Introduction
Approximately 10 million people worldwide suffer
from corneal blindness (1,2). Limbal stem cell deficiency (LSCD) is
among the leading causes of corneal damage since these cells are
essential for maintaining the integrity of the corneal surface and
transparency of the cornea (3,4).
Injury or inflammation in the cornea causes LSCD (5), an extremely debilitating human eye
disease that causes painful vision loss and other clinical
manifestations including corneal neovascularization, chronic
inflammation, erosions, ulceration, and stromal scarring (6,7).
Hereditary aniridia is a primary LSCD, which is characterized by
congenital erythrokeratoderma, keratitis, inadequate nutrition or
cytokine supply, neurotrophic keratopathy, peripheral inflammation,
and sclerocornea (8). LSCD may
also be caused by secondary external factors such as trauma,
chemical acid or alkali, or thermal injuries and radiation
(9). Stevens-Johnson syndrome
results in secondary LSCD (10),
and LSCD may be unilateral or bilateral depending upon the
cause.
LSCD is often diagnosed by clinical assessment or
detection of conjunctival goblet cells on the corneal surface using
impression cytology (3,9,11).
Under conditions of limbal stem cell loss, replacement with healthy
limbal cells is necessary, which can be achieved by transplanting
healthy limbal tissue containing nourishing cells. Cells can be
transplanted from the other eye of the patient if it is healthy
(autograft) or from the eye of a living or cadaveric donor
(allograft) to the eye with LSCD (12,13).
When taking excess graft from other healthy eye or living donor,
stem cell deficiency may occur in the donor eye. In the late 1990s,
cultured, autologous, limbal epithelial cell implants were used
successfully to improve vision in patients with chemical
injury-induced LSCD (14). Since
then, transplantation of cultivated epithelial stem cells has
become a treatment of choice for numerous patients with LSCD.
Bilateral LSC deficiency is frequently observed in the general
population in which no residual stem cells are available for ex
vivo culture. Allograft is associated with a high risk of
rejection, neoplasia, and disease transmission (15). Thus, allogenic cell populations
from other regions in the patient may replace the use of allogenic
material. Various approaches have been developed for deriving
limbal stem cells from different sources such as oral mucosal
epithelial cells, conjunctival epithelial cells, hair
follicle-derived epithelial stem cells, amniotic epithelial cells,
human embryonic stem cells, induced pluripotent stem cells,
umbilical cord lining epithelial stem cells, Wharton's jelly
mesenchymal stem cells (16–19),
and human immature dental pulp stem cells (DPSCs) (20,21).
DPSCs are a relatively convenient resource, as teeth are easy to
access and potentially superior to other types of adult stem cells
(22). More than 20 years have
passed since the first limbal stem cell transplantation, however,
its standardization and application in India has not reached some
populations, particularly those in rural regions. In the present
study, DPSCs were cultured in limbal stem cell media and these
cells were characterized against limbal stem cells, revealing the
significance of using dental pulp stem cell treatment in bilateral
LSCD.
Materials and methods
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM),
phosphate-buffered saline (PBS), antibiotic and antimycotic
solution, fetal bovine serum, Trypsin-EDTA, and tissue culture
plastics were procured from HiMedia Laboratories Pvt. Ltd. (Mumbai,
India). Insulin, transferrin, selenium (ITS), epidermal growth
factor (EGF), hydrocortisone, RNA extraction TRIzol®,
and immunocytochemistry secondary fluorescence antibodies [Alexa
Fluor® 647-conjugated goat-anti-rabbit immunoglobulin G
(IgG; 2 mg/ml; cat. no. A21244) and Alexa Fluor 488-conjugated
goat-anti-mouse IgG (2 mg/ml; cat. no. A11001)] were obtained from
Molecular Probes (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Western blot protein markers, loading gel, chemiluminescence
developers, and nitrocellulose membranes were procured from Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Antibodies (200 µg/ml)
against limbal stem cell and corneal epithelial markers, including
ATP-binding cassette super-family G member 2 (ABCG2; cat. no.
sc-377176), cytokeratin 12 (cat. no. sc-25722), E-cadherin (cat.
no. sc-7870), vimentin (cat. no. sc-32322) and loading control
GAPDH (cat. no. sc-166574) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The polymerase chain
reaction (PCR) primers were obtained from GeNoRime (Chennai,
India), and PCR reagents were from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA) and Takara Bio, Inc. (Otsu, Japan).
