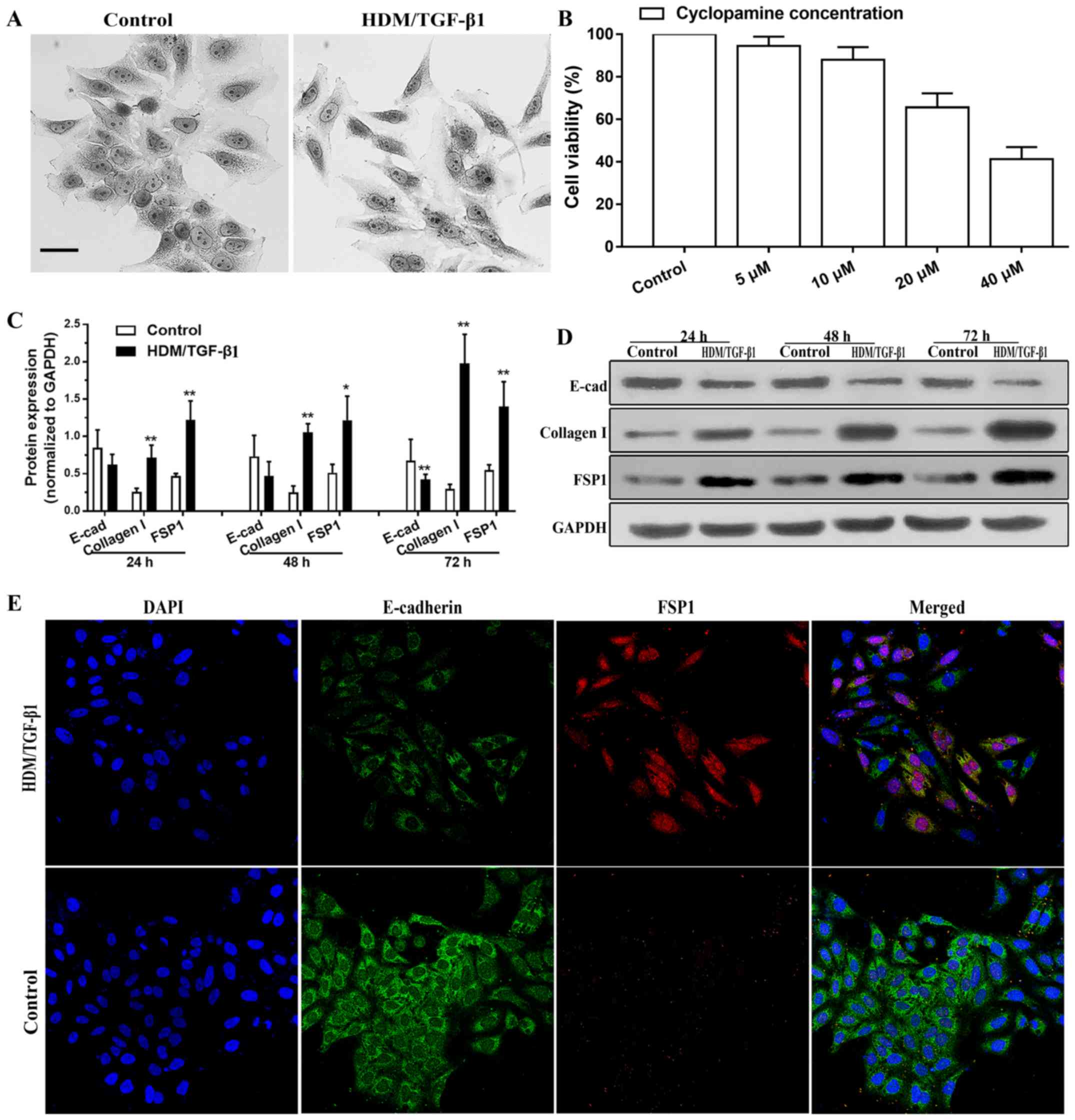
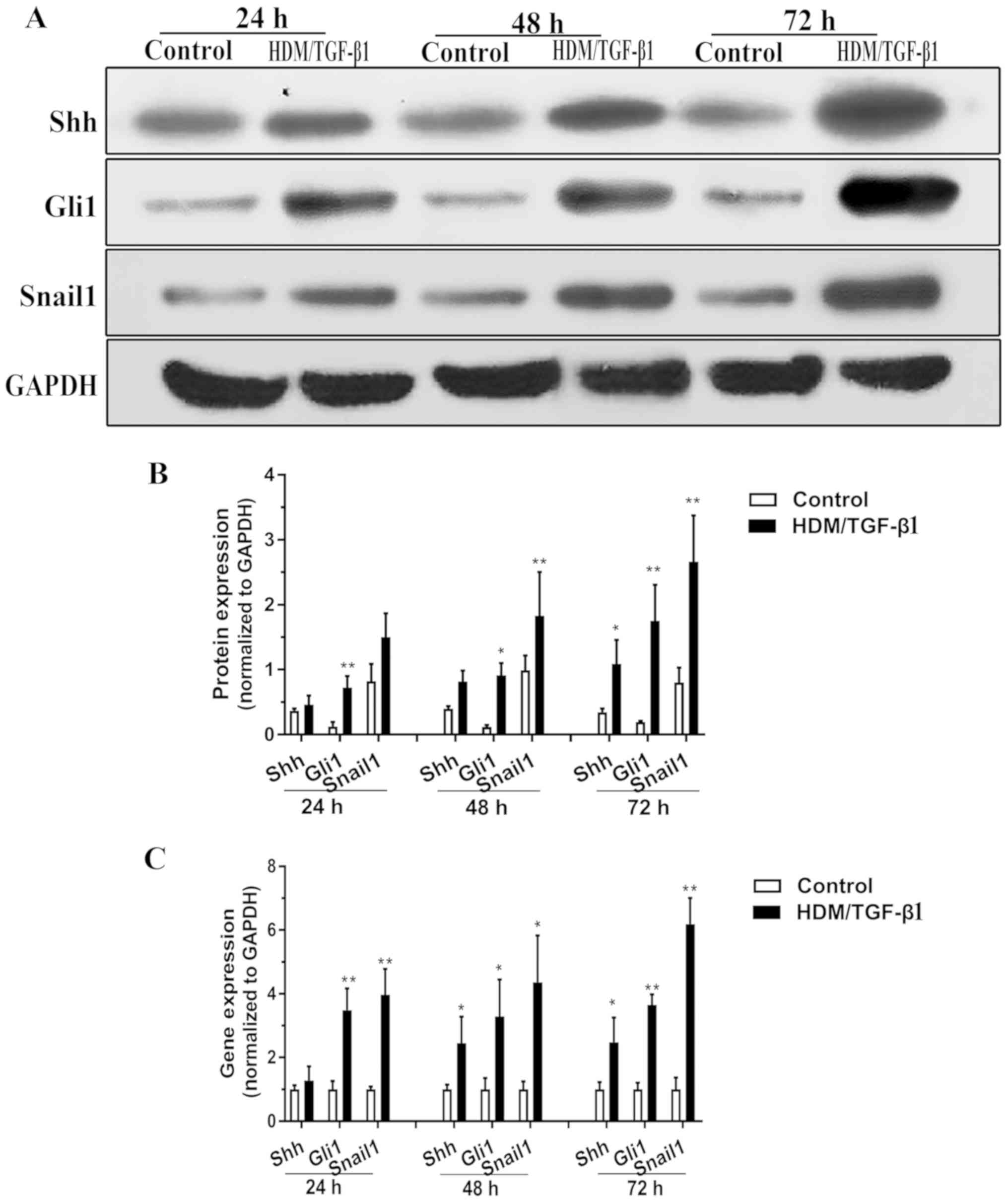
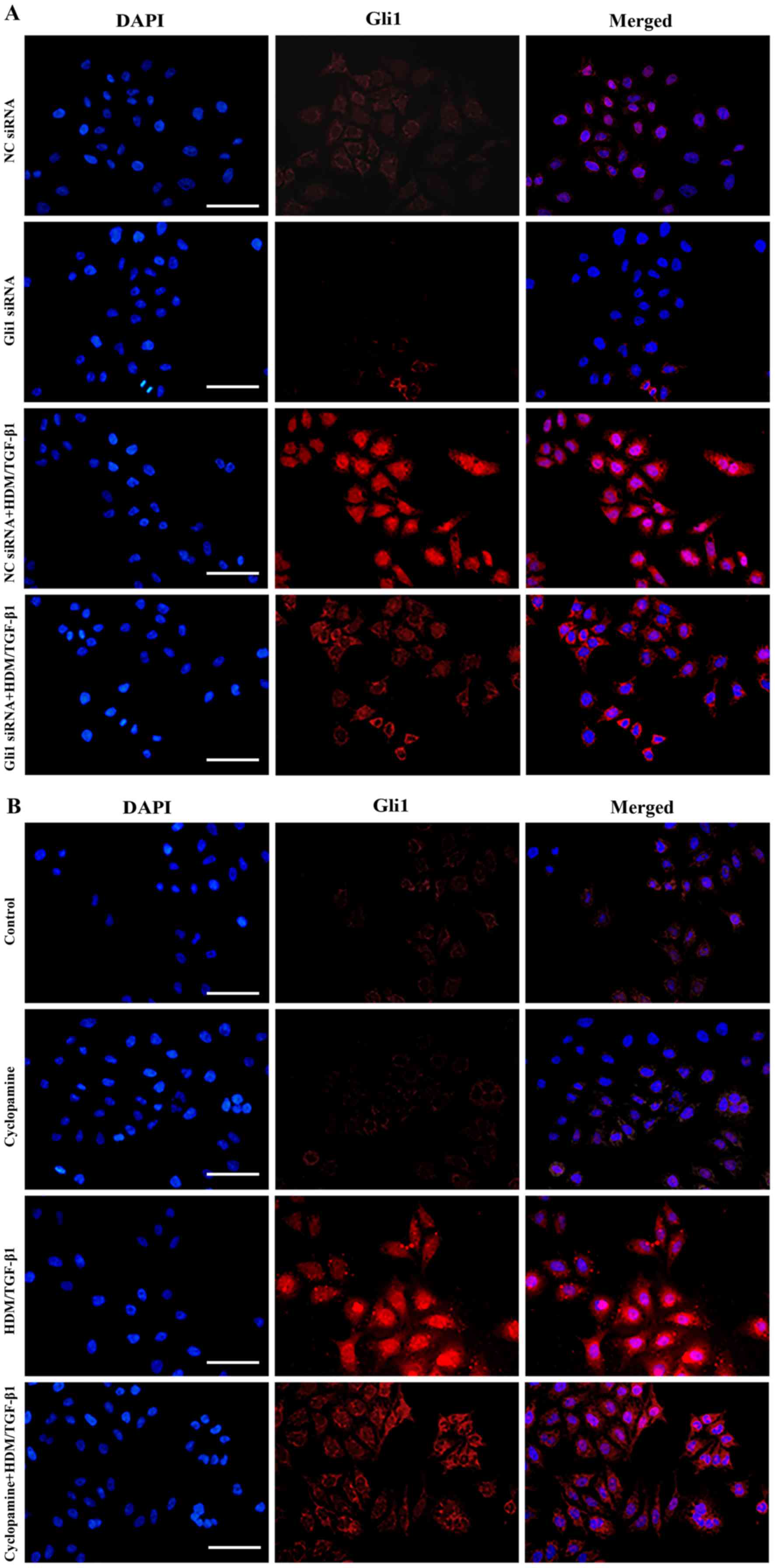
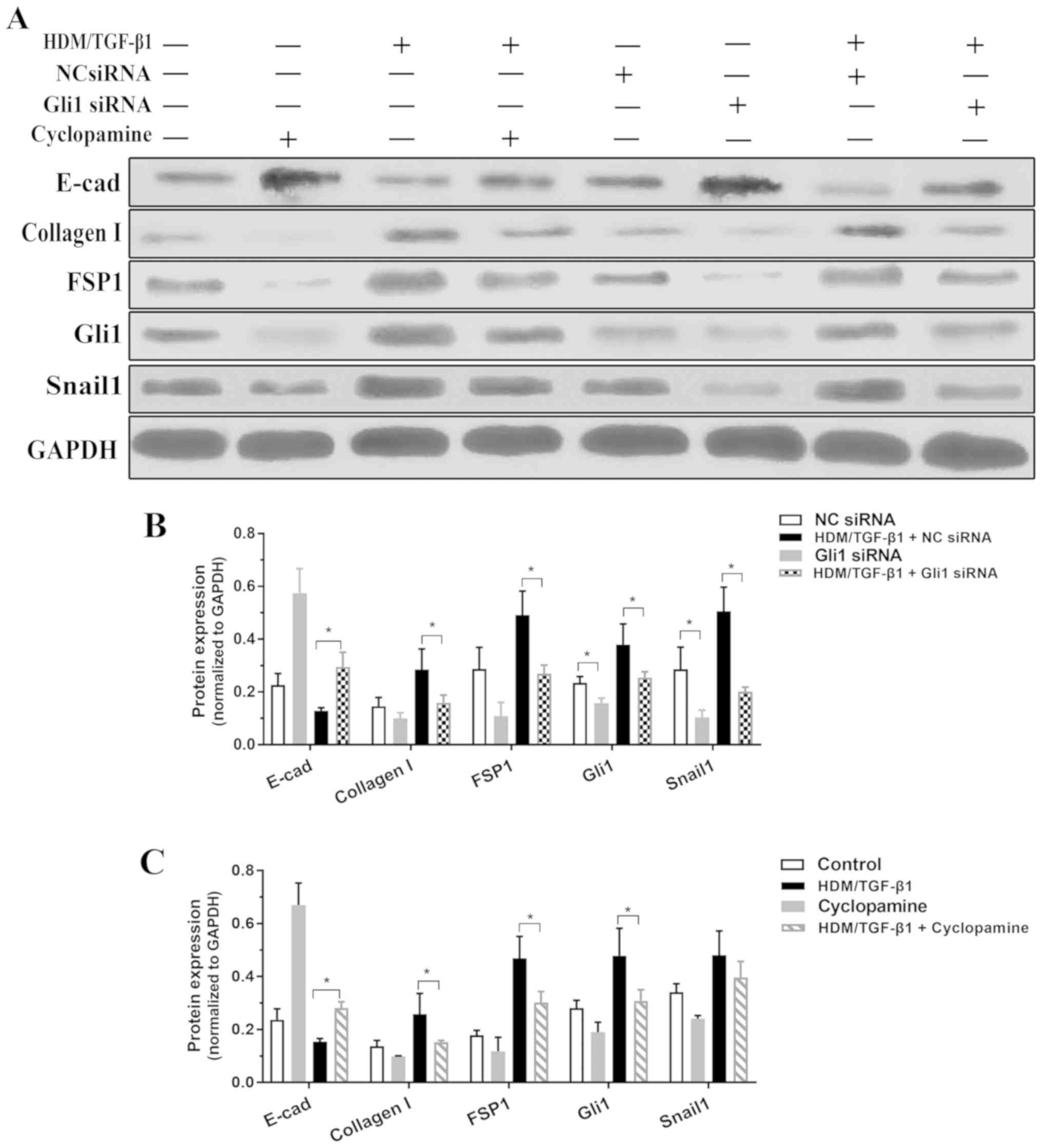
|
1
|
Broide DH: Immunologic and inflammatory
mechanisms that drive asthma progression to remodeling. J Allergy
Clin Immunol. 121:560–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holgate ST, Davies DE, Puddicombe S,
Richter A, Lackie P, Lordan J and Howarth P: Mechanisms of airway
epithelial damage: Epithelial-mesenchymal interactions in the
