Introduction
Immune-mediated liver injury is one of the most
troublesome diseases clinically. Autoreactive T cells destroy
hepatocytes or cholangiocytes in autoimmune liver diseases and
virus-specific T cells destroy infected hepatocytes in viral
hepatitis (1). The pathogenesis of
immune-mediated liver injury has not yet been fully clarified
(2), and immunosuppressive
treatments or antiviral drugs are the main therapeutic methods used
clinically for treating these diseases (3). However, the questionable
effectiveness and strong side-effects of these treatments and the
high possibility of disease relapse limit the utility of these
therapeutic approaches. Hence, finding new treatments to ameliorate
immune-mediated liver damage in these patients remains an urgent
issue.
An imbalance between
CD4+CD25+Foxp3+ regulatory T cells
(Tregs) and T helper 17 (Th17) cells has recently been suggested to
be important in the pathogenesis of immune-mediated liver diseases
(4). Tregs are essential in
maintaining peripheral immunological tolerance, as they control
autoreactive T cells and inhibit inflammation by releasing
anti-inflammatory cytokines (5).
Th17 cells are a subtype of helper CD4+ T cells that
produce interleukin (IL)-17, which induces immune cell infiltration
and liver damage, driving hepatic inflammation and contributing to
autoimmune liver disease (6,7).
Moreover, an increase in the Th17/Treg ratio also accelerates liver
fibrosis (8). A recent study has
indicated that blocking Th17 cells allows CD25− Treg
cells to differentiate into functionally stable immune inhibitory
cells; this may be a novel therapy for patients with autoimmune
hepatitis (AIH) (7). The
developmental pathways for Th17 cells and Tregs are reciprocally
interconnected (9), suggesting
that the factors that affect the balance between these two cell
types may influence the outcome of the immune responses.
Concanavalin A (ConA) can induce a typical T
cell-mediated hepatitis in mice, which is characterized by
significantly increased plasma levels of transaminase and severe
liver cell inflammation or even necrosis within 8–24 h (10,11).
ConA has the ability to stimulate the activation of T lymphocytes,
mostly CD4+T cells, and animal models of ConA-induced
liver injury are ideal tools to investigate T-cell-dependent
immune-mediated liver injury (12). The infiltration of T cells,
macrophages, and neutrophils into the mouse liver and the elevated
levels of pro-inflammatory cytokines produced from these cells
indicate that this mouse model closely mimics the pathogenic
mechanisms and pathological changes associated with AIH (13,14).
Moreover, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α)
are the two most important cytokines mediating ConA-induced
immune-mediated hepatitis; they are also the most crucial cytokines
associated with autoimmune liver diseases in the human body
(15,16). However, the ConA-induced mouse
model is an acute immune-mediated mouse model. The liver
inflammation and lymphocyte infiltration disappear in 48 h. For
decades, researchers have been investigated the chronic
immune-mediated mouse model (17,18).
Cytochrome P450 2D6 (CYP2D6) is the major human autoantigen in type
2 AIH and recognized by type 1 liver kidney microsomal antibodies
(LKM-1s) (19). Overexpression of
the human CYP2D6 gene in mice can result in a chronic form of
severe, autoimmune liver damage, and autoantibody generation
(20,21). Chronic hepatitis and liver fibrosis
can also be observed in a CYP2D6 mouse model, suggesting that it
could be an appropriate tool to investigate chronic immune-mediated
hepatitis.
Members of the Janus kinase (JAK) family, including
JAK1, JAK2, JAK3, and tyrosine kinase 2 (Tyk2), are involved in the
growth, survival, development, and differentiation of a variety of
cells, but are critically important for immune cells (22). JAKs phosphorylate signal
transducers and activators of transcription (STATs) to regulate the
expression of different downstream genes (23). JAKs are essential for
cytokine-induced intracellular signaling of lymphocytes, and their
dysfunction contributes to the impairment of immune cell function
(24). Cytokines that bind to
receptors containing the common γ chains, such as IL-2, IL-4, IL-7,
IL-9, IL-15, and IL-21, are crucial for the function of T cells;
JAK1 is activated via these cytokine-binding chains, while the
common γ chains activate JAK3 (25). Nowadays, the clinical use of JAK
inhibitors has been confirmed to improve many inflammation-driven
diseases.