Limbal stem cell separation and
culture
The Institutional Ethics Committee Board of SDM
College of Medical Sciences and Hospital (Manjushree Nagar,
Dharwad, Karnataka, India; permit no. 065, ECR/683/INST/KA/2014)
approved the study protocol. Informed consent was obtained from the
donor patient family. After using the cadaveric donor eye for the
graft, spare limbal tissues were collected from the SDM College of
Medical Sciences Eye Bank (Dharwad, India) from May 2015 to March
2016. Concurrently, donor blood was collected and screened for
hepatitis B and C and human immunodeficiency virus antigens. The
corneo-scleral button of a cadaver was excised from fresh globes
and limbal tissue with adjacent peripheral cornea was treated with
trypsin to remove all epithelial cells from the peripheral cornea.
The intact limbal basal epithelium was removed by incubation with
Dispase II purchased from HiMedia Laboratories Pvt. Ltd.
Concurrently, an explant culture of limbal stem cells was prepared
with the spare palisade of Vogt tissue in the respective
medium.
Amniotic membranes used as scaffolds were harvested
from the placenta of healthy women delivered by elective cesarean
section at full-term. Consent for the use of the placenta was
obtained from the mothers before delivery.
Dental pulp stem cell separation and
culture
Human permanent teeth for dental pulp extraction
were obtained during prophylactic orthodontic treatment or wisdom
tooth extraction. Informed consent was obtained from the patients
and their parents. The pulps were minced into small pieces 0.1–0.2
mm in diameter in DMEM. Small pieces of pulp tissue were dispersed
in dispase II and digested by trypsin. These dispersed cells were
seeded into 6-well plates containing DMEM. DPSCs were cultured at
37°C with 5% CO2 and then characterized for mesenchymal
stem cell surface markers Thy-1, homing cell adhesion molecule
(HCAM), C-kit, cluster of differentiation (CD)-24 and CD-45 by
western blot analysis (data not shown).
Total cell extraction and western
blotting
For the stem cell surface marker expression analysis
of limbal and DPSCs, cells were collected and lysed in extraction
buffer (25 mM Tris-HCl, pH 7.9, 0.5 mM EDTA, and 0.1 mM PMSF) with
several freeze-thaw cycles. The lysates were then centrifuged at
12,000 × g for 15 min at 4°C, and the supernatants were collected.
The protein content was measured via a bicinchoninic acid protein
assay using various concentrations of serum albumin as standards as
previously described (23). Total
protein (40 µg/lane) was separated on a 10% Bis-Tris polyacrylamide
gel in Tris-HCl buffer. Separated proteins were transferred to
nitrocellulose membranes, blocked with 1% bovine serum albumin
(BSA) from HiMedia Laboratories Pvt. Ltd. at room temperature for 1
h. The membranes were hybridized with limbal stem cell and corneal
epithelial marker antibodies (diluted at 1:1,000 in 1% BSA) against
ABCG2 (cat. no. sc-377176), vimentin (cat. no. sc-32322),
cytokeratin 12 (cat. no. sc-25722), E-cadherin (cat. no. sc-7870),
Thy-1 (cat. no. sc-59396), HCAM (cat. no. sc-65265), c-kit (cat.
no. sc-13508), CD24 (cat. no. sc-70598), CD45 (cat. no. sc-1178)
and the loading control GAPDH (cat. no. sc-166574; all from Santa
Cruz Biotechnology, Inc.) overnight at 4°C. The appropriate
horseradish peroxidase-conjugated goat-anti-mouse (cat. no.
170-6516; Bio-Rad Laboratories, Inc.) or goat-anti-rabbit (cat. no.
1706515, Bio-Rad Laboratories, Inc.) secondary antibodies (1:3,000)
were incubated with the respective membranes for 2 h at room
temperature. The membranes were developed using ECL Plus (Bio-Rad
Laboratories, Inc.), and images were obtained by autoradiography
using a G:BOX ChemiXX9 system fom Syngene Europe (Cambridge, UK).
GeneSys V1.6.5.0 software (Syngene, Frederick, MD, USA) was used to
quantify protein expression.
