Introduction
Asthma is a complex type of allergic inflammation
that principally involves the conducting airways and is often
related to airway remodeling (1).
The importance of tissue remodeling, which involves extracellular
matrix (ECM) deposition and fibroblast proliferation instead of
eosinophilic infiltration in the airway walls, is an early and
persistent component of asthma that has been emphasized (2). Fibroblasts may play a key role in
peribronchial fibrosis. Nevertheless, the origin of these
fibroblasts and the exact pathogenesis of airway remodeling in
asthma remain unclear.
The destruction of epithelial integrity is an
important event in airway remodeling and airway hyperresponsiveness
(3). In recent years, studies have
suggested that epithelial-mesenchymal transition (EMT) provides a
direct source of fibroblasts (4–6). EMT
is a biological process in which epithelial cells undergo multiple
biochemical changes to acquire a mesenchymal cell phenotype. House
dust mite (HDM) is the major indoor allergen and is associated with
allergic response in asthma patients. A previous study demonstrated
that HDM combined with transforming growth factor β1 (TGF-β1) can
induce marked EMT characteristics (7). Chronic HDM exposure leads to TGF-β
expression in the airway epithelium and the induction of EMT
(8). These results suggest that
EMT may play a unique role in the airway remodeling of asthma.
However, the exact molecular mechanism of EMT in allergic asthma
remains unclear.
Sonic hedgehog (SHH) signaling is an evolutionarily
conserved pathway involved in a variety of biological processes
during normal embryonic development and adult tissue homeostasis
(9). SHH signaling molecules
include a receptor Patched (Ptch) and a signal transducer
Smoothened (Smo). Once Hedgehog binds to Ptch, the inhibition of
Smo is relieved, leading to the translocation of full-length Gli
proteins into the nucleus and the activation of the expression of
hedgehog target genes (10). The
Sonic hedgehog pathway is significantly upregulated in the airway
epithelium of children with asthma (11). Snail1 is a major target of the SHH
signaling pathway, which regulates EMT and fibroblast motility
(12). Shh is expressed in the
lung and regulates epithelial-mesenchymal crosstalk (13). A genome-wide association study
linked Hedgehog interacting protein (HHIP) and Ptch1 mutations to
lung function decline and different asthma phenotypes (14). HHIP acts in a negative feedback
loop to attenuate Hedgehog signaling by affecting Hedgehog
proteins. However, the mechanism of SHH signaling that contributes
to the pathophysiology of asthma is still unclear.
To the best of our knowledge, there has been no
systematic study of the possible molecular mechanisms of HDM that
may be involved in EMT in human bronchial epithelial cells (HBECs).
A previous study demonstrated that HDM alone could not induce cell
morphological and phenotypic changes in HBECs, but HDM combined
with TGF-β1 can induce marked EMT characteristics (7). Therefore, the present study was
designed to examine whether HDM/TGF-β1 could trigger EMT in HBECs
via the SHH signaling pathway. The identification of the exact
molecular mechanism that underlies EMT may facilitate the search
for new targets to prevent airway remodeling in asthma.
Materials and methods
Cell culture
An HBEC cell line (16HBECs; American Type Culture
Collection/ATCC) was cultured in 2 ml of DMEM (Sigma-Aldrich; Merck
KGaA) containing 10% foetal bovine serum (FBS) and penicillin (100
U/ml). Prior to treatment, cells were seeded at a density of
5–6×105 cells per well in 6-well plates. The cells were
maintained at 37°C in a humidified atmosphere with 5%
CO2. The confluent 16HBECs were serum-starved overnight
and were incubated with or without HDM (30 µg/ml; cat. no.
XPB82D3A2.5; Greer Laboratories, Lenoir, NC, USA) and TGF-β1 (5
ng/ml; cat. no. T7039; Sigma-Aldrich; Merck KGaA) for 24–72 h.
Cell viability assay
To determine a suitable concentration for
cyclopamine treatment, a Counting Kit-8 assay (CCK-8) was used to
monitor cellular viability. In brief, 16HBECs were seeded at a
density 1×103 cells/ml in 96-well plates and were
treated with cyclopamine at different concentrations (0, 5, 10, 20
and 40 µM) for 72 h. At the end of the treatment, CCK-8 reagent
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added
to each well of the plates, and then the plates were incubated at
37°C in an incubator for 4 h. Finally, the absorbance values at 450
nm were measured using a microplate reader (FLX800TBID, Bio-Tek
Instruments, USA). The cell viability was analyzed in
triplicate.