pathogenesis of asthma. Eur Respir J Suppl. 44:24S–29S. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies DE: The role of the epithelium in
airway remodeling in asthma. Proc Am Thorac Soc. 6:678–682. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pain M, Bermudez O, Lacoste P, Royer PJ,
Botturi K, Tissot A, Brouard S, Eickelberg O and Magnan A: Tissue
remodelling in chronic bronchial diseases: From the epithelial to
mesenchymal phenotype. Eur Respir Rev. 23:118–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borthwick LA, Parker SM, Brougham KA,
Johnson GE, Gorowiec MR, Ward C, Lordan JL, Corris PA, Kirby JA and
Fisher AJ: Epithelial to mesenchymal transition (EMT) and airway
remodelling after human lung transplantation. Thorax. 64:770–777.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han Q, Lin L, Zhao B, Wang N and Liu X:
Inhibition of mTOR ameliorates bleomycin-induced pulmonary fibrosis
by regulating epithelial-mesenchymal transition. Biochem Biophys
Res Commun. 500:839–845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heijink IH, Postma DS, Noordhoek JA,
Broekema M and Kapus A: House dust mite-promoted
epithelial-to-mesenchymal transition in human bronchial epithelium.
Am J Respir Cell Mol Biol. 42:69–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson JR, Roos A, Berg T, Nord M and
Fuxe J: Chronic respiratory aeroallergen exposure in mice induces
epithelial-mesenchymal transition in the large airways. PLoS One.
6:e161752011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choudhry Z, Rikani AA, Choudhry AM, Tariq
S, Zakaria F, Asghar MW, Sarfraz MK, Haider K, Shafiq AA and
Mobassarah NJ: Sonic hedgehog signalling pathway: A complex
network. Ann Neurosci. 21:28–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rimkus TK, Carpenter RL, Qasem S, Chan M
and Lo HW: Targeting the Sonic Hedgehog Signaling Pathway: Review
of Smoothened and GLI Inhibitors. Cancers (Basel). 8(pii): E222016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu C, Zou C, Hussain M, Shi W, Shao Y,
Jiang Z, Wu X, Lu M, Wu J, Xie Q, et al: High expression of Sonic
hedgehog in allergic airway epithelia contributes to goblet cell
metaplasia. Mucosal Immunol. 11:1306–1315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signalling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kugler MC, Joyner AL, Loomis CA and Munger
JS: Sonic hedgehog signaling in the lung. From development to
disease. Am J Respir Cell Mol Biol. 52:1–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou W, Zou Y, Zhao Z, Li B and Ran P:
Nicotine-induced epithelial-mesenchymal transition via
Wnt/β-catenin signaling in human airway epithelial cells. Am J
Physiol Lung Cell Mol Physiol. 304:L199–L209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmad A, Maitah MY, Ginnebaugh KR, Li Y,
Bao B, Gadgeel SM and Sarkar FH: Inhibition of Hedgehog signaling
sensitizes NSCLC cells to standard therapies through modulation of
EMT-regulating miRNAs. J Hematol Oncol. 6:772013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding H, Zhou D, Hao S, Zhou L, He W, Nie
J, Hou FF and Liu Y: Sonic hedgehog signaling mediates
epithelial-mesenchymal communication and promotes renal fibrosis. J
Am Soc Nephrol. 23:801–813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steelant B, Farre R, Wawrzyniak P, Belmans
J, Dekimpe E, Vanheel H, Van Gerven L, Kortekaas Krohn I, Bullens
DMA, Ceuppens JL, et al: Impaired barrier function in patients with
house dust mite-induced allergic rhinitis is accompanied by
decreased occludin and zonula occludens-1 expression. J Allergy
Clin Immunol. 137:1043–1053.e5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bolanos AL, Milla CM, Lira JC, Ramírez R,
Checa M, Barrera L, García-Alvarez J, Carbajal V, Becerril C,
Gaxiola M, et al: Role of sonic hedgehog in idiopathic pulmonary
fibrosis. Am J Physiol Lung Cell Mol Physiol. 303:L978–L990. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grindley JC, Bellusci S, Perkins D and
Hogan BL: Evidence for the involvement of the Gli gene family in
embryonic mouse lung development. Dev Biol. 188:337–348. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zavadil J and Bottinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwano M, Plieth D, Danoff TM, Xue C, Okada
H and Neilson EG: Evidence that fibroblasts derive from epithelium
during tissue fibrosis. J Clin Invest. 110:341–350. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pu Y, Liu Y, Liao S, Miao S, Zhou L and
Wan L: Azithromycin ameliorates OVA-induced airway remodeling in
Balb/c mice via suppression of epithelial-to-mesenchymal
transition. Int Immunopharmacol. 58:87–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Standing ASI, Yanez DC, Ross R, Crompton T
and Furmanski AL: Frontline Science: Shh production and Gli
signaling is activated in vivo in lung, enhancing the Th2 response
during a murine model of allergic asthma. J Leukoc Biol.
102:965–976. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yue D, Li H, Che J, Zhang Y, Tseng HH, Jin
JQ, Luh TM, Giroux-Leprieur E, Mo M, Zheng Q, et al: Hedgehog/Gli
promotes epithelial-mesenchymal transition in lung squamous cell
carcinomas. J Exp Clin Cancer Res. 33:342014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hills CE and Squires PE: TGF-beta1-induced
epithelial-to-mesenchymal transition and therapeutic intervention
in diabetic nephropathy. Am J Nephrol. 31:68–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao C, Chen A, Jamieson CH, Fereshteh M,
Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al:
Hedgehog signalling is essential for maintenance of cancer stem
cells in myeloid leukaemia. Nature. 458:776–779. 2009. View Article : Google Scholar : PubMed/NCBI
|