malekpour F, Volinia
S, Croce CM, Najmabadi H, et al: Epigenetically deregulated
microRNA-375 is involved in a positive feedback loop with estrogen
receptor alpha in breast cancer cells. Cancer Res. 70:9175–9184.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou Q, Yi W, Huang J, Fu F, Chen G and
Zhong D: MicroRNA-375 targets PAX6 and inhibits the viability,
migration and invasion of human breast cancer MCF-7 cells. Exp Ther
Med. 14:1198–1204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu H, Fu L, Xie C, Zuo WS, Liu YS, Zheng
MZ and Yu JM: miR-375 inhibits cancer stem cell phenotype and
tamoxifen resistance by degrading HOXB3 in human ER-positive breast
cancer. Oncol Rep. 37:1093–1099. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schrauder MG, Strick R, Schulz-Wendtland
R, Strissel PL, Kahmann L, Loehberg CR, Lux MP, Jud SM, Hartmann A,
Hein A, et al: Circulating micro-RNAs as potential blood-based
markers for early stage breast cancer detection. PLoS One.
7:e297702012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamoto Y, Yoshioka Y, Minoura K,
Takahashi RU, Takeshita F, Taya T, Horii R, Fukuoka Y, Kato T,
Kosaka N and Ochiya T: An integrative genomic analysis revealed the
relevance of microRNA and gene expression for drug-resistance in
human breast cancer cells. Mol Cancer. 10:1352011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Srivastava N, Manvati S, Srivastava A, Pal
R, Kalaiarasan P, Chattopadhyay S, Gochhait S, Dua R and Bamezai
RN: miR-24-2 controls H2AFX expression regardless of gene copy
number alteration and induces apoptosis by targeting antiapoptotic
gene BCL-2: A potential for therapeutic intervention. Breast Cancer
Res. 13:R392011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YM, Lee JY, Ho CC, Hong QS, Yu SL,
Tzeng CR, Yang PC and Chen HW: miRNA-34b as a tumor suppressor in
estrogen-dependent growth of breast cancer cells. Breast Cancer
Res. 13:R1162011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ke H, Zhao L, Feng X, Xu H, Zou L, Yang Q,
Su X, Peng L and Jiao B: NEAT1 is required for survival of breast
cancer cells through fus and mir-548. Gene Regul Syst Bio.
10:11–17. 2016.PubMed/NCBI
|
|
31
|
Shi Y, Qiu M, Wu Y and Hai L: miR-548-3p
functions as an anti-oncogenic regulator in breast cancer. Biomed
Pharmacother. 75:111–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lv ZD, Kong B, Liu XP, Jin LY, Dong Q, Li
FN and Wang HB: miR-655 suppresses epithelial-to-mesenchymal
transition by targeting Prrx1 in triple-negative breast cancer. J
Cell Mol Med. 20:864–873. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang HQ, Wang RJ, Diao CF, Li JW, Su JL
and Zhang S: The PTTG1-targeting miRNAs miR-329, miR-300, miR-381,
and miR-655 inhibit pituitary tumor cell tumorigenesis and are
involved in a p53/PTTG1 regulation feedback loop. Oncotarget.
6:29413–29427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
An N, Luo X, Zhang M and Yu R:
MicroRNA-376b promotes breast cancer metastasis by targeting Hoxd10
directly. Exp Ther Med. 13:79–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li LJ, Huang Q, Zhang N, Wang GB and Liu
YH: miR-376b-5p regulates angiogenesis in cerebral ischemia. Mol
Med Rep. 10:527–535. 2014. View Article : Google Scholar : PubMed/NCBI
|