Introduction
Non-traumatic osteonecrosis (ON) arises from
increased intraosseous pressure caused by fat cell hypertrophy and
abnormal adipogenesis in the bone marrow, which subsequently
results in ischemia which leads to osteocyte and bone marrow cell
(BMC) death (1). The incidence of
ON has been increasing, especially in men between 30 and 50 years
of age (2–4). The underlying pathogenesis of ON is
multifactorial, including adipogenesis or fat hypertrophy,
imbalance of osteoblasts, angiogenesis inhibition and apoptosis of
endothelial cells (5–7). ON therapy primarily includes
conventional core decompression and joint replacement surgery.
However, this treatment only achieves a moderate decrease in
intra-medullary pressure, with few clinical benefits and a high
expense (8).
Based on the theory that mesenchymal stem cells
(MSCs) are pluripotent cells which can differentiate into a range
of cell types, transplantation of MSCs is used as a novel treatment
strategy. For example, implantation of autologous BMCs has been
successfully applied in treating early stages of ON (9,10).
However, several studies indicate that decreased osteogenic
differentiation and replication capacity of MSCs in the bone marrow
also have crucial roles in non-traumatic ON (11), and abnormal MSCs are observed after
transplantation of MSCs (12,13),
thus limiting its application.
MicroRNAs (miRNAs/miRs) are small, endogenous,
noncoding RNAs that potentially regulate a large number of genes
(14–16). miRNAs are thought to modulate 30%
of the human genome through base-pairing with 3′untranslated
regions (UTRs) of their target mRNAs and ultimately cleaving mRNA
or repressing translation mRNAs (14–17).
Although miRNAs serve as regulators in a number of biological
processes, including morphogenesis, differentiation, proliferation
and tumorigenesis (18), there are
relatively fewer reports examining the role of miRNA in the
pathogenesis of ON (19,20).
miR-186 has been reported to serve as an inhibitor
of tumor cell growth and migration in various types of cancer such
as lung cancer (21,22) and colorectal cancer (23). In the present study, the role of
miR-186-5p in osteoblastic differentiation and cell growth of ON
patients was determined. miR-186-5p expression was relatively
higher in MSCs of patients with non-traumatic ON and inhibited the
viability and differentiation of osteoblastic cells in human
mesenchymal stem cells (HMSCs). CXCL13 was a functional target of
miR-186-5p in HMSCs. In addition, miR-186-5p modulated protein
kinase B (AKT) signaling in HMSCs. These findings suggest that
miR-186-5p may be a potential molecular target for treating
patients with non-traumatic ON.
Materials and methods
Clinical samples
The present study was approved by the Ethics
Committee of Wuhan Union Hospital, bone marrow samples were
obtained with informed consent from 15 ON patients and 15
osteoarthritis (OA) patients receiving surgery between October 2010
and July 2013 at Wuhan Union Hospital, Huazhong University of
Science and Technology. Patient characteristics are shown in
Table I.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Group | Number of
patients | Age, years | Sex, F/M |
|---|
| Osteoarthritis | 15 | 52±6 | 8/7 |
| Osteonecrosis |
|
|
|
| Alcohol | 5 | 53±10 | 1/4 |
| Steroid | 9 | 46±14 | 4/5 |
| Idiopathic | 1 | 52 | 1/0 |
Bone marrow samples of 5–10 ml from the proximal end
of the femur of each patient were taken by inserting a tapered awl
into the femoral canal during total hip arthroplasty. OA was
diagnosed using radiographic images, and the diagnostic criteria
were satisfied for all patients. Non-traumatic ON was confirmed
based on radiographic and magnetic resonance imaging. Of the 15
patients with non-traumatic ON, nine were treated with
corticosteroids, five suffered from alcoholism and one was
diagnosed as idiopathic ON. The clinical characteristics of
patients are shown in Table I. To
obtain mesenchymal stem cells (MSCs), mononuclear cells from the
bone marrow were obtained using Ficoll-Hypaque gradient
centrifugation (Fresenius Kabi I Norge AG), suspended in
low-glucose Dulbecco's modified Eagle's medium (DMEM; HyClone; GE
Healthcare Life Sciences) containing 10% fetal bovine serum (FBS;
Gibco: Thermo Fisher Scientific, Inc.) and seeded at a density of
1×106 cells per cm2.
Cell culture
293T cells and HMSC-bm cells were obtained from the
Type Culture Collection of the Chinese Academy of Sciences and
cultured in MSC growth medium consisting of low-glucose DMEM
supplemented with 10% FBS and maintained in a cell incubator at
37°C with 5% CO2. Cells were cultured in flasks and upon
reaching 90% confluency, cells were passaged at 1:2 dilution. Cells
between the 4 and 7th passages were used for the following
experiments. Osteoblastic differentiation was accomplished using
medium containing 100 ng/ml BMP-2 (R&D Systems China, Co.,
Ltd.) and 10% FBS. For AKT activation, cells were incubated with 10
mM LiCl (7447-41-8, ApexBio, Houston, TX) for 48 h after
transfection.
RNA-seq and data analysis
RNA sequencing was performed at Guangzhou RiboBio
Co., Ltd., using an Illumina HiSeq 2500. RNA-seq data was aligned
to the Ensembl transcript annotations (GenBank Assembly ID
GCA_000001405.25) using bowtie and RSEM as previously described
(24). Cufflinks (version 2.2.1)
(25) was used to estimate gene
expression abundance (number of fragments per kb of each gene per
million mapped reads). Differentially expressed genes were detected
using DESeq (version 1.16.0) (26)
and a false discovery rate <0.01 and a fold-change >1.25 were
used as thresholds to define significantly differentially expressed
genes. Other bioinformatics analyses were performed using glbase
(27) and additional; details are
provided in Fig. S1 and Table SIII. Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathway analysis was employed to determine the
significant pathways (http://www.genome.jp/kegg/).