Immunocytochemistry
Cells were detached from 100-mm plates with 0.25%
trypsin-EDTA (Hi Media) and washed twice with PBS. Cover slips
sterilized with methanol were placed into 6-well plates;
1–3×105 cells were seeded into each well and grown
overnight in respective growth media at 37°C with 5%
CO2. Cells were fixed and permeabilized with 100%
methanol for 15 min at room temperature. The cells were then washed
twice with PBS and blocked with 1% BSA in PBS. After 30 min of
incubation on ice, the aforementioned antibodies against ABCG2,
E-cadherin, cytokeratin 12 and vimentin were added at 1:50 dilution
in 1% BSA, and the cells were incubated at 4°C overnight. The
following steps were performed in the dark. Subsequently, secondary
antibodies (Alexa Fluor 647 goat anti-rabbit IgG and Alexa Fluor
488 goat anti-mouse IgG) was added at 1:200 dilution in 1% BSA,
incubated for 1 h at room temperature and then washed three times
with PBS for 5 min in the dark. Counterstaining was performed using
DAPI (1 µg/ml) for 2 min in room temperature at dark. The cover
slips were transferred upside down onto glass slides with one drop
of polyvinyl alcohol in PBS, and coverslips were mounted with
Vectashield mounting medium (Vector Laboratories, Inc., Burlingame,
CA, USA). Images were acquired using a Zeiss LSM 510-META confocal
microscope (Zeiss AG, Oberkochen, Germany).
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. After DNase treatment, 1 µg total RNA
was reverse-transcribed using a RevertAid First Strand cDNA
synthesis kit (Thermo Fischer Scientific, Inc.) in a total volume
of 20 µl reaction mixture according to the manufacturer's
instructions. qPCR was performed with Verso SYBR Green I dye
(Thermo Fischer Scientific, Inc. A) as described by the
manufacturer in a final volume of 25 µl in a Cepheid SmartCycler
II. qPCR was conducted as follows: 5 min at 95°C, followed by 40
cycles of 95°C for 30 sec and primer melting temperature for 30 sec
(Connexin 43, 63°C; Keratin 12, Keratin 14 and PAX6, 60°C;
E-Cadherin, 55°C; and GAPDH, 54°C), and a final step of 68°C for 30
sec. The expression of each gene was normalized to that of GAPDH,
which served as a loading control. The 2−∆∆Cq method was
used to determine the relative gene expression (24). The primer sequences used to amplify
each gene are listed in Table
I.
 | Table I.List of primers and their sequences
used in the study. |
Table I.
List of primers and their sequences
used in the study.
| Gene name | Primer sequence
(5′-3′) | Length (bp) |
|---|
| E-Cadherin | FP:
TGGGCTGGACCGAGAGAGTT | 20 |
|
| RP:
ATCTCCAGCCAGTTGGCAGT | 20 |
| Connexin 43 | FP:
GCGTGAGGAAAGTACCAAAC | 20 |
|
| RP:
GGGCAACCTTGAGTTCTTCC | 20 |
| Keratin 12 | FP:
CTACCTGGATAAGGTGCGAGCT | 22 |
|
| RP:
TCTCGCATTGTCAATCTGCA | 20 |
| Keratin 14 | FP:
CATGAGTGTGGAAGCCGACAT | 21 |
|
| RP:
CCCTCTCAGGGCATTCATCTC | 21 |
| Pax 6 | FP:
GTGTCCAACGGATGTGTGAG | 20 |
|
| RP:
CTAGCCAGGTTGCGAAGAAC | 20 |
| GAPDH | FP:
GAGCGAGATCCCTCCAAA | 18 |
|
| RP:
ACTGTGGTCATGAGTCCTTC | 20 |
Statistical analysis
qPCR analysis results are presented as mean values,
with error bars representing the 95% confidence intervals.
Immunocytochemistry and western blot quantification analyses are
presented as averages, with error bars representing the standard
deviation. Where applicable, the results were compared using
Tukey's and Sidak's multiple comparison tests following two-way
analysis of variance (ANOVA) with P<0.05 as the level of
significance. ImageJ 1.52h (National Institutes of Health,
Bethesda, MD, USA) was used to analyse and quantify the
immunocytochemistry data and western blot data was quantified using
densitometric scanning. GraphPad Prism 6 (GraphPad Software, Inc.,
La Jolla, CA, USA) was used to compare the data sets.