Western blot analysis
The cells were lysed on ice using RIPA buffer
containing protease inhibitors. The cytoplasmic extract (CE) buffer
was composed of 1% Nonidet P-40, 150 mM NaCl, 50 mM Tris pH 8.0, 1
mM sodium orthovanadate and 5 mM NaF. The nuclear and cytoplasmic
proteins were extracted according to the manufacturer's protocol,
and proteins (30 µg/lane) were separated by 10% SDS-PAGE,
subsequently electro-transferred onto PVDF membranes. The detection
of blotted proteins was performed with anti-E-cadherin (1:500; cat.
no. sc-8426), anti-type I collagen (1:1,000; cat. no. sc-59772),
anti-Gli1 (1:1,000; cat. no. sc-20687), anti-Snail1 (1:1,000; cat.
no. sc-271977), anti-GAPDH (1:1,000; cat. no. sc-47724) (Santa Cruz
Biotechnology, Inc.) and anti-FSP1 (1:1,000; cat. no. ab124805;
Abcam) antibodies. Primary antibodies were added to the membrane in
5% nonfat dry milk at 4°C overnight and were then incubated with a
horseradish peroxidase-linked anti-rabbit or anti-mouse secondary
antibody (1:2,000; cat. no. sc-2004, sc-2005; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Immunodetection
was performed by chemiluminescence (ECL, Millipore, Billerica, MA,
USA). GAPDH was used as an internal control. The gray levels of the
blots were measured using ImageJ software version 1.48 (National
Institutes of Health, Bethesda, MD, USA). The experiment was
repeated three times.
Immunofluorescence staining
Immunofluorescence staining was performed as
described previously (15). HDM
and TGF-β1 were added to the wells. The cells were incubated with
anti-E-cadherin (1:50; cat. no. sc-8426), anti-Gli1 (dilution
1:100; cat. no. sc-20687; Santa Cruz Biotechnology, Inc.), and
anti-FSP1 (1:150; cat. no. ab124805; Abcam) antibodies at 4°C
overnight, and were subsequently stained with secondary antibodies
conjugated with Alexa Green 488 (1:1,000; cat. no. A-11001;
Molecular Probes) or with Cy3-labelled secondary antibodies
(1:1,000; cat. no. 111-136-144; Jackson ImmunoResearch
Laboratories) for 1 h at room temperature. Cells were also stained
with DAPI. The slides were visualized with a Zeiss Axio Imager 2
microscope (Carl Zeiss AG).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was isolated and reverse transcribed into
cDNA using a ReverTra Ace qPCR RT kit (Toyobo Biotechnology, Tokyo,
Japan). Gene expression was determined by SYBR Green Real-Time PCR
Master Mix (Toyobo Biotechnology). The primer pairs were
synthesized by Invitrogen; Thermo Fisher Scientific, Inc. The
primer sequences used were as follows: SHH (sense
5′-AAGGTATGAAGGGAAGATCT-3′ and antisense
5′-CCAAAGCGTTCAACTTGTC-3′); Gli1 (sense 5′-GTGGAAATGACTGGCAATGC-3′
and antisense 5′-TGCGGCGTTCAAGAGAGACT-3′); Snail1 (sense
5′-TCCTTCGTCCTTCTCCTCTA-3′ and antisense 5′-GCTTCGGATGTGCATCTTG-3′)
and GAPDH (sense 5′-GCCTTCCGTGCCCCACTGC-3′ and antisense
5′-GGCTGGTGGTCCAGGGGTCT-3′). GAPDH was used as an internal control.
Gene expression was calculated using the 2−ΔΔCq method
(16).
Blockade of SHH signaling
Small-interfering RNA (siRNA) for Gli1 or
cyclopamine was used to inhibit SHH signaling. Cyclopamine is an
isolated alkaloid that shows strong potential to bind to SMO and
inhibit the SHH signaling pathway (10). Cells (16HBECs) were seeded in
24-well plates at a density of 3×104 cells/well and were
transfected with siRNA for Gli1 (5′-AACUCCACAGGCAUACAGGAU-3′) and a
negative control siRNA (5′-AACGUACGCGGAAUACAACGA-3′) purchased from
Sangon Biotech (Shanghai, China), which were used in a previous
study (17). siRNA was introduced
into cells using Lipofectamine LIXV reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Twenty-four hours later, the cells were
stimulated with HDM/TGF-β1. After 72 h, cells were harvested for
the detection of downstream target genes in the SHH signaling
pathway. All of the siRNA experiments were performed in
triplicate.
Cyclopamine (Sigma-Aldrich; Merck KGaA) was
dissolved in dimethyl sulfoxide (DMSO) (at 100 mmol/l stock
solution) (18). The cells were
transfected in 24-well plates at a density of 3×104
cells/well. After 24 h, the 16HBECs were stimulated with HDM/TGF-β1
and were then treated with cyclopamine for 72 h. Control wells were
treated with only 0.1% (v/v) DMSO. The total amount of DMSO in the
medium never exceeded 1% (v/v). Finally, the cells were harvested
for further experiments.
Statistical analysis
All data are expressed as the mean ± the standard
error of the mean (SEM). Group differences were analyzed by a
Student's t-test or one-way ANOVA with Tukey post hoc analysis.
Statistical significance was set at P<0.05.
Results
HDM combined with TGF-β1 induces EMT
in HBECs
The HDM and TGF-β1 concentrations were chosen
according to previous studies (7,19).