Tofacitinib is a potent, selective JAK inhibitor
that preferentially inhibits JAK1 and JAK3 (26). It is a new JAK inhibitor that is
under investigation for the treatment of rheumatoid arthritis
(27); according to recent
studies, it has also been reported to be helpful against psoriatic
arthritis (28), ulcerative
colitis (29), and alopecia areata
(30). Tofacitinib inhibits STAT-1
activation, resulting in the downregulation of IL-6 and IFN-γ in
naïve CD4+ T cells (31). Evidence has revealed that
tofacitinib may improve autoimmune diseases by suppressing the
differentiation of pathogenic Th1 and Th17 cells, as well as innate
immune cell signaling (31).
However, the exact immune processes affected by tofacitinib and the
influence of tofacitinib on gene expression in situ are
unknown (27). Furthermore, there
are no studies reporting the effects of tofacitinib on the balance
of Tregs and Th17 cells or immune-mediated hepatitis. The present
study aimed to investigate the effects of tofacitinib on
immune-mediated liver injury in mice and the mechanisms underlying
these effects.
Materials and methods
Reagents
Tofacitinib was purchased from Dalian Meilun
Biotechnology Co., Ltd. ConA and dimethyl sulfoxide (DMSO) were
purchased from Sigma-Aldrich; Merck KGaA. The antibodies used for
western blotting and immunohistochemistry in this study were
purchased from Santa Cruz Biotechnology, Inc., R&D Systems, and
Cell Signaling Technology, Inc., including antibodies against
STAT1, phosphorylated STAT1 (p-STAT1), TNF-α, and IFN-γ. The
antibodies used for flow cytometry, such as those recognizing CD4,
CD25, Foxp3, and IL-17A were purchased from BioLegend, Inc. and BD
Pharmingen; BD Biosciences. Fetal bovine serum (FBS) was purchased
from Gibco; Thermo Fisher Scientific, Inc.
Plasmid and in vivo gene
transfection
Plasmid pCYP2D6, the expression vector carrying the
cDNA encoding human CYP2D6, was constructed by the insertion of
cDNA into plasmid pcDNA3.1 (Invitrogen; Thermo Fisher Scientific,
Inc.) in our laboratory. For in vivo gene transfection,
pCYP2D6 was injected to mice via the tail vein using the
hydrodynamics-based gene delivery technique (32).
Animals and experimental protocol
Specific pathogen-free (SPF) male C57BL/6 mice (6–8
weeks old; 18–20 g) were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. The mice were housed in an
SPF environment at 24±2°C with an alternating 12-h light/dark cycle
at the Experimental Animal Center of the Tongji Medical College. A
total of 120 mice were used in the whole experiment and 6 mice were
assigned to each experimental group except when otherwise indicated
in the figure legends. Ninety-six of those mice were used to study
the effect of tofacitinib in the ConA-induced immune-mediated liver
injury and the remaining mice were used to confirm the effect of
tofacitinib on liver fibrosis in an AIH mouse model. For all mouse
experiments, the method of euthanasia was cervical dislocation. The
protocol was approved by the Ethics Committee of Animal Experiments
of Tongji Medical College and monitored by the Department of
Experimental Animals of Tongji Medical College.