MTT assays
For the cell viability assay, cells were seeded into
a 96-well plate (1×103 cells/well) and used to perform
MTT assays (Sigma-Aldrich; Merck KGaA). MTT diluted in PBS (5
mg/ml) was added to each well (20 µl per well) and incubated for 4
h. Formazan was then dissolved in DMSO (200 µl per well) and the
absorbance value at 490 nm (optical density, 490 nm) was
determined.
Reverse transcription-quantitative PCR
(RT-qPCR)
The total RNA of samples or cells was extracted
using a miRNeasy mini kit or RNeasy kit (Qiagen China Co., Ltd.).
RNA was reverse transcribed using Transcriptor (Roche Diagnostics)
with an oligo-dT primer or the Bulge-loop™ miRNA qRT-PCR Primer
Sets specific for miR-miR-186-5p or U6 (Guangzho Ribobio Co., Ltd.)
according to the manufacturer's protocol. Briefly, the reactions
were incubated for 60 min at 42°C and 10 min at 70°C and were then
stored at −20°C. RT-qPCR analysis was performed using a LightCycler
96 System (Roche Diagnostics) with SYBR Green PCR Master Mix
(Takara Bio, Inc.). In brief, the reactions were incubated at 95°C
for 20 sec, followed by 40 cycles of 95°C for 10 sec and 60°C for
35 sec. A dissociation stage was performed at the end of the
amplification procedure to determine potential non-specific
amplifications. U6 and GAPDH were used as the internal control.
Relative gene expression in functional assay was analyzed using the
2−∆∆Cq method (28) and
present with fold-changes. Relative gene expression in results of
clinical samples was present with -ΔCq [ΔCq=Cq (Gene)-Cq (U6)].
Primer used for RT-qPCR were listed in Table SI.
Transfection
To determine the effects of miR-186-5p on cellular
function, a miR-186-5p mimic, miRNA mimic control, anti-miR-186-5p,
nonspecific anti-miRNA control and small interfering (si)RNAs
targeting human CXCL13 (si-CXCL13) were all synthesized by
Guangzhou RiboBio Co., Ltd. For transfection,
INTERFERin® (Polyplus-transfection SA) and 20 nM of the
RNAs mentioned above were prepared according to the manufacturer's
protocol and mixed with cells in 24-well cell culture plates when
the cells were at 50% confluence. The medium was replaced with
fresh medium 12 h after transfection.
MicroRNA target prediction and
luciferase reporter assays
TargetScan version 7.2 (http://www.targetscan.org/vert_72/) was used to
predict the targets of miR-186-5p. The complete computational
protocol is available at the above website. The miR-186-5p target
region containing the CXCL13 3′UTR (5′-GGACUCUGGUAUCUAAUUCUUUA-3′)
was inserted into the pmirGLO luciferase reporter vector (Promega
Corporation). As a control, a plasmid containing mutant sites
(sequence 5′-GGACUCUGGUAUCUAUAAGAAAA-3′) of the miR-186-5p target
region in the CXCL13 3′UTR was also inserted into the pmirGLO
luciferase reporter vector. Co-transfection of 200 ng wild type
(WT) or mutant (mut) CXCL13 3′UTR with 100 nM of miR-186-5p mimic
or anti-miR-186-5p and their respective negative controls into 293T
cells was performed using INTERFERin® in 48-well plates.
Relative luciferase activity was analyzed using the dual-luciferase
reporter system (Promega Corporation) and normalized with
Renilla luciferase activity. Each experiment was repeated
four times.
Western blotting
Western blotting was performed as described
previously (29). Briefly,
whole-cell extracts were prepared in lysis buffer (20 mM Tris-HCl,
pH 7.4, 150 mM NaCl, 10% glycerol, 0.2% Nonidet P-40, 1 mM EDTA, 1
mM EGTA, 1 mM PMSF, 10 mM NaF, 5 mg/ml aprotinin, 20 mM leupeptin,
and 1 mM sodium orthovanadate) and centrifuged at 13,000 × g for 20
min at 4°C. Protein concentrations were determined using the
bicinchoninic acid (BCA, Invitrogen; Thermo Fisher Scientific,
Inc.) assay. Immunoblotting was performed using 30 µg protein
lysate with specific primary antibodies (CXCL13: Proteintech
10927-1-AP; AKT: Proteintech 10176-2-AP; p-AKT (Ser473): CST 4060;
ERK1/2: Proteintech 16443-1-AP; p-ERK1/2: CST 4370;) which were
diluted in a SignalBoost immunoreaction enhancer buffer (Millipore,
407207-1) at 4°C overnight. Immunocomplexes were incubated with the
fluorescein-conjugated secondary antibody (Thermo Fisher
Scientific, Inc., A32735 and A32730) at room temperature for 2 h
and then detected using an Odyssey fluorescence scanner
(Li-Cor).
Osteogenic differentiation and
evaluation
Osteogenic differentiation was evaluated using
alkaline phosphatase (ALP) staining and osteoblast-specific gene
analyses. For ALP staining, adherent cells were prefixed with 4%
formaldehyde for 30 min and Western blue stabilized substrate was
added (Promega Corporation) for 30 min at room temperature. ALP
activity was examined using p-nitrophenyl phosphate (Sigma-Aldrich;
Merck KGaA) as described previously (30). For Alizarin Red S (ARS) staining,
the cells were washed with PBS and fixed with 4% formaldehyde for
30 min and then stained for 20 min with 40 mM ARS solution (Merck
Millipore, TMS-008-C). Finally, they were rinsed three times with
PBS to reduce nonspecific staining. The absorbance value at 570 nm
was measured to quantify the osteogenic differentiation. Expression
levels of osteoblast-specific genes, such as RUNX2 and COL1A1 were
also determined using RT-qPCR. Measurements were performed in three
times for each experiment.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent repeats. Differences between groups
were compared using analysis of variance followed by a Tukey's
post-hoc test when applicable, while continuous variables were
compared using Student t-test. To assess the correlation between
miR-186-5p and CXCL13 expression levels, Pearson's rank correlation
coefficient was used. Statistical analyses were performed with
using IBM SPSS statistics version 19 (IBM Corp). P<0.05 was
considered to indicate a statistically significant difference.