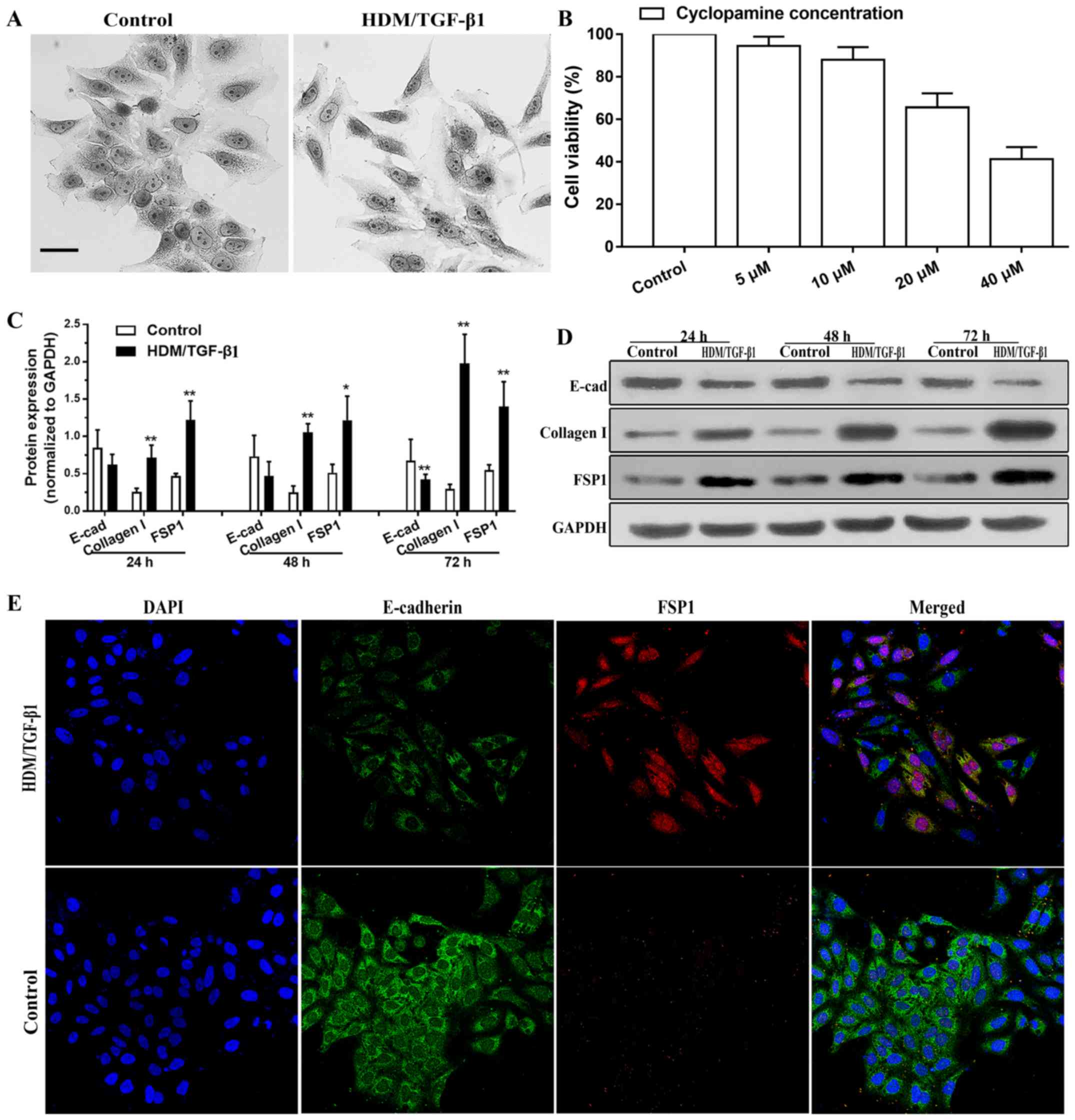
First, we examined whether HDM/TGF-β1 could induce a morphological
change that was characteristic of EMT. Cells (16HBECs) in culture
exhibited a cobblestone morphology. However, after stimulation by
HDM/TGF-β1, the cells acquired an elongated and mesenchymal-like
morphology with a loss of cell-cell contact (Fig. 1A). After 72 h of exposure to
HDM/TGF-β1, western blot analysis demonstrated that the protein
level of E-cadherin as decreased; however, the expression levels of
mesenchymal markers type I collagen and FSP1 were markedly
upregulated compared to that in the controls (Fig. 1C and D). As shown by
immunofluorescence staining, the expression of E-cadherin was
decreased in the 16HBECs after 72 h of treatment with HDM/TGF-β1,
and the expression of FSP1 was increased compared to that in the
controls (Fig. 1E). These results
indicated that HDM/TGF-β1 induces EMT characteristics in the
HBECs.