The treatment was as follows for the ConA mouse
model: The mice were injected with a single dose (15 mg/kg of body
weight) of ConA via the tail vein to induce acute immune-mediated
liver injury, as described in a previous study (12). Three days before the injection of
ConA, the mice in the treatment groups were administered with
tofacitinib (5, 10 and 15 mg/kg/day) by gavage based on the
recommended dose described in a previous study (33). The mice were sacrificed at 12, 24
and 48 h post-ConA injection. The blood was collected from the
angular vein and the livers were collected for hematoxylin and
eosin (H&E) staining, immunohistochemistry (IHC), western blot
analysis, and quantitative polymerase chain reaction (qPCR). For
the AIH mouse model: To detect the effects of tofacitinib on the
mouse model of chronic immune-mediated hepatitis, adenovirus
(109 pfu; Viraltherapy Technology) was injected once
initially and then pCYP2D6 plasmid was transfected several times
(50 µg per injection) into mice to induce the AIH mouse model, as
described in our previous studies (34,35).
Thirty-six days after the adenovirus injection, the AIH mice were
administered with tofacitinib (10 mg/kg/day, 2 days apart) by
gavage and were then sacrificed two weeks later to observe the
level of liver fibrosis by Sirius red staining.
Histopathology and
immunohistochemistry
The entire left lobe of the mouse livers was excised
and fixed in 4% paraformaldehyde for at least 24 h, embedded in
paraffin, and cut to yield 5-µm-thick sections. Following hydration
in a decreasing ethanol gradient, all sections were deparaffinized
and stained with Harris hematoxylin solution for 5 min at 37°C. For
IHC, paraffin-embedded liver tissue samples were cut into
5-µm-thick consecutive sections, dewaxed in xylene, and rehydrated
in graded ethanol solutions. Then, the nonspecific binding sites in
the tissues were blocked, and the steam cooking method was used for
antigen retrieval. The sections were incubated with the following
primary antibodies: Anti-p-STAT1 (1:50; product no. 7649; Cell
Signaling Technology, Inc.); anti-IL-6 (1:100; cat. no. GB11117)
and anti-IL-10 (1:100; cat. no. GB11108; both from Servicebio
Technology Co., Ltd.) overnight at 4°C. Then, they were incubated
with horseradish peroxidase-conjugated polyclonal goat anti-rabbit
secondary antibodies (1:200; GB23303; Servicebio Technology Co.,
Ltd.) for 1 h at room temperature. Finally, the sections were
counterstained with hematoxylin.
Protein extraction and western blot
analysis
Cell extracts and liver tissue samples were digested
in RIPA buffer containing a phosphatase inhibitor cocktail and PMSF
(Wuhan Boster Biological Technology, Ltd.). These samples were
centrifuged at 12,000 × g for 30 min and the supernatant was
retained. The protein concentration was determined using the
bicinchoninic acid method. Then, 30 µg of each protein sample was
separated on 10% SDS polyacrylamide gels; the protein bands were
then transferred onto PVDF membranes. The membranes were blocked
with 5% non-fat milk (non-fat dry milk powder dissolved in
Tris-buffered saline with Tween-20) for 1 h at room temperature and
then incubated overnight at 4°C on shaking tables with the
following primary antibodies: Anti-STAT1 (1:1,000; product no.
14994) and anti-p-STAT1 (1:1,000; product no. 7649; both from Cell
Signaling Technology, Inc.); anti-IFN-γ (1:2,000; cat. no. AF-485;
R&D Systems); anti-TNF-α (1:500; cat. no. sc-12744; Santa Cruz
Biotechnology, Inc.); and anti-β-actin (1:2,000; Promoter
Biotechnology Ltd.). Next, the membranes were washed and incubated
with horseradish peroxidase-conjugated secondary antibodies
(1:1,000; Promoter Biotechnology Ltd.) for 1 h at room temperature.
β-actin was used as a loading control. The expression of the
antibody-linked proteins was visualized by enhanced
chemiluminescence using an ECL assay kit (Wuhan Boster Biological
Technology, Ltd.) and analyzed using ImageJ V1.48 (National
Institutes of Health).