Results
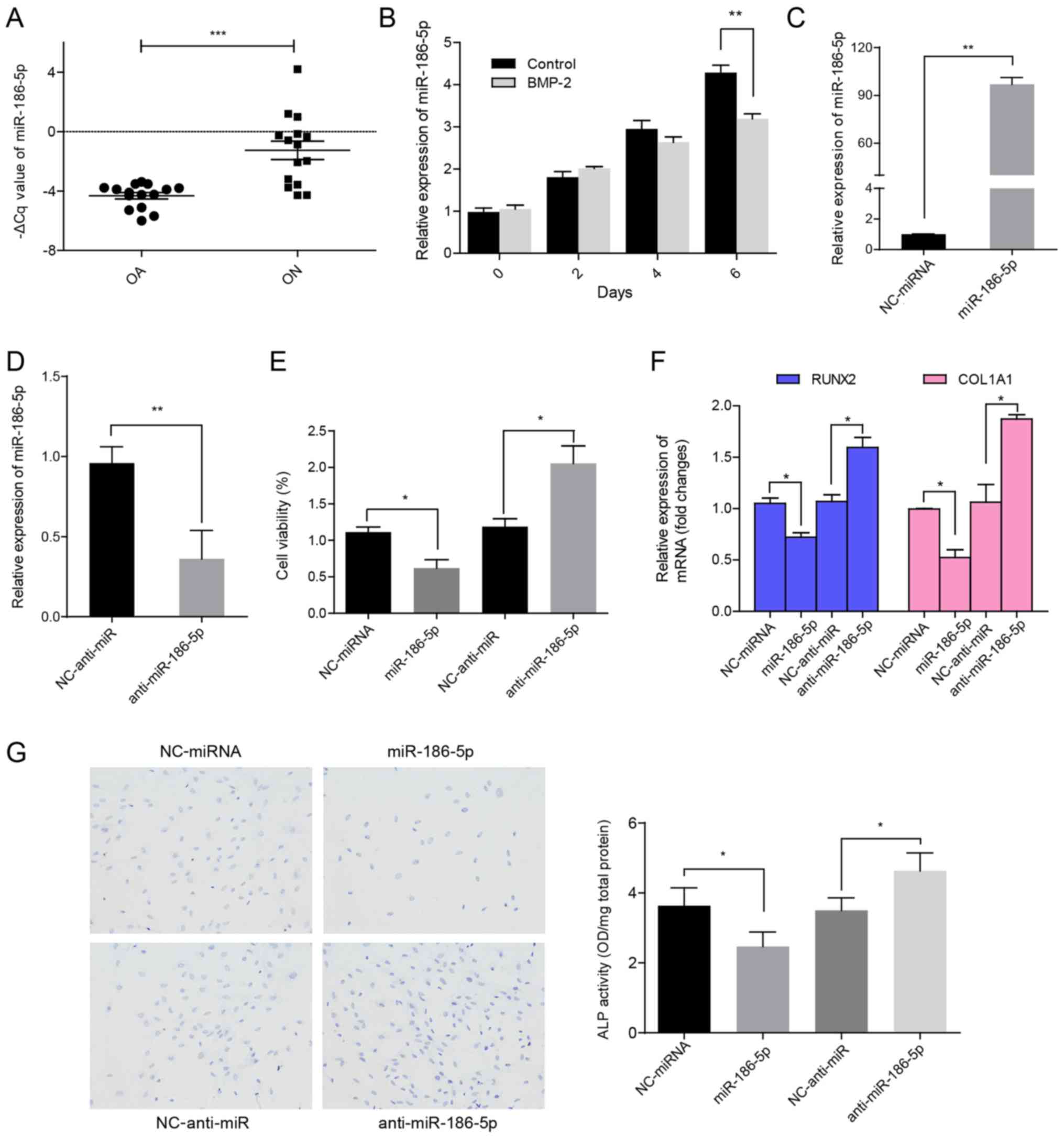
miR-186-5p expression is significantly
increased in ON of formal head (ONFH) and modulates MSC growth and
differentiation
To identify novel functional miRNAs in ONFH
progression, miR-186-5p expression levels were determined in HMSCs
from patients with OA and non-traumatic ON. RT-qPCR analysis showed
that miR-186-5p levels were significantly increased in HMSCs from
the ON group Compared with the patients with OA (P<0.0001;
Fig. 1A). To determine the
underlying roles of miR-186-5p during osteoblastic differentiation
of HMSCs, the expression of miR-186-5p was analyzed in HMSC-bm. As
shown in Fig. 1B, compared with
MSCs from the negative control group, miR-186-5p expression levels
increased at a slower rate when treated with BMP-2 for 6 days,
suggesting that miR-186-5p may affect cell viability during MSC
differentiation. These results also indicated that during growth
and differentiation of HMSCs cells, the expression of miR-186-5p
was elevated and cell differentiation and viability was negatively
regulated by miR-186-5p. When the cells reached confluence,
miR-186-5p expression levels decreased. Consistent with a previous
study (21) showing that
miR-186-5p negatively modulates cell proliferation, overexpression
of miR-186-5p significantly suppressed HMSC-bm viability
(P<0.05; Fig. 1C-E) and
osteoblastic differentiation (Fig. 1F
and G), whereas inhibition of miR-186-5p exerted the opposite
effects (Fig. 1C-G). Taken
together, these results demonstrate that miR-186-5p participates in
the disease course of OA through negatively regulating viability
and differentiation of MSCs.
CXCL13 is a direct target of
miR-186-5p in HMSCs
To determine the mechanism of how miR-186-5p
suppressed differentiation of MSCs, the targets of miR-186-5p in
HMSCs were determined. Bioinformatics analysis predicted that the
3′UTR of CXCL13 mRNA was a potential target of miR-186-5p (Fig. 2A and Table SII). Furthermore, RNA-seq was
performed on MSCs from three patients with ON and three patients
with OA patients. The potential functions of these differentially
expressed mRNAs were identified using KEGG pathway analyses. The
differentially abundant genes between OA and ON patients were
significantly associated with Cytokine-cytokine receptor
interaction pathways (Fig. S2).
Specifically, expression of CXCL13 was downregulated in patients
with ON (Fig. 2B; Table SIII). In addition, miR-186-5p
expression was negatively correlated with CXCL13 mRNA levels in
bone marrow samples from patients with ON patients (Fig. 2C). The dual-luciferase reporter
assays suggested that transfection with miR-186-5p mimic or
anti-miR-186-5p significantly reduced or increased, respectively
the luciferase activity of WT CXCL13 3′UTR (P<0.05), whereas
neither had any significant effects on the mut 3′UTR (Fig. 2D). These results suggest that
miR-186-5p directly targets the 3′UTR of CXCL13. Transfection of
miR-186-5p mimics or anti-miR-186-5p mimics into HMSCs cells or
primary MSCs from patients with OA resulted in both mRNA and
protein expression levels of CXCL13 being significantly reduced or
increased, respectively (P<0.05; Fig. 2E-G). These results suggested that
miR-186-5p directly targeted and abrogated CXCL13 expression in
HMSCs.
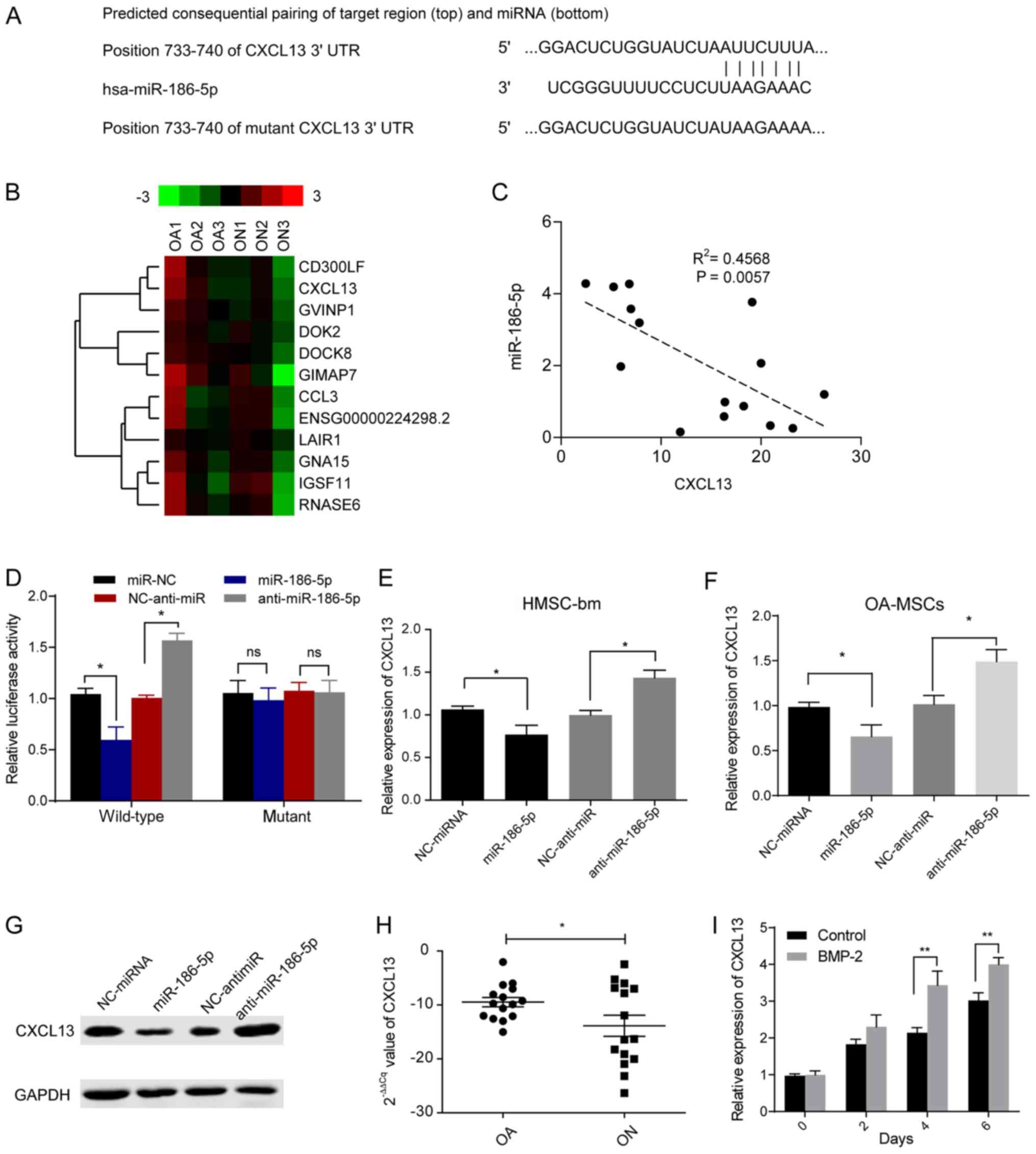 | Figure 2.CXCL13 is a direct target of
miR-186-5p in HMSCs. (A) Schematic diagram of the predicted
miR-186-5p seed sequences in the 3′-UTRs of the wild type CXCL13
and mutant CXCL13 mRNAs. (B) A total of 12 genes were
differentially expressed, out of 1,016 genes, at the mRNA level in
clinical samples between patients with ON compared with OA. Gene
expression data is presented as a matrix, in which columns
represent individual mRNA samples and rows represent individual
genes. Relative gene expression is presented as differing
intensities of green and red according to the key. (C) Correlation
analysis of miR-186-5p and CXCL13 expression in patients with ON.