HDM combined with TGF-β1 induces an
increase in the expression of SHH signaling in HBECs
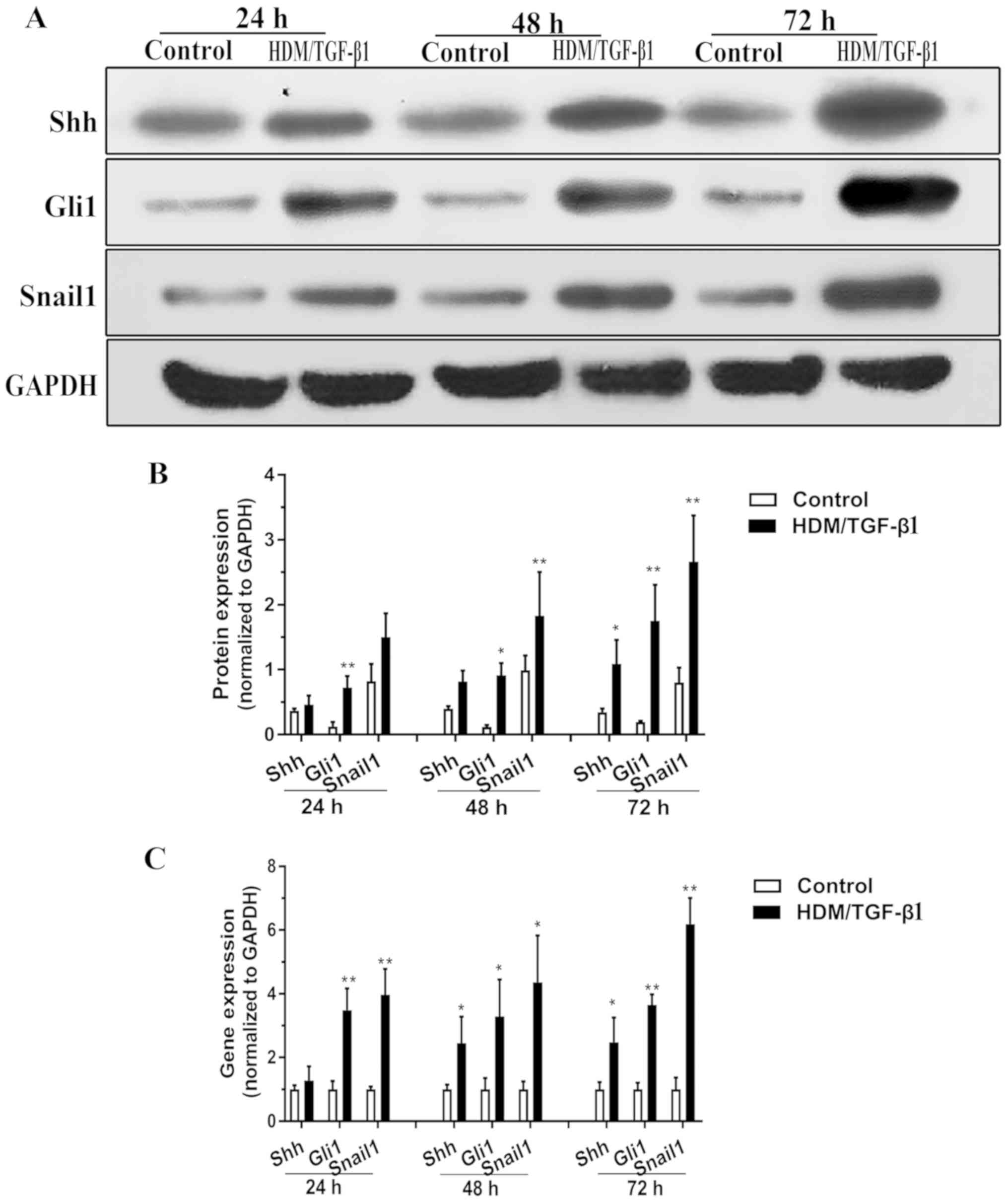
As shown by western blotting, the cells exposed to
HDM/TGF-β1 for 24 h exhibited slightly increased Shh secretion
compared to that in the control cells, and this difference
gradually increased when cells were exposed for 72 h (Fig. 2A and B). qPCR data demonstrated a
significant increase in Shh mRNA expression after 48 h of
HDM/TGF-β1 treatment (Fig. 2C).
These findings demonstrated that the gene expression and protein
levels of Shh were increased. Since Shh has repeatedly been shown
to activate SHH-Gli1 signaling in vitro (20,21),
we explored whether HDM/TGF-β1 could induce an accumulation of
Gli1. Indeed, exposure to HDM/TGF-β1 for 24 h resulted in an
accumulation of the total Gli1 levels compared to the control
(Fig. 2C). Snail1 is a well-known
target of SHH signaling that regulates EMT and fibroblast motility
(12). The expression of Snail1
was significantly increased in the 16HBECs exposed to HDM/TGF-β1
for 48 h compared with its expression in the control cells
(Fig. 2A-C).