RNA extraction and real-time qPCR
Total RNA was isolated from the liver tissue samples
and cells using TRIzol reagent (Takara Bio, Inc.) and transcribed
into cDNA using a reverse transcription kit (cat. no. RR036A;
Takara Bio, Inc.), according to the manufacturer's protocols. qPCR
was performed according to the following steps: 40 cycles at 95°C
for 30 sec and 60°C for 30 sec; the PCR analyses were performed
using Maxima SYBR-Green qPCR Master Mixes (Takara Bio, Inc.) on an
ABI StepOne Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Fold significance was determined by the
2ΔΔCq method, as described in a previous study (36). The primers used for real-time qPCR
are listed in Table I.
 | Table I.Primer sequences for PCR. |
Table I.
Primer sequences for PCR.
| Genes | Primer
sequences |
|---|
| IFN-γ | F:
5′-TGCTGATGGCCTGATTGTCTT-3′ |
|
| R:
5′-GCCACGGCACAGTCATTGA-3′ |
| TNF-α | F:
5′-CTGAACTTCGGGGTGATCGG-3′ |
|
| R:
5′-GGCTTGTCACTCGAATTTTGAGA-3′ |
| β-actin | F:
5′-GCTCTTTTCCAGCCTTCCTT-3′ |
|
| R:
5′-TGATCCACATCTGCTGGAAG-3′ |
Assay for serum transaminase activity
or inflammatory cytokines
Venous blood was collected from the angular vein of
the mice and then centrifuged at 500 × g for 10 min to obtain serum
as the supernatant. The activities of liver enzymes, including
aspartate transaminase (AST) and alanine transaminase (ALT), in the
collected mouse sera, were determined using an automatic
biochemistry analysis apparatus at the Clinical Laboratory of
Tongji Hospital. The cytokines (IL-2, IL-4, IL-6, IFN-γ, TNF-α,
IL-17A, and IL-10) in the serum were detected by flow cytometry
using a mouse cytometric bead array (CBA) kit (560485; BD
Biosciences).
Cell isolation
Isolation of liver mononuclear cells
(MNCs)
To isolate the non-parenchymal cells (NPCs) from the
liver, first, in situ perfusion with DMEM/F12 (Thermo Fisher
Scientific, Inc.) containing type IV collagenase (Sigma-Aldrich;
Merck KGaA) was performed. Mice in each experimental group were
anesthetized using a mixture of ketamine (100 mg/kg) and xylazine
(10 mg/kg) by intraperitoneal injection. The liver was then
perfused, ground, digested, and filtered. The filtrate was
centrifuged at 20 × g for 5 min and washed in DMEM/F12. The
resuspended cells were processed with 30% Percoll (Sigma-Aldrich;
Merck KGaA) and gently overlaid onto 70% Percoll. After density
gradient centrifugation at 1,000 × g for 30 min, liver MNCs were
harvested from the interface of the Percoll gradient (37). The MNCs collected were used for
further fluorescence-activated cell sorting (FACS) analysis.
Isolation of splenocytes
Mice spleens were ground and filtered with 70 µm
cell strainer in PBS. After elimination of erythrocytes by Red
Blood Cell Lysis Buffer (BD Biosciences), splenocytes were washed
and resuspended in PBS for further FACS analysis.
Flow cytometry
A single-cell suspension of MNCs (at least
106 cells/tube) was resuspended in phosphate-buffered
saline (PBS) containing 1% BSA. To reduce nonspecific fluorescent
staining, the cells were incubated with anti-mouse CD16/CD32 (cat.
no. 101319; BioLegend, Inc.), which blocked the Fcγ III/II
receptor. Then, the surfaces of the cells were stained with
fluorochrome-conjugated antibodies for 30 min on ice. The following
antibodies were used: FITC-conjugated anti-CD4 (cat. no. 557307),
APC-conjugated anti-CD25 (cat. no. 557192), BV421-conjugated
anti-Foxp3 (cat. no. 562996), and PE-conjugated anti-IL-17A (cat.
no. 559502; all from BD Biosciences). The cell samples were
assessed on a FACS Calibur flow cytometer (BD Immunocytometry
Systems) and the data were analyzed using the FlowJo software V10
(Tree Star, Inc.).