(D) Wild-type or mutant CXCL13 3′UTR was co-transfected with
miR-186-5p mimics or anti-miR-186-5p in HMSC-bm cells and the
relative activity of the luciferase reporter genes were measured
after 24 h. *P<0.05. mRNA expression levels of CXCL13 in (E)
HMSC-bm cells or (F) MSCs from OA patients (OA-MSCs, n=3)
transfected with either miR-186-5p mimics or anti-miR-186-5p.
*P<0.05. (G) Protein expression levels of CXCL13 in HMSC-bm
cells transfected with either miR-186-5p mimics or anti-miR-186-5p.
(H) mRNA expression levels of CXCL13 in cultured primary cells
obtained from patients with non-traumatic ON or OA. *P<0.05. (I)
Expression levels of CXCL13 in HMSC-bm cells induced with BMP-2.
**P<0.01. UTR, untranslated region; ON, osteonecrosis; OA,
osteoarthritis; BMP-2, bone morphogenetic protein-2; MSC,
mesenchymal cells; NC, negative control; HMSC, human mesenchymal
stem cells; miR, microRNA; ns, non-significant. |
miR-186-5p suppresses cell viability
and osteoblastic differentiation by inhibiting CXCL13
Downregulation of CXCL13 mRNA levels was first
observed in the RNA-seq data (Fig.
2B) and the results were validated in bone marrow tissues from
patients with ON and OA patients using RT-qPCR (Fig. 2H). Compared with MSCs from the
negative control group, CXCL13 expression levels increased faster
when treated with BMP-2 for 6 days, suggesting that CXCL13 may
serve an antagonistic role against miR-186-5p in MSC
differentiation (Fig. 2I).
Furthermore, the results showed that CXCL13 overexpression promoted
cell viability and osteoblastic differentiation of MSCs, and
reduction of CXCL13 mRNA expression levels had the opposite effects
(Fig. 3A-E). To further
investigate whether miR-186-5p modulated CXCL13 expression levels,
rescue assays were performed. HMSCs cells were co-transfected with
miR-186-5p mimic and a plasmid expressing CXCL13. As shown in
Fig. 3, the inhibitory effects of
miR-186-5p on CXCL13 were partially reversed after transfection
with the plasmid (Fig. 3F-I).
These results suggest that miR-186-5p targets and regulates CXCL13
in MSCs.
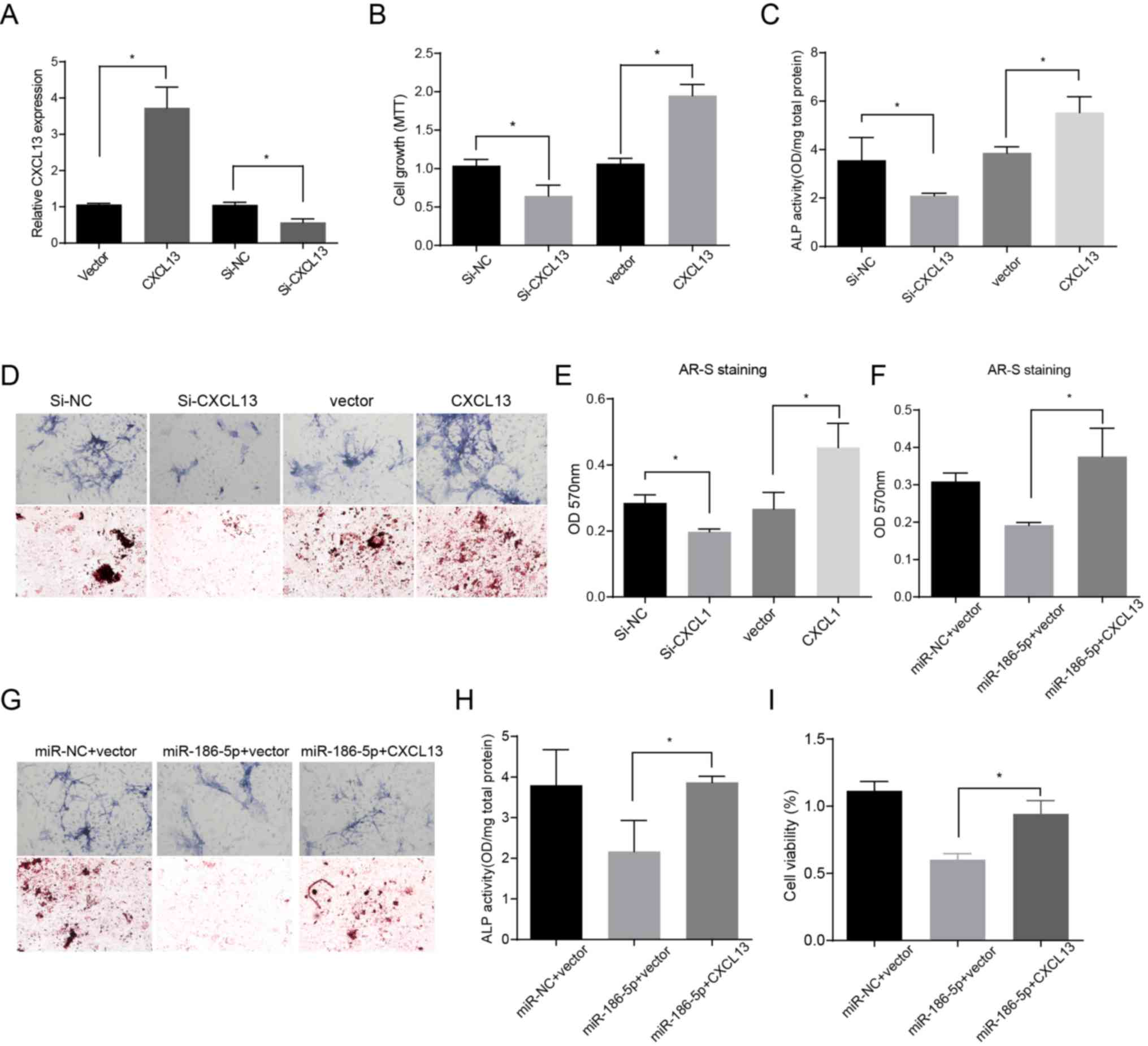 | Figure 3.miR-186-5p decreases the viability of
HMSC-bm cells and bone formation by negatively modulating CXCL13
expression. (A) Relative mRNA expression levels of CXCL13 48 h
after transfection with CXCL13, si-CXCL13 or negative control in
HMSC-bm cells induced with BMP-2. A total of 6 days after treatment
with BMP-2, the (B) cell viability, (C) ALP activity analysis and
(D) cytology images and (E) ARS staining analysis were shown.
Control and miR-186-5p mimics were co-transfected with or without
pcDNA3.1-CXCL13 in HMSC-bm cells treated with BMP-2. After 6 days,
(F) ARS staining and (G) cytology images, (H) ALP activity and (I)
viability of cells were shown. Magnification, ×100. *P<0.05. si,
small interfering; NC, negative control; miR, microRNA; ALP,
alkaline phosphatase; OD, optical density; ARS, Alizarin red S;
BMP-2, bone morphogenetic protein-2; HMSC, human mesenchymal stem
cells. |
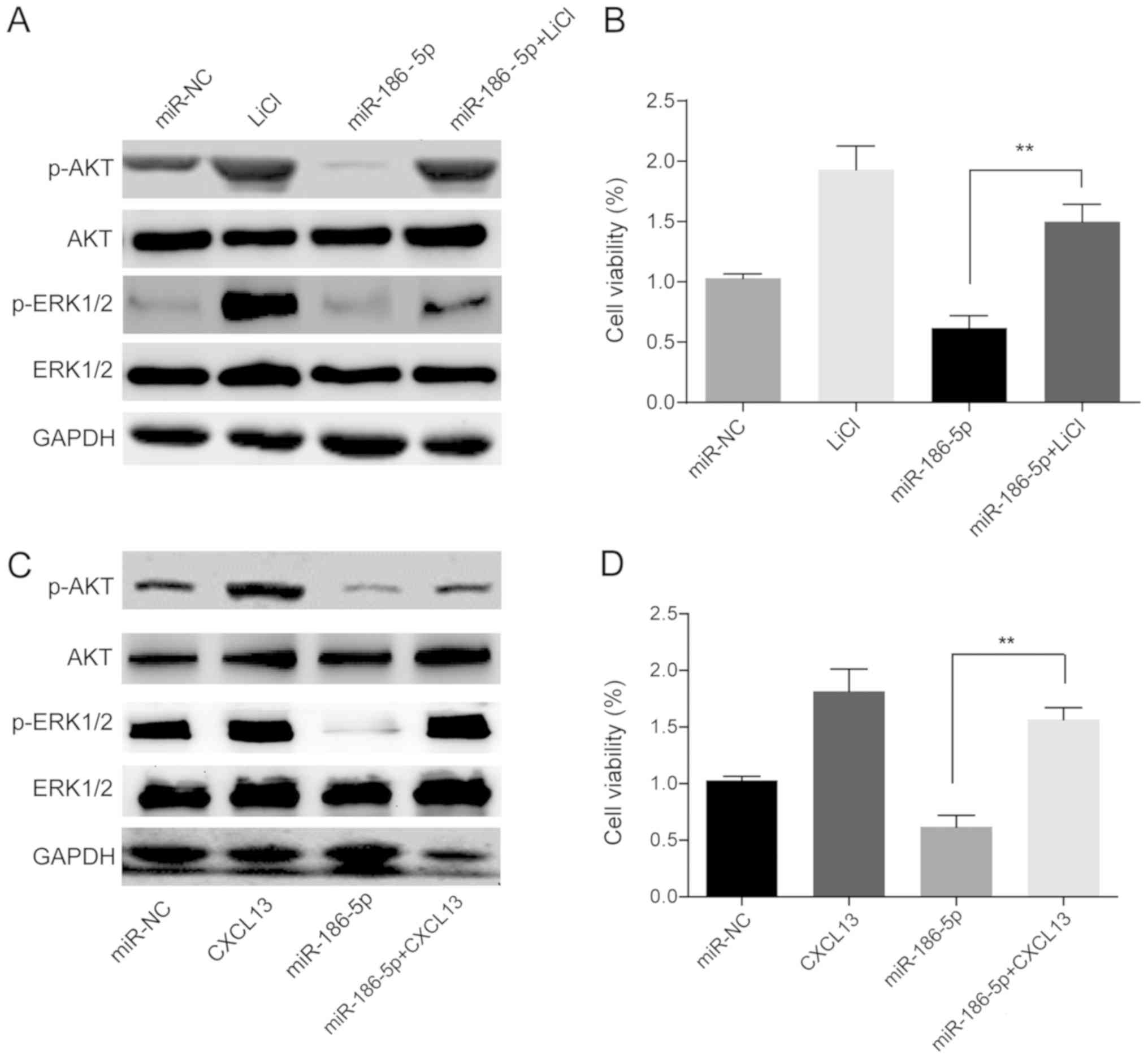
miR-186-5p/CXCL13 modulates AKT/ERK
signaling to regulate cell growth and osteoblastic
differentiation
It has been reported that CXCL13 promotes growth and
invasion of colon cancer cells via the PI3K/AKT signaling pathway
(31). To determine the underlying
mechanism by which the miR-186-5p/CXCL13 axis in ON regulated cell
growth and differentiation, the AKT signaling pathway in HMSC-bm
cells was examined. Western blot analysis showed that
overexpression of miR-186-5p significantly suppressed AKT/ERK
signaling (Fig. 4). In addition,
when the AKT signaling activator, LiCl, was added to
miR-186-5p-overexpressing HMSC-bm cells, the MTT assays suggested
that LiCl could restore the negative effects of miR-186-5p on
HMSC-bm cell viability (Fig. 4A and
B). Furthermore, transfection with the CXCL13 vector also
partially rescued the growth of HMSC-bm cells when co-transfected
with miR-186-5p (Fig. 4C and D).
These data suggest that miR-186-5p/CXCL13 affects MSC growth
through modulating PI3K/AKT signaling.
Discussion
Accumulating evidence has shown that miRNAs function
by modulating various biological processes in human MSCs. In the
etiopathogenesis of non-traumatic ON, certain microRNAs, such as
miR-17-5p, miR-125 and miR-135 have been identified as modulators
of cell growth and differentiation (32,33),
highlighting the importance of miRNAs in osteonecrosis diagnosis
and treatment. However, the functional significance of miRNAs is
not yet completely understood. In the present study, it was
demonstrated that expression of miR-186-5p was increased in the
HMSCs of patients with ON and served important roles in regulating
cell growth and osteoblastic differentiation through directly
targeting CXCL13. The data of the present study demonstrated that
the expression of miR-186-5p was altered when CXCL13 expression was
increased during osteoblast differentiation of HMSCs, suggesting
their involvement in bone development. Furthermore, miR-186-5p
overexpression suppressed activation of the AKT/ERK signaling
pathway. Specific activation of AKT/ERK signaling rescued the
effects of miR-186-5p in HMSCs.
Chemokines are the largest family of cytokines, with
at least 47 members which are divided into four major categories,
the CX3C, CXC, CC and C subfamilies, based on the spacing and
number of conserved cysteine residues in the amino acid sequences.
Chemokines bind to seven transmembrane-spanning G-protein-coupled
receptors to exert influence on various biological functions
(34). CXCL13 directly modulates
cellular proliferation and viability induces migration of MSCs to
move to the injured area and induces differentiation of osteoblasts
(35–37). Although CXCL13 was considered to be
a critical regulator of MSC differentiation, none of
characteristics were significantly correlated with CXCL13
expression in the 15 patients enrolled in the present study (Data
not shown), given the small sample size. This result should be
further explored within a larger cohort.
The RNA-seq analysis results showed that CXCL13
expression was downregulated in HMSCs of patients with ON. In
addition, CXCL13 mRNA expression levels were negatively correlated
with miR-186-5p expression levels in bone marrow samples in
patients with ON. miR-186-5p downregulated mRNA as well as the
protein expression levels of CXCL13 in HMSCs and increasing the
levels of CXCL13 partially restored miR-186-5p-hindered
osteoblastic differentiation, and cell viability. The present study
also predicted the underlying functions of differentially abundant
mRNAs using KEGG pathway analyses. These findings corroborated the
hypothesis that one of the most significantly important pathways in
the development of osteonecrosis was cytokine-cytokine receptor
interaction, which was also identified by previous studies
(38,39). Taken together, the present study
highlighted the important role of miRNA-CXCL13 regulation in
patients with non-traumatic ON and may serve as potential
therapeutic targets for treating patients with ON.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors thank Professor HL Jin from Cancer
Centre, Wuhan Union Hospital for helpful and valuable discussions,
Dr Xiao-Bo Feng and Dr Wei Tong from Department of Orthopedics,
Wuhan Union Hospital for the technical assistance.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81702193, 81572161,
81602107 and 81672155).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WHX and JL obtained human specimens, provided
support with experimental techniques and wrote the manuscript. HTT
prepared all samples and clinical data. RYW performed
bioinformatics analysis of experimental data. YF performed
bioinformatics analysis of clinical data. JT and JJ designed
research, performed all experiments, conceived the study and
supervised all experiments.
Ethics approval and consent to
participate
The experimental protocol was established, according
to the ethical guidelines of the Helsinki Declaration and was
approved by the Human Ethics Committee of Wuhan Union Hospital
(approval no. 17S001).
Patient consent for publication
Written informed consent was obtained from all
individual participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Wu J, Zhu Y and Han J: Therapeutic
application of mesenchymal stem cells in bone and joint diseases.
Clin Exp Med. 14:13–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mont MA, Marulanda GA, Jones LC, Saleh KJ,
Gordon N, Hungerford DS and Steinberg ME: Systematic analysis of
classification systems for osteonecrosis of the femoral head. J
Bone Joint Surg Am. 88 (Suppl 3):S16–S26. 2006. View Article : Google Scholar
|
|
3
|
Zhao DW, Yu M, Hu K, Wang W, Yang L, Wang
BJ, Gao XH, Guo YM, Xu YQ, Wei YS, et al: Prevalence of
Nontraumatic osteonecrosis of the femoral head and its associated
risk factors in the Chinese population: Results from a nationally
representative survey. Chin Med J (Engl). 128:2843–2850. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto T, DiCarlo EF and Bullough PG:
The prevalence and clinicopathological appearance of extension of
osteonecrosis in the femoral head. J Bone Joint Surg Br.
81:328–332. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan G, Kang PD and Pei FX: Glucocorticoids
affect the metabolism of bone marrow stromal cells and lead to
osteonecrosis of the femoral head: A review. Chin Med J (Engl).
125:134–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seamon J, Keller T, Saleh J and Cui Q: The
pathogenesis of nontraumatic osteonecrosis. Arthritis.
2012:6017632012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng Y, Yang SH, Xiao BJ, Xu WH, Ye SN,
Xia T, Zheng D, Liu XZ and Liao YF: Decreased in the number and
function of circulation endothelial progenitor cells in patients
with avascular necrosis of the femoral head. Bone. 46:32–40. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Liao W, Zhao Q, Liu M, Xia W, Yang Y
and Shao N: Angiogenesis and bone regeneration by allogeneic
mesenchymal stem cell intravenous transplantation in rabbit model
of avascular necrotic femoral head. J Surg Res. 183:193–203. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang
L, Liu B and Yu X: Treatment of early stage osteonecrosis of the
femoral head with autologous implantation of bone marrow-derived
and cultured mesenchymal stem cells. Bone. 50:325–330. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gangji V, De Maertelaer V and Hauzeur JP:
Autologous bone marrow cell implantation in the treatment of
non-traumatic osteonecrosis of the femoral head: Five year
follow-up of a prospective controlled study. Bone. 49:1005–1009.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan Z, Hang D, Guo C and Chen Z: Fate of
mesenchymal stem cells transplanted to osteonecrosis of femoral
head. J Orthop Res. 27:442–446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang S, Zu Y, Fu Y, Zhang Y and Efferth
T: Activation of the mitochondria-driven pathway of apoptosis in
human PC-3 prostate cancer cells by a novel hydrophilic paclitaxel
derivative, 7-xylosyl-10-deacetylpaclitaxel. Int J Oncol.
33:103–111. 2008.PubMed/NCBI
|
|
13
|
Nishimori S, Tanaka Y, Chiba T, Fujii M,
Imamura T, Miyazono K, Ogasawara T, Kawaguchi H, Igarashi T, Fujita
T, et al: Smad-mediated transcription is required for transforming
growth factor-beta 1-induced p57(Kip2) proteolysis in osteoblastic
cells. J Biol Chem. 276:10700–10705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gustafson D, Tyryshkin K and Renwick N:
microRNA-guided diagnostics in clinical samples. Best Pract Res
Clin Endocrinol Metab. 30:563–575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamasaki K, Nakasa T, Miyaki S, Yamasaki
T, Yasunaga Y and Ochi M: Angiogenic microRNA-210 is present in
cells surrounding osteonecrosis. J Orthop Res. 30:1263–1270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Wijnen AJ, van de Peppel J, van
Leeuwen JP, Lian JB, Stein GS, Westendorf JJ, Oursler MJ, Im HJ,
Taipaleenmäki H, Hesse E, et al: MicroRNA functions in osteogenesis
and dysfunctions in osteoporosis. Curr Osteoporos Rep. 11:72–82.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai J, Wu J, Zhang H, Fang L, Huang Y,
Yang Y, Zhu X, Li R and Li M: miR-186 downregulation correlates
with poor survival in lung adenocarcinoma, where it interferes with
cell-cycle regulation. Cancer Res. 73:756–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Yin C, Zhang B, Sun Y, Shi L, Liu N,
Liang S, Lu S, Liu Y, Zhang J, et al: PTTG1 promotes migration and
invasion of human non-small cell lung cancer cells and is modulated
by miR-186. Carcinogenesis. 34:2145–2155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SY, Lee YH and Bae YS: MiR-186,
miR-216b, miR-337-3p, and miR-760 cooperatively induce cellular
senescence by targeting α subunit of protein kinase CKII in human
colorectal cancer cells. Biochem Biophys Res Commun. 429:173–179.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pike KA, Hutchins AP, Vinette V, Théberge
JF, Sabbagh L, Tremblay ML and Miranda-Saavedra D: Protein tyrosine
phosphatase 1B is a regulator of the interleukin-10-induced
transcriptional program in macrophages. Sci Signal. 7:ra432014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anders S and Huber W: Differential
expression analysis for sequence count data. Genome Biol.
11:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hutchins AP, Jauch R, Dyla M and
Miranda-Saavedra D: Glbase: A framework for combining, analyzing
and displaying heterogeneous genomic and high-throughput sequencing
data. Cell Regen (Lond). 3:12014.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression using real-time quantitative PCR and the
2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu L, Chen L, Li L, Sun H, Yang G, Chang
Y, Tu Q, Wu M and Wang H: Hepatitis B virus X protein enhances
cisplatin-induced hepatotoxicity via a mechanism involving
degradation of Mcl-1. J Virol. 85:3214–3228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu K, Zeng D, Zhang Y, Xia L, Xu L, Kaplan
DL, Jiang X and Zhang F: BMP-2 gene modified canine bMSCs promote
ectopic bone formation mediated by a nonviral PEI derivative. Ann
Biomed Eng. 39:1829–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Z, Zhang X, Guo H, Fu L, Pan G and Sun
Y: CXCL13-CXCR5 axis promotes the growth and invasion of colon
cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 400:287–295.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia J, Feng X, Xu W, Yang S, Zhang Q, Liu
X, Feng Y and Dai Z: MiR-17-5p modulates osteoblastic
differentiation and cell proliferation by targeting SMAD7 in
non-traumatic osteonecrosis. Exp Mol Med. 46:e1072014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jing D, Hao J, Shen Y, Tang G, Li ML,
Huang SH and Zhao ZH: The role of microRNAs in bone remodeling. Int
J Oral Sci. 7:131–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rüegg C, Hasmim M, Lejeune FJ and Alghisi
GC: Antiangiogenic peptides and proteins: From experimental tools
to clinical drugs. Biochim Biophys Acta. 1765:155–177.
2006.PubMed/NCBI
|
|
35
|
Lisignoli G, Toneguzzi S, Piacentini A,
Cristino S, Grassi F, Cavallo C and Facchini A: CXCL12 (SDF-1) and
CXCL13 (BCA-1) chemokines significantly induce proliferation and
collagen type I expression in osteoblasts from osteoarthritis
patients. J Cell Physiol. 206:78–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cristino S, Piacentini A, Manferdini C,
Codeluppi K, Grassi F, Facchini A and Lisignoli G: Expression of
CXC chemokines and their receptors is modulated during chondrogenic
differentiation of human mesenchymal stem cells grown in
three-dimensional scaffold: Evidence in native cartilage. Tissue
Eng Part A. 14:97–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tian F, Ji XL, Xiao WA, Wang B and Wang F:
CXCL13 promotes osteogenic differentiation of mesenchymal stem
cells by inhibiting miR-23a expression. Stem Cells Int.
2015:6323052015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adapala NS and Kim HW: Comprehensive
genome-wide transcriptomic analysis of immature articular cartilage
following ischemic osteonecrosis of the femoral head in piglets.
PLoS One. 11:e01531742016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yuan C and Cai J: Time-series expression
profile analysis of fracture healing in young and old mice. Mol Med
Rep. 16:4529–4536. View Article : Google Scholar : PubMed/NCBI
|