Blockade of SHH signaling inhibits the
nuclear translocation of Gli1
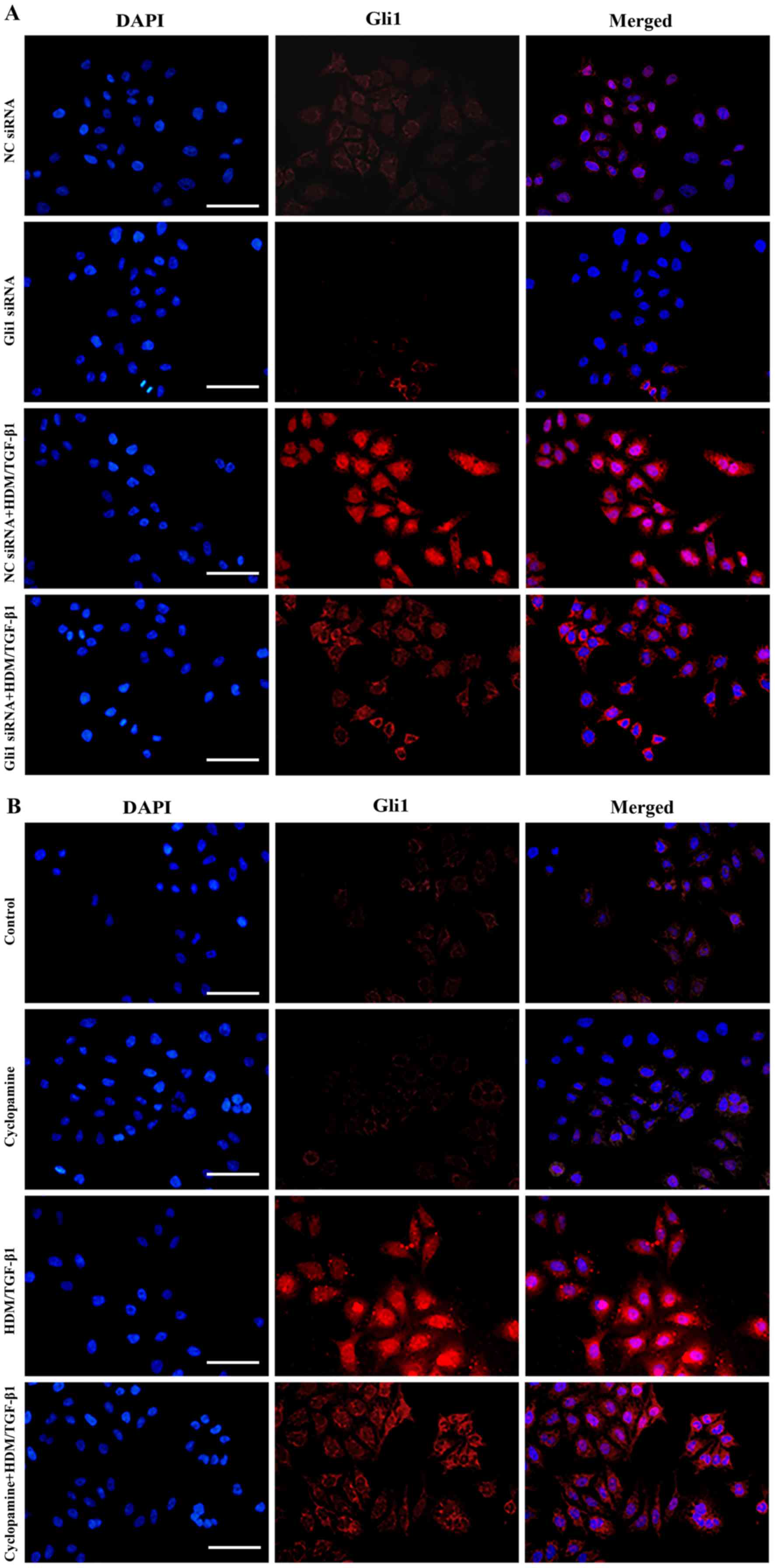
The nuclear translocation of Gli1 is a key inducer
of Snail1 protein expression (22). The nuclear localization and
accumulation of Gli1 are necessary for its transcriptional
activity. By immunofluorescence staining, we found that Gli1
protein expression was increased in cells that were treated with
HDM/TGF-β1 for 72 h compared to that in untreated cells and was
mainly localized in the nucleus of the HDM/TGF-β1-stimulated
16HBECs (Fig. 3A and B).
Using a CCK-8 assay, cyclopamine treatment was shown
to lead to the inhibition of 16HBEC survival in a dose-dependent
manner (Fig. 1B). In the present
study, cyclopamine (10 µM) was used to specifically block the SHH
signaling pathway. After silencing of Gli1 using siRNA or
cyclopamine, the nuclear translocation and protein expression of
Gli1 were significantly decreased (Figs. 3A and B, 4A-C).
Inhibition of SHH signaling attenuates
HDM/TGF-β1-induced EMT in 16HBECs
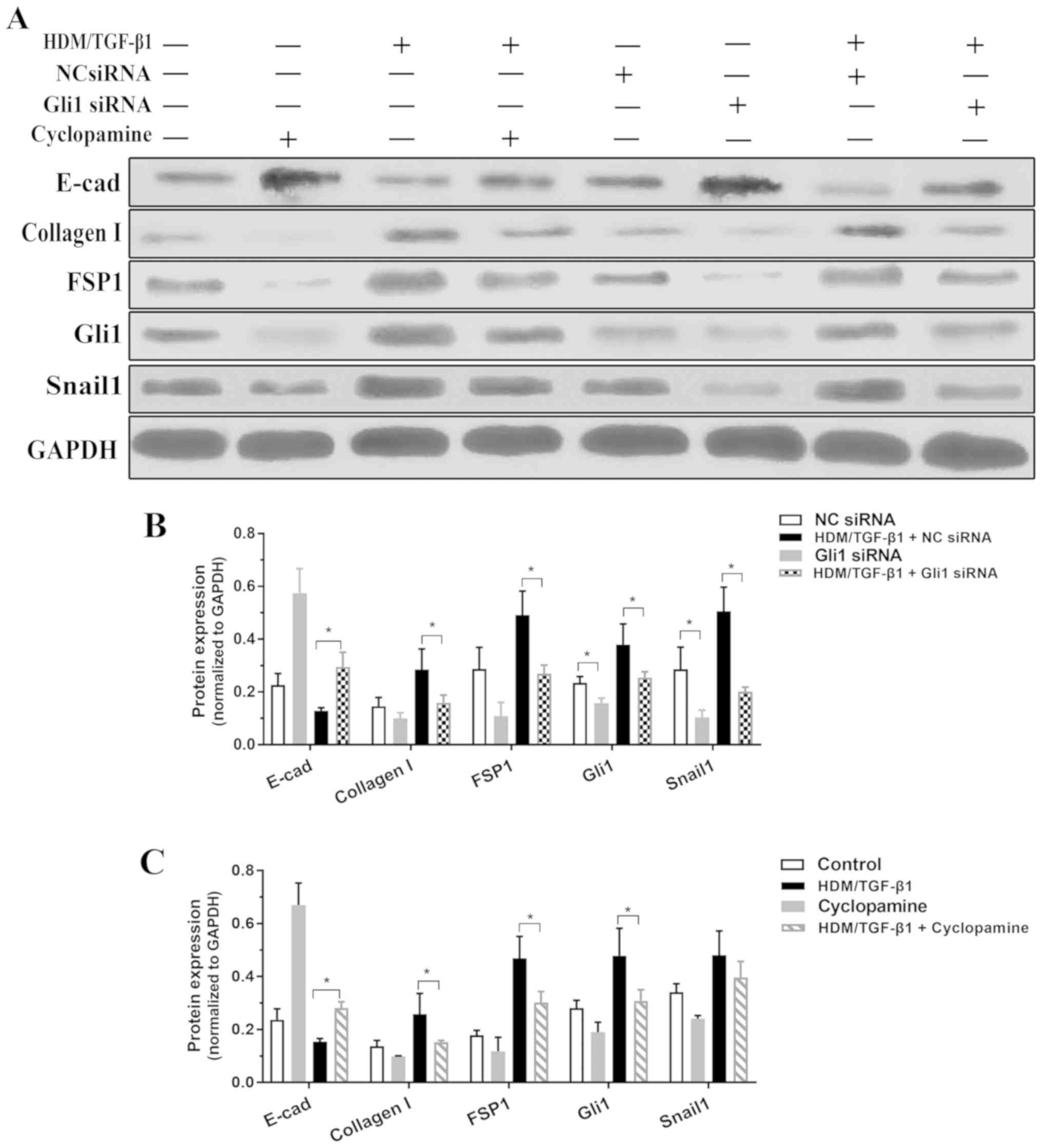
To examine whether SHH signaling mediates
HDM/TGF-β1-induced EMT, siRNA was used to silence Gli1. Gli1 siRNA
was transfected into 16HBECs and reduced Gli1 protein expression in
16HBECs that were treated or untreated by HDM/TGF-β1. Compared to
transfection with a negative control siRNA, transfection with
Gli1-specific siRNA significantly decreased the EMT process, as
demonstrated by the decreased expression of type I collagen and
FSP1 and the increased expression of E-cadherin (Fig. 4A and B). Cultured 16HBECs were
treated with cyclopamine following HDM/TGF-β1 stimulation. As shown
in Fig. 4A and C, this inhibitor
of SHH signaling effectively antagonized the EMT process in
vitro.
Discussion
The bronchial epithelium is an initial barrier and
may be more susceptible to inhaled noxious agents (7). Airway remodeling is a marked feature
of asthma that aims to maintain epithelial integrity. The present
study revealed that in human bronchial epithelial cells (HBECs),
exposure to HDM/TGF-β1-induced epithelial-mesenchymal transition
(EMT), which was identified by the upregulation of mesenchymal
markers (FSP1 and type I collagen) and the loss of the adherens
junction protein E-cadherin. We believe these findings provide
evidence for the upregulation of the Sonic hedgehog (SHH) signaling
pathway in house dust mite (HDM)/transforming growth factor β1
(TGF-β1)-mediated EMT in HBECs. Furthermore, SHH activated the
transcription factor Snail1 and was sufficient to induce matrix
production in HBECs in vitro. These results demonstrated
that HDM/TGF-β1 may induce the upregulation of SHH expression along
with the cytoplasmic accumulation and nuclear translocation of
glioma-associated antigen-1 (Gli1). Moreover, the genetic knockout
or pharmacological antagonism of Gli1 ameliorated EMT. In general,
these findings suggest that HDM/TGF-β1 may trigger the induction of
EMT in HBECs via an SHH signaling mechanism. The inhibition of SHH
signaling may be a new therapeutic method for preventing airway
remodeling in asthma.