Statistical analysis
All data are expressed as the mean ± standard error
and all experiments were performed independently in triplicate.
One-way analysis of variance (one-way ANOVA) with Tukey's multiple
comparisons test or Dunnett's multiple comparisons test if the
P-value was significant, were used. Statistical analysis was
performed with GraphPad Prism 5.0 (GraphPad Software). P<0.05
was considered to indicate a statistically significant
difference.
Results
The JAK1/STAT1 pathway is activated in
mice with ConA-induced hepatitis
To establish a mouse model of immune-mediated liver
injury, mice were injected with ConA (15 mg/kg) via the tail vein
and sacrificed at 0, 6, 12, 24, and 48 h to observe the degree of
inflammation in the livers. The liver tissues were harvested, and
the levels of inflammation were analyzed by H&E staining
(Fig. 1A). As revealed in the
representative images, a handful of inflammatory cells began to
infiltrate around the portal area 6 h post-injection, and more
inflammatory cells had accumulated at 12 h post-injection. In
addition, abundant necrotic areas were observed at 24 h after the
ConA injection. The plasma ALT and AST levels increased 6 h after
the intravenous ConA administration and peaked at 12 h (Fig. 1B and C). Real-time PCR was also
used to detect the mRNA levels of the important inflammatory
cytokines TNF-α and IFN-γ. Their expression patterns revealed clear
tendencies, peaking at 12 h after the ConA injection (Fig. 1D).
 | Figure 1.Immune-mediated hepatitis is induced
by ConA injection. (A) C57 BL/6 mice were injected with ConA (15
mg/kg) via the tail vein and sacrificed at 0, 6, 12, 24, and 48 h
post-injection. The liver tissues were harvested and analyzed by
H&E staining (n=6 in each group; ×100 and ×200 magnification).
(B and C) The plasma ALT and AST levels of the mice were detected
using an automatic biochemistry analysis apparatus (n=6). (D) The
mRNA expression of TNF-α and IFN-γ in the mouse livers was analyzed
by real-time PCR (n=6). **P<0.01, ***P<0.001 vs. the 0-h
group. ConA, concanavalin A; ALT, alanine transaminase; AST,
aspartate transaminase; TNF-α, tumor necrosis factor-α; IFN-γ,
interferon-γ. |
To further explore whether the JAK1/STAT1 pathway is
involved in ConA-induced acute immune-mediated liver injury,
western blotting and immunohistochemical staining were used to
detect the expression of STAT1 and p-STAT1 in the mouse livers at
different time-points (Fig. 2A and
B). The expression of p-STAT1 increased at the 12-h time-point,
and further significantly increased at 24 h. However, the p-STAT1
levels revealed a decreasing trend at the 48-h time point,
indicating that the JAK1/STAT1 pathway plays an important role in
the progression and development of ConA-induced acute liver injury
in mice. These results revealed that ConA could in fact induce
acute hepatitis in mice and that the JAK1/STAT1 pathway was
activated in this immune-mediated hepatitis mouse model, indicating
that this pathway may play a key role in ConA-induced mouse
hepatitis.