Results
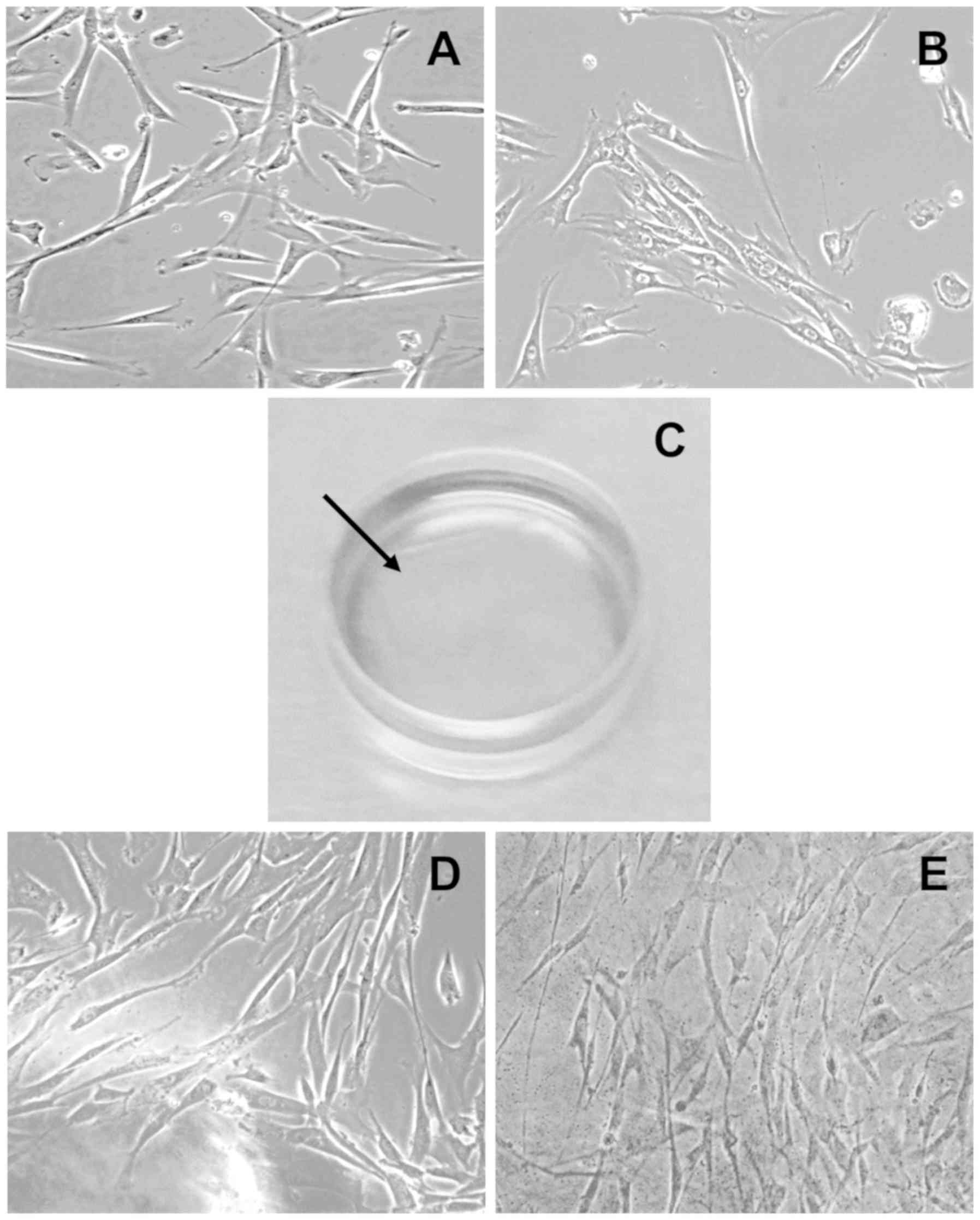
Morphological characteristics of
limbal and dental pulp stem cells
Morphologically, limbal and dental pulp stem cells
formed an epithelial-like migrated monolayer of cells in different
regions of the culture flask within a few hours after seeding. The
shape and culture pattern of both cell phenotypes were similar
(Fig. 1A and B). The cells growing
on the culture flask in DMEM became confluent within three weeks of
seeding; however, cells growing in limbal stem cell (LSC) media
with an amniotic membrane scaffold (Fig. 1C) took longer to become confluent,
but exhibited the same morphological phenotypes (Fig. 1D and E). The approximate cell yield
after three weeks of growth was 1×104
cells/mm3 (counted manually using Neubauer's
chamber).
Molecular characterization of limbal
and dental pulp stem cells
To verify the potential of DPSCs to function as
limbal stem cells in the respective microenvironment, the relative
expression of specific molecular markers such as e-cadherin,
connexin 43, keratin 12, keratin 14, and pax6 genes (25,26)
to GAPDH gene in limbal and dental pulp stem cells grown in DME
medium, LSC media, and LSC media with an amniotic membrane as
scaffold (LSC-AM) was evaluated by RT-PCR (Fig. 2). As expected, irrespective of the
medium in which the cells were grown, DPSCs expressed all stem cell
markers at similar levels as in LSCs. However, dental pulp and
limbal stem cells grown in LSC media with the AM exhibited good
expression of stem cell markers compared with cells grown in DMEM
and LSC media only, with the exception of keratin 12 and
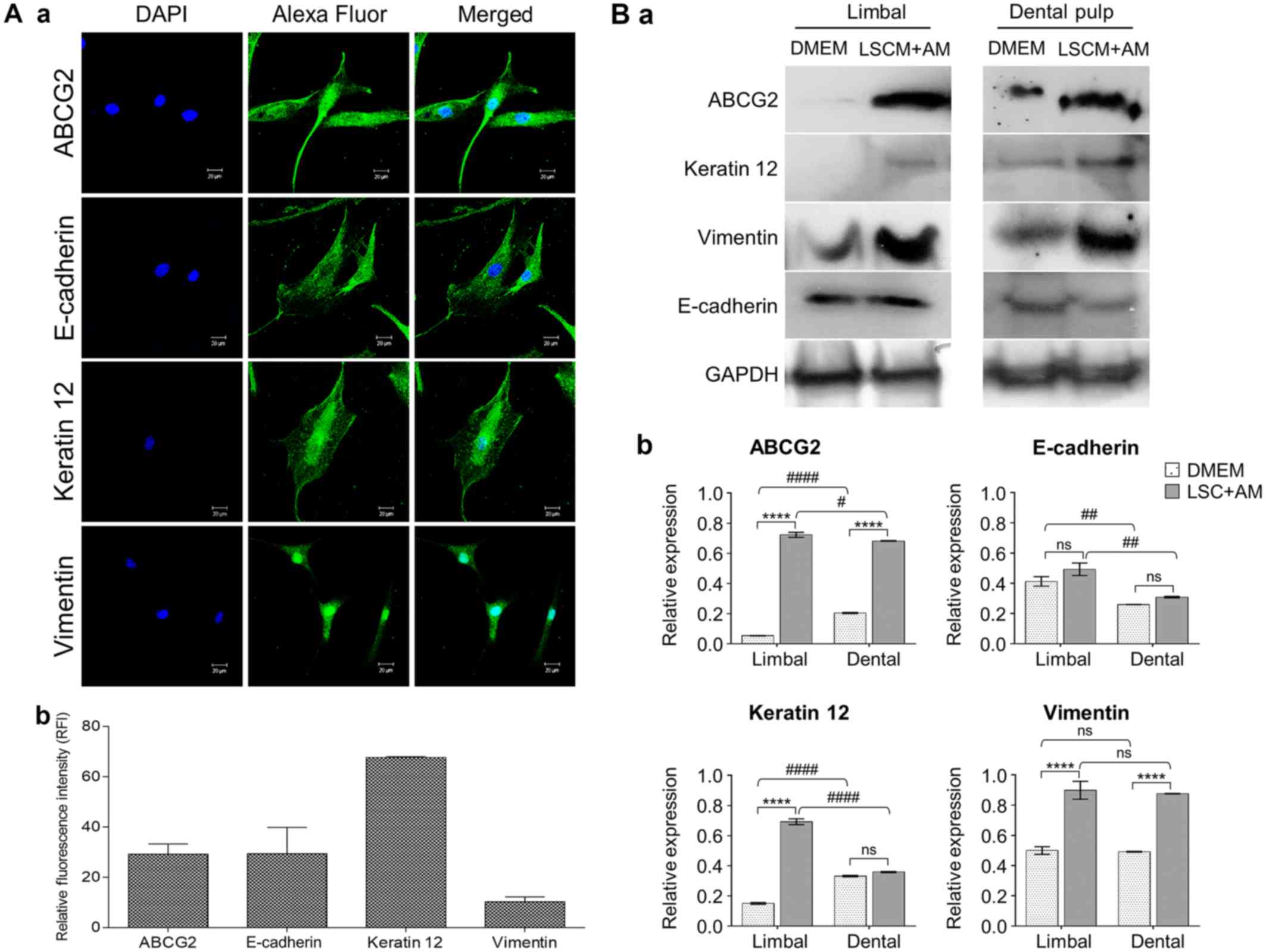
E-cadherin. Immunofluorescence analysis of DPSCs grown in LSC media
revealed positive expression for limbal-specific epithelial stem
cell markers such as vimentin, ABCG2, and keratin 12 (Fig. 3A) (25). Similarly, expression of all stem
cell markers in limbal and dental pulp stem cells was confirmed by
western blot analyses (Fig. 3B).
Although molecular expression is quite low at the transcript level
in DPSCs, the functional protein level expression according to
immunocytochemistry and western blot analyses revealed that stem
cell and corneal differentiation molecule levels were significantly
(P≤0.05) high. Thus, these cells can function as limbal stem cells
in the appropriate microenvironment.
Discussion
Stem cell deficiency in the limbus region of the eye
can reduce vision and seriously affect the quality of life. Several
treatment modalities have been developed for limbal stem cell
deficiency (LSCD). The development of methods for ex vivo
engineering of stem cells provided better treatment options for
LSCD patients involving transplantation of in vitro cultured
LSCs (14,27). Studies have revealed that cells
with high in vitro self-renewal and multi-potential
differentiation capacity are good candidates for cell therapy.
Therefore, various tissues including adipose, umbilical cord blood,
amniotic fluid, and placental tissues, peripheral blood, Wharton's
jelly, dental pulp, oral mucosa, corneal epithelium, dermal
fibroblasts, cartilage and bone marrow, have been evaluated for
deriving stem cells for autogenic transplantation (16–19).
Amniotic membrane transplantation (AMT) has become a popular
therapy for ocular surface damage and can be used to treat partial
LSCD on its own or to treat total LSCD by limbal allografting
(13). Drug therapy can be
combined with AMT, such as the use of antivirals and steroid
therapies to arrest inflammation and treat ocular herpes (28). Additionally, the development of a
suitable culture system using different carriers of sheet, culture
medium, or feeder layers are essential to avoid immune reactivity
and produce high-quality stem cells.
Many procedures have been developed for treating
LSCD and restoring at least some functional vision. In the present
study, DPSCs were cultured in LSC media supplemented with an
amniotic membrane to induce regeneration of limbal stem cells.
DPSCs can be easily accessed during tooth extraction or minimally
invasive pulpectomy. Thus, there is no risk of limbal grafting and
harvesting epithelial/limbal cells from a healthy eye is
unnecessary (29). Previously,
Karaöz et al (30) revealed
that DPSCs have properties of epithelial stem cells and that the
expression of several markers was similar to that in bone marrow
stem cells. Furthermore, they found that DPSCs have a higher rate
of proliferation and stronger neural and epithelial stem cell
properties, including the expression of corneal epithelial specific
cytokeratin 12 (31). Spath et
al (32) revealed that the
explant method of DPSC isolation enhanced the cell differentiation
abilities compared to the enzymatic digestion method, whereas
Kerkis and Caplan (33) revealed
no difference between the two methods. The methods of isolation and
selection are key determinants in the transdifferentiation success
of stem cell lines (34,35). Therefore, the explant protocol was
used successfully and demonstrated the differentiation of DPSCs to
limbal stem cells in the present study. DPSCs grown in the presence
of the amniotic membrane revealed similar morphological and
molecular characteristics as limbal stem cells. In the present
study, the dental pulp stem cells were characterized with the
respective marker analysis by western blot assay. However,
fluorescence-activated cell sorting should be conducted to ensure
the homogeneous cell population of DPSCs, which may further enhance
specific epithelial regeneration. Multipotency of isolated DPSCs
through differentiation along adipogenic, chondrogenic, and
osteogenic lineages is not elucidated as it is out of the scope of
the present preliminary study. Although the data presented
evaluates the use of dental pulp stem cells in bilateral LSCD
cases, further additional information concerning the functionality
of LSC is required before its application for potential therapeutic
use. In recent studies, researchers used different media factors
and in other studies different scaffolds such as contact lenses
were used (30,32). Although use of amniotic membrane in
corneal treatment is an old practice, combination of amniotic
membrane as scaffold to grow and tune these dental pulp stem cells
to limbal stem cells is unique and an easy standardization
protocol, which was adapted in the present study (31,36).
In summary, it was demonstrated that stem cells can
be easily isolated from dental pulp and used for limbal stem cell
differentiation. Additionally, these transdifferentiated DPSCs
could provide a good barrier between corneal and conjunctival
epithelia and prevent conjunctivalization of the cornea compared to
other cell sources.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
Advanced Research Unit of Rajiv Gandhi University of Health
Sciences (grant no. 14M006; Bengaluru, Karnataka. India).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SP, MP and PS conceived and planned the experiments.
SP, PS, CD, AB, MP and SK conducted the experiments. PP, SP, VP, VK
and PS contributed to the analysis and interpretation of the
results. SP and PS investigated and supervised the findings of this
study. PS wrote the manuscript with support from SP, PP and VK. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study protocol was approved by SDM
College of Medical Sciences and Hospital (Manjushree Nagar,
Dharwad, Karnataka, India; permit no. 065, ECR/683/INST/KA/2014).
Informed consent was obtained from the donor patient family.
Consent for the use of the placenta was obtained from the mothers
before delivery. Informed consent was obtained from the patients
and their parents for dental pulp extraction.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Vashist P, Gupta N, Tandon R, Gupta S and
Sreenivas V: Burden of corneal blindness in India. Indian J
Community Med. 38:198–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oliva MS, Schottman T and Gulati M:
Turning the tide of corneal blindness. Indian J Ophthalmol.
60:423–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Q, Xu J and Deng SX: The diagnosis of
limbal stem cell deficiency. Ocul Surf. 16:58–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barut Selver Ö, Yağcı A, Eğrilmez S,
Gürdal M, Palamar M, Çavuşoğlu T, Ateş U, Veral A, Güven Ç and
Wolosin JM: Limbal stem cell deficiency and treatment with stem
cell transplantation. Turk J Ophthalmol. 47:285–291. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmad S: Concise review: Limbal stem cell
deficiency, dysfunction, and distress. Stem Cells Transl Med.
1:110–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebrahimi M, Taghi-Abadi E and Baharvand H:
Limbal stem cells in review. J Ophthalmic Vis Res. 4:40–58.
2009.PubMed/NCBI
|
|
7
|
Lim P, Fuchsluger TA and Jurkunas UV:
Limbal stem cell deficiency and corneal neovascularization. Semin
Ophthalmol. 24:139–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skeens HM, Brooks BP and Holland EJ:
Congenital aniridia variant: Minimally abnormal irides with severe
limbal stem cell deficiency. Ophthalmology. 118:1260–1264.
2011.PubMed/NCBI
|
|
9
|
Sejpal K, Bakhtiari P and Deng SX:
Presentation, diagnosis and management of limbal stem cell
deficiency. Middle East Afr J Ophthalmol. 20:5–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
White ML, Chodosh J, Jang J and Dohlman C:
Incidence of Stevens-Johnson syndrome and chemical burns to the
eye. Cornea. 34:1527–1533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Araújo AL, Ricardo JR, Sakai VN, Barros JN
and Gomes JÁ: Impression cytology and in vivo confocal microscopy
in corneas with total limbal stem cell deficiency. Arq Bras
Oftalmol. 76:305–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Medical Advisory Secretariat, . Limbal
stem cell transplantation: An evidence-based analysis. Ont Health
Technol Assess Ser. 8:1–58. 2008.
|
|
13
|
Liang L, Sheha H, Li J and Tseng S: Limbal
stem cell transplantation: New progresses and challenges. Eye
(Lond). 23:1946–1953. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burman S and Sangwan V: Cultivated limbal
stem cell transplantation for ocular surface reconstruction. Clin
Ophthalmol. 2:489–502. 2008.PubMed/NCBI
|
|
15
|
Dua HS and Azuara-Blanco A: Corneal
allograft rejection: Risk factors, diagnosis, prevention, and
treatment. Indian J Ophthalmol. 47:3–9. 1999.PubMed/NCBI
|
|
16
|
Pirjali T, Azarpira N, Ayatollahi M,
Aghdaie MH, Geramizadeh B and Talai T: Isolation and
characterization of human mesenchymal stem cells derived from human
umbilical cord Wharton's Jelly and amniotic membrane. Int J Organ
Transplant Med. 4:111–116. 2013.PubMed/NCBI
|
|
17
|
Haagdorens M, Van Acker SI, Van Gerwen V,
Ní Dhubhghaill S, Koppen C, Tassignon MJ and Zakaria N: Limbal stem
cell deficiency: Current treatment options and emerging therapies.
Stem Cells Int. 2016:97983742016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhamodaran K, Subramani M, Ponnalagu M,
Shetty R and Das D: Ocular stem cells: A status update! Stem Cell
Res Ther. 5:562014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
He H and Yiu SC: Stem cell-based therapy
for treating limbal stem cells deficiency: A review of different
strategies. Saudi J Ophthalmol. 28:188–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pisciotta A, Carnevale G, Meloni S, Riccio
M, De Biasi S, Gibellini L, Ferrari A, Bruzzesi G and De Pol A:
Human dental pulp stem cells (hDPSCs): Isolation, enrichment and
comparative differentiation of two sub-populations. BMC Dev Biol.
15:142015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferro F, Spelat R and Baheney CS: Dental
pulp stem cell (DPSC) isolation, characterization, and
differentiation. Methods Mol Biol. 1210:91–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang AH, Chen YK, Lin LM, Shieh TY and
Chan AW: Isolation and characterization of dental pulp stem cells
from a supernumerary tooth. J Oral Pathol Med. 37:571–574. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shetty PK, Thamake SI, Biswas S, Johansson
SL and Vishwanatha JK: Reciprocal regulation of annexin A2 and EGFR
with Her-2 in Her-2 negative and herceptin-resistant breast cancer.
PLoS One. 7:e442992012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Polisetti N, Agarwal P, Khan I, Kondaiah
P, Sangwan VS and Vemuganti GK: Gene expression profile of
epithelial cells and mesenchymal cells derived from limbal explant
culture. Mol Vis. 16:1227–1240. 2010.PubMed/NCBI
|
|
26
|
Greco D, Vellonen KS, Turner HC, Häkli M,
Tervo T, Auvinen P, Wolosin JM and Urtti A: Gene expression
analysis in SV-40 immortalized human corneal epithelial cells
cultured with an air-liquid interface. Mol Vis. 16:2109–2120.
2010.PubMed/NCBI
|
|
27
|
Nam SM, Maeng YS, Kim EK, Seo KY and Lew
H: Ex vivo expansion of human limbal epithelial cells using human
placenta-derived and umbilical cord-derived mesenchymal stem cells.
Stem Cells Int. 2017:42061872017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi W, Chen M and Xie L: Amniotic membrane
transplantation combined with antiviral and steroid therapy for
herpes necrotizing stromal keratitis. Ophthalmology. 114:1476–1481.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Atallah MR, Palioura S, Perez VL and
Amescua G: Limbal stem cell transplantation: Current perspectives.
Clin Ophthalmol. 10:593–602. 2016.PubMed/NCBI
|
|
30
|
Karaöz E, Demircan PC, Sağlam O, Aksoy A,
Kaymaz F and Duruksu G: Human dental pulp stem cells demonstrate
better neural and epithelial stem cell properties than bone
marrow-derived mesenchymal stem cells. Histochem Cell Biol.
136:455–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kushnerev E, Shawcross SG, Sothirachagan
S, Carley F, Brahma A, Yates JM and Hillarby MC: Regeneration of
corneal epithelium with dental pulp stem cells using a contact lens
delivery system. Invest Ophthalmol Vis Sci. 57:5192–5199. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spath L, Rotilio V, Alessandrini M,
Gambara G, De Angelis L, Mancini M, Mitsiadis TA, Vivarelli E, Naro
F, Filippini A and Papaccio G: Explant-derived human dental pulp
stem cells enhance differentiation and proliferation potentials. J
Cell Mol Med. 14:1635–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kerkis I and Caplan AI: Stem cells in
dental pulp of deciduous teeth. Tissue Eng Part B Rev. 18:129–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhan X, Dravid G, Ye Z, Hammond H,
Shamblott M, Gearhart J and Cheng L: Functional antigen-presenting
leucocytes derived from human embryonic stem cells in vitro.
Lancet. 364:163–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Graziano A, d'Aquino R, Laino G and
Papaccio G: Dental pulp stem cells: A promising tool for bone
regeneration. Stem Cell Rev. 4:21–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Syed-Picard FN, Du Y, Lathrop KL, Mann MM,
Funderburgh ML and Funderburgh JL: Dental pulp stem cells: A new
cellular resource for corneal stromal regeneration. Stem Cells
Transl Med. 4:276–285. 2015. View Article : Google Scholar : PubMed/NCBI
|