Is EMT important in lung disease? EMT is estimated
to contribute 36% of the fibroblasts in fibrotic lesions (23). In vitro, the stimulation of
the bronchial airway epithelium by HDM synergized with TGF-β1 and
resulted in reduced E-cadherin expression (7). Johnson and colleagues (8) described airway epithelial cell
transdifferentiation in a mouse model of allergic asthma. In our
study, HBECs stimulated by HDM/TGF-β1 exhibited morphological
features of EMT; by immunofluorescence staining, the expression of
an epithelial marker was decreased, while expression of mesenchymal
markers was increased compared to those in the controls. The
exposure of HBECs to HDM/TGF-β1 induced EMT. These findings are in
line with a previous study that was performed in an HDM-challenged
mouse model (24). This study
showed that EMT may contribute to asthma, but the role of EMT in
asthma needs to be further studied in the future.
The Hedgehog signaling pathway plays an important
role during vertebrate embryonic development and tumorigenesis.
Patched (Ptch) was found to impede Smoothened (Smo) activity when
the Hedgehog ligand is lacking and then prevents the activation of
Gli (14). In the present study,
we first detected the gene and protein expression of the core
molecular components of the SHH signaling pathway in HBECs
stimulated by allergens. By western blot analysis, we found that
Shh and Gli1 secretion was significantly increased in 16HBECs
exposed to HDM/TGF-β1 for 48 h compared to their secretion in
control cells, and real-time PCR revealed the significant
upregulation of SHH and Gli1 mRNA expression. Our results suggested
that the gene expression and protein levels of Shh/Gli1 were
upregulated in response to HDM/TGF-β1. By immunofluorescence
staining, we found that Gli1 protein expression was increased, and
Gli1 was localized primarily to the nucleus of 16HBECs treated with
HDM/TGF-β1. The nuclear localization and accumulation of Gli1 is
essential to its transcriptional activity. The efficiency of Gli1
siRNA or cyclopamine in the inhibition of Gli1 expression was
reported in a previous study (18). After silencing of Gli1 using siRNA
or cyclopamine, the nuclear translocation and protein expression of
Gli1 were significantly decreased. These findings demonstrated that
SHH signaling may play a role in asthma. Our findings are
consistent with two previous studies that showed that SHH was
significantly increased in the bronchial epithelium in asthma
patients (11,25). A recent study reported that Gli
protein expression was strongly associated with EMT biomarkers in
lung carcinomas (26).
In the present study, the expression of the Snail1
protein was significantly increased in 16HBECs exposed to
HDM/TGF-β1. Some studies have emphasized the important roles of the
transcription factor Snail1 during the EMT process (27). To examine the role of Shh/Gli1 in
HDM/TGF-β1-induced Snail1 activation and the EMT process, we
treated HBECs with Gli1 siRNA or cyclopamine. Following the
blockade of SHH signaling, 16HBECs stimulated by HDM/TGF-β1 had
lower cytoplasmic Snail1 expression as well as reduced EMT
biomarker alterations than untreated cells. Based on these data, we
proposed that the combination of HDM and TGF-β1 activated Shh/Gli1,
which upregulated the nuclear translocation of Gli1 and in turn
increased Snail1 expression, finally resulting in EMT.
If SHH signaling is important for EMT induction, the
inhibition of SHH signaling may have antifibrotic effects. The use
of cyclopamine, a well-characterized Smo inhibitor, is limited
in vivo due to its short half-life and off-target effects
occur at high doses (28). Ding
et al (18) demonstrated
that cyclopamine could prevent fibroblast proliferation and matrix
synthesis in renal tubulointerstitial fibrosis. The present study
showed that cyclopamine antagonized the EMT process and reduced ECM
production in vitro. However, the antifibrotic effects of
cyclopamine in asthma warrant further study.
In summary, these findings demonstrate that the
combination of HDM and TGF-β1 may induce EMT in HBECs. The effect
was mainly triggered by the SHH signaling pathway, and the
inhibition of SHH signaling abolished SHH-induced EMT and ECM
synthesis. Therefore, SHH signaling may play a key role in EMT and
airway remodeling in asthma, and pharmacological antagonism of SHH
signaling may have a therapeutic effect in asthma. In the future,
the role of the in vivo blockade of Shh/Gli1 signaling
during EMT requires further study.
Acknowledgements
Not applicable.
Funding
This study was supported by Zhejiang Provincial
Natural Science Foundation of China (grant no. LY16H010002,
2016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and WH designed the research study. YZ, WH, LZ
and YM performed the experiments, WS and YM analyzed the data and
prepared the figures. YZ drafted the manuscript. YZ and YM edited
and revised manuscript. YZ and WH interpreted the results of the
experiments. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Broide DH: Immunologic and inflammatory
mechanisms that drive asthma progression to remodeling. J Allergy
Clin Immunol. 121:560–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holgate ST, Davies DE, Puddicombe S,
Richter A, Lackie P, Lordan J and Howarth P: Mechanisms of airway
epithelial damage: Epithelial-mesenchymal interactions in the
pathogenesis of asthma. Eur Respir J Suppl. 44:24S–29S. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies DE: The role of the epithelium in
airway remodeling in asthma. Proc Am Thorac Soc. 6:678–682. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pain M, Bermudez O, Lacoste P, Royer PJ,
Botturi K, Tissot A, Brouard S, Eickelberg O and Magnan A: Tissue
remodelling in chronic bronchial diseases: From the epithelial to
mesenchymal phenotype. Eur Respir Rev. 23:118–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borthwick LA, Parker SM, Brougham KA,
Johnson GE, Gorowiec MR, Ward C, Lordan JL, Corris PA, Kirby JA and
Fisher AJ: Epithelial to mesenchymal transition (EMT) and airway
remodelling after human lung transplantation. Thorax. 64:770–777.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han Q, Lin L, Zhao B, Wang N and Liu X:
Inhibition of mTOR ameliorates bleomycin-induced pulmonary fibrosis
by regulating epithelial-mesenchymal transition. Biochem Biophys
Res Commun. 500:839–845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heijink IH, Postma DS, Noordhoek JA,
Broekema M and Kapus A: House dust mite-promoted
epithelial-to-mesenchymal transition in human bronchial epithelium.
Am J Respir Cell Mol Biol. 42:69–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson JR, Roos A, Berg T, Nord M and
Fuxe J: Chronic respiratory aeroallergen exposure in mice induces
epithelial-mesenchymal transition in the large airways. PLoS One.
6:e161752011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choudhry Z, Rikani AA, Choudhry AM, Tariq
S, Zakaria F, Asghar MW, Sarfraz MK, Haider K, Shafiq AA and
Mobassarah NJ: Sonic hedgehog signalling pathway: A complex
network. Ann Neurosci. 21:28–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rimkus TK, Carpenter RL, Qasem S, Chan M
and Lo HW: Targeting the Sonic Hedgehog Signaling Pathway: Review
of Smoothened and GLI Inhibitors. Cancers (Basel). 8(pii): E222016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu C, Zou C, Hussain M, Shi W, Shao Y,
Jiang Z, Wu X, Lu M, Wu J, Xie Q, et al: High expression of Sonic
hedgehog in allergic airway epithelia contributes to goblet cell
metaplasia. Mucosal Immunol. 11:1306–1315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signalling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kugler MC, Joyner AL, Loomis CA and Munger
JS: Sonic hedgehog signaling in the lung. From development to
disease. Am J Respir Cell Mol Biol. 52:1–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou W, Zou Y, Zhao Z, Li B and Ran P:
Nicotine-induced epithelial-mesenchymal transition via
Wnt/β-catenin signaling in human airway epithelial cells. Am J
Physiol Lung Cell Mol Physiol. 304:L199–L209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmad A, Maitah MY, Ginnebaugh KR, Li Y,
Bao B, Gadgeel SM and Sarkar FH: Inhibition of Hedgehog signaling
sensitizes NSCLC cells to standard therapies through modulation of
EMT-regulating miRNAs. J Hematol Oncol. 6:772013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding H, Zhou D, Hao S, Zhou L, He W, Nie
J, Hou FF and Liu Y: Sonic hedgehog signaling mediates
epithelial-mesenchymal communication and promotes renal fibrosis. J
Am Soc Nephrol. 23:801–813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steelant B, Farre R, Wawrzyniak P, Belmans
J, Dekimpe E, Vanheel H, Van Gerven L, Kortekaas Krohn I, Bullens
DMA, Ceuppens JL, et al: Impaired barrier function in patients with
house dust mite-induced allergic rhinitis is accompanied by
decreased occludin and zonula occludens-1 expression. J Allergy
Clin Immunol. 137:1043–1053.e5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bolanos AL, Milla CM, Lira JC, Ramírez R,
Checa M, Barrera L, García-Alvarez J, Carbajal V, Becerril C,
Gaxiola M, et al: Role of sonic hedgehog in idiopathic pulmonary
fibrosis. Am J Physiol Lung Cell Mol Physiol. 303:L978–L990. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grindley JC, Bellusci S, Perkins D and
Hogan BL: Evidence for the involvement of the Gli gene family in
embryonic mouse lung development. Dev Biol. 188:337–348. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zavadil J and Bottinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwano M, Plieth D, Danoff TM, Xue C, Okada
H and Neilson EG: Evidence that fibroblasts derive from epithelium
during tissue fibrosis. J Clin Invest. 110:341–350. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pu Y, Liu Y, Liao S, Miao S, Zhou L and
Wan L: Azithromycin ameliorates OVA-induced airway remodeling in
Balb/c mice via suppression of epithelial-to-mesenchymal
transition. Int Immunopharmacol. 58:87–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Standing ASI, Yanez DC, Ross R, Crompton T
and Furmanski AL: Frontline Science: Shh production and Gli
signaling is activated in vivo in lung, enhancing the Th2 response
during a murine model of allergic asthma. J Leukoc Biol.
102:965–976. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yue D, Li H, Che J, Zhang Y, Tseng HH, Jin
JQ, Luh TM, Giroux-Leprieur E, Mo M, Zheng Q, et al: Hedgehog/Gli
promotes epithelial-mesenchymal transition in lung squamous cell
carcinomas. J Exp Clin Cancer Res. 33:342014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hills CE and Squires PE: TGF-beta1-induced
epithelial-to-mesenchymal transition and therapeutic intervention
in diabetic nephropathy. Am J Nephrol. 31:68–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao C, Chen A, Jamieson CH, Fereshteh M,
Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al:
Hedgehog signalling is essential for maintenance of cancer stem
cells in myeloid leukaemia. Nature. 458:776–779. 2009. View Article : Google Scholar : PubMed/NCBI
|