Tofacitinib effectively ameliorates
inflammation in the ConA-induced hepatitis mouse model
To detect the effects of tofacitinib on ConA-induced
hepatitis in vivo, the mice were randomly divided into
different experimental groups. The mice in the treatment groups
received different doses of tofacitinib (5, 10, and 15 mg/kg/day)
three days before the ConA injection to ascertain whether
tofacitinib exerts protective effects and whether its effects are
dose-dependent. The mice in each group were sacrificed 24 h
post-ConA injection. The mice injected with ConA developed
hypothermia, becoming unresponsive and inactive. They curled up in
the cage and were unusually quiet. However, the body temperature of
the mice in the treatment group was much higher and these mice were
in better condition. H&E staining revealed that tofacitinib
alleviated the histological liver damage (Fig. 3A) and significantly decreased the
upregulated serum transaminase expression (Fig. 3B) in the ConA-injected mice. The
gross appearance of the liver also provided strong evidence of the
inflammation-relieving effect of tofacitinib from another
perspective (Fig. 3C). Moreover,
liver damage was alleviated to a greater extent in the mice
administered with 10 or 15 mg/kg/day of tofacitinib than in those
administered with 5 mg/kg/day of tofacitinib (data not shown).
However, there was no significant difference between the
experimental groups comprised of mice administered with 10
mg/kg/day of tofacitinib and those administered with 15 mg/kg/day
of tofacitinib; thus, only the results of the group comprised of
the mice that were administered with 10 mg/kg/day of tofacitinib
were presented.
Tofacitinib suppresses the expression
of pro-inflammatory cytokines in mice with ConA-induced
hepatitis
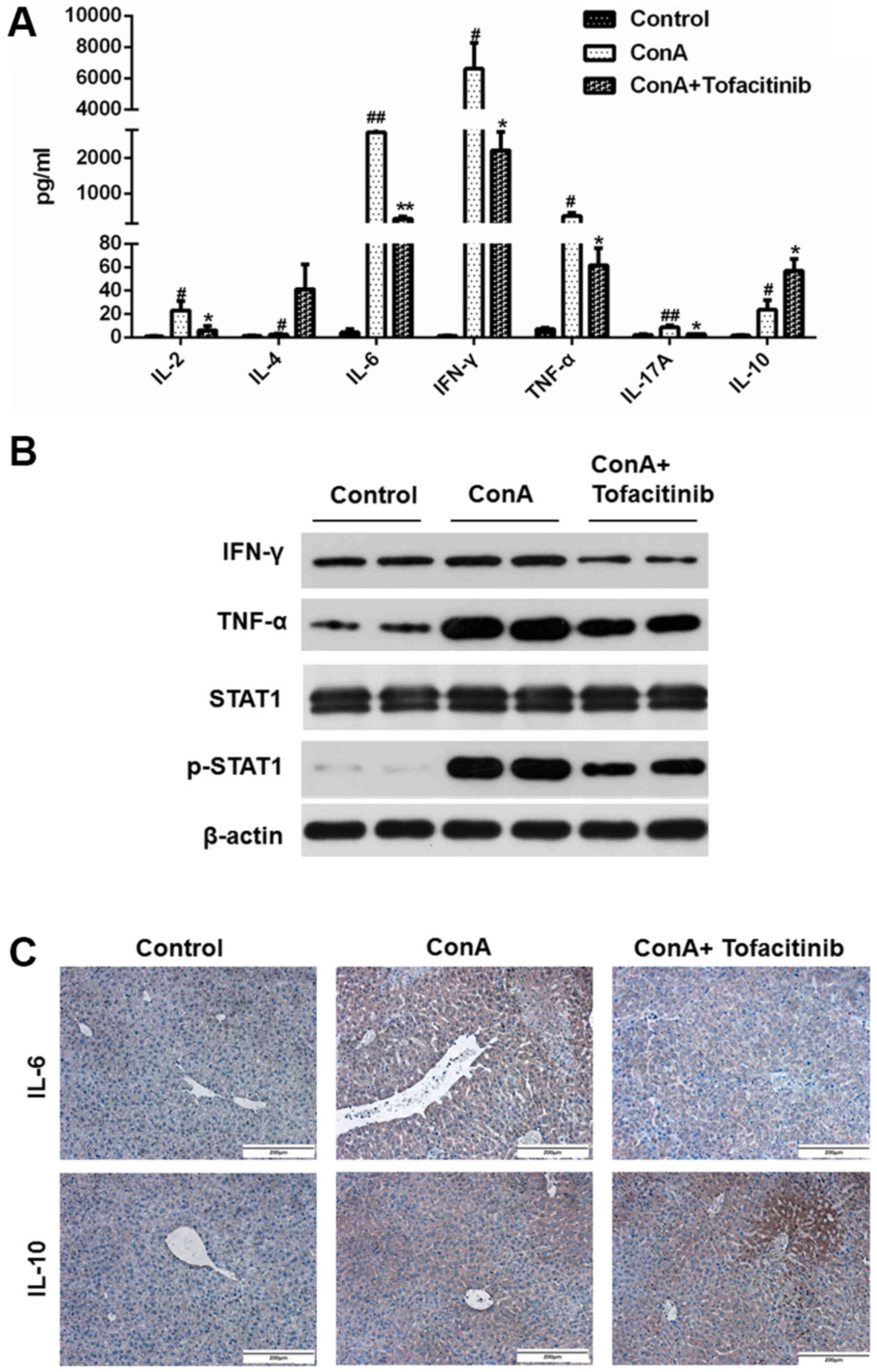
A mouse CBA kit was used to detect important
cytokines (IL-2, IL-4, IL-6, IFN-γ, TNF-α, IL-17A and IL-10) in the
serum of mice from each experimental group; the data are presented
in Fig. 4A. As observed in the
results, the expression of some pro-inflammatory cytokines, such as
IL-2, IL-6, IFN-γ, TNF-α, and IL-17A, was upregulated after the
ConA injection and revealed a downward trend in mice from the
tofacitinib treatment group, while the expression of the
anti-inflammatory cytokines, such as IL-4 and IL-10, increased
after the tofacitinib treatment. The protein expression levels of
TNF-α and IFN-γ significantly increased in the whole liver extracts
from mice in the ConA-treated groups, while the pretreatment with
tofacitinib downregulated these expression levels (Fig. 4B). Considering that IL-6 and IL-10
are the representative cytokines contributing to the distinct
differentiation of T cells, immunohistochemical staining was
further used to confirm the results presented in Fig. 4A (Fig.
4C). These results collectively confirmed that tofacitinib
regulated the secretion level of anti- and pro-inflammatory
cytokines under conditions of immune-mediated hepatitis.
Tofacitinib restores the impaired
Treg/Th17 cell ratio under conditions of ConA-induced
hepatitis
The imbalance of Tregs and Th17 cells plays a key
role in the pathogenesis of many immune-mediated diseases; this
imbalance may be regulated by different types of cytokines. ConA
can induce severe inflammation in the mouse liver and cause the
upregulation of pro-inflammatory cytokine expression in the serum.
In addition, many autoimmune diseases are characterized by systemic
disorders. Therefore, the ratio of Treg/Th17 cells was detected not
only in the mouse liver, but also in the spleen, which is
representative of the situation in the peripheral regions. ConA
induced an increase in the number of Th17 cells in both the liver
and spleen (Fig. 5A and C); the
number of Tregs also increased. This increase occurred because
Tregs, which are immunoregulatory cells, respond to the acute
inflammation caused by ConA. Notably, the number of Tregs in mice
from the treatment group exhibited an even greater increase, while
the number of Th17 cells in the same group exhibited a downward
trend. Although the number of both Tregs and Th17 cells exhibited
an increasing tendency, the ratio of Treg/Th17 cells decreased
following ConA injection (Fig. 5B and
D). However, in the tofacitinib treatment group, the impaired
Treg/Th17 cell ratio was significantly recovered.
Tofacitinib relieves liver fibrosis
under conditions of AIH
Although the mouse model of ConA-induced
immune-mediated liver injury resembles conditions of AIH in humans
(38), chronic inflammation and
liver fibrosis are not observed in this model. Evidence has
revealed that the balance of Tregs and Th17 cells is involved in
the liver fibrosis associated with chronic immune-mediated
hepatitis (39,40). Therefore, an AIH mouse model was
used to further explore the effects of tofacitinib under conditions
of liver fibrosis during immune-mediated hepatitis. Evident liver
fibrosis appeared in the livers of mice with AIH and was in fact
alleviated following tofacitinib treatment; representative Sirius
red staining images are presented in Fig. 6. These data confirmed, from another
point of view, that tofacitinib could suppress immune-mediated
liver injury.
Discussion
Recently, an increase in the number of cases of
immune-mediated liver injury, such as those involving autoimmune
liver diseases, has been reported; this has attracted a great
amount of attention from researchers (2). However, there is no effective therapy
for these diseases apart from immunosuppressants (41). Moreover, the side effects of
long-term standard immunosuppression and the lack of response to
standard immunosuppressive therapy are the major clinical
challenges associated with this treatment strategy (42). In recent years, some researchers
have also considered whether certain interventions used to treat
other autoimmune diseases such as rheumatic disorders, including
treatment with CTLA-4 Ig, recombinant IL-10, and anti-TNF-α
antibodies, and the adoptive transfer of Tregs, can be used for
treating immune-mediated hepatitis (43). However, these alternative
strategies have still not been applied widely in clinical
settings.
Tofacitinib is an inhibitor of the members of the
JAK family, which are intracellular proteins crucial to the
downstream regulation of many inflammatory mediators (44). Tofacitinib is used to treat
rheumatoid arthritis and is effective in methotrexate-naïve and
DMARD-experienced patients, including those who are unresponsive to
TNF inhibitors (26,45,46).
Recently, some studies revealed that tofacitinib also has a
protective effect against other diseases caused by immune
disorders, such as inflammatory bowel disease (47) and alopecia universalis (48,49).
It was observed that cytokines and the JAK/STAT pathways they
participate in also play important roles in patients with AIH
(50) and mice with ConA-induced
hepatitis (51); this has led to
the investigation of whether tofacitinib can also play a protective
role against immune-mediated hepatitis. However, no studies related
to this aspect have been reported. In the present study, to the
best of our knowledge, for the first time, the protective effects
of tofacitinib against immune-mediated liver injury were detected
in a mouse model of ConA-induced immune-mediated hepatitis, which
is a well-established animal model for the study of T cell-mediated
hepatitis (14).
In the present study, a mouse model of ConA-induced
immune-mediated hepatitis was first established and the JAK1/STAT1
pathway in the mouse livers was detected. It was revealed that the
plasma transaminase levels and the expression levels of TNF-α and
IFN-γ peaked at 12 h after ConA injection, but the activation of
the JAK1/STAT1 pathway appeared to occur later, perhaps because the
release of the inflammatory cytokines that activate the JAK/STAT
pathway proteins or the expression of JAK/STAT pathway proteins was
delayed. It was revealed that the selective JAK inhibitor
tofacitinib could in fact ameliorate mouse liver inflammation
effectively. The CBA results revealed significant changes in the
levels of several cytokines in the mouse serum. TNF-α and IFN-γ are
regarded as the most important mediators of ConA-induced hepatitis
(52,53), and their expression levels revealed
a decreasing tendency in the mice from the tofacitinib treatment
groups. The level of IL-6, which is essential for the
differentiation of Th17 cells, was elevated after the ConA
injection, but decreased following tofacitinib treatment; similar
results were observed in case of IL-17A. Collectively, these
results indicated that Th17 cells are involved in the protective
effect of tofacitinib. However, the expression of IL-10, an
anti-inflammatory cytokine related to tissue remodeling, Treg
differentiation, and immune homeostasis, also increased after the
ConA injection, perhaps due to a reaction elicited by the severe
inflammation. Furthermore, IL-10 expression exhibited a further
increase in mice from the tofacitinib treatment group, indicating
that tofacitinib enhanced immune suppression in the mice.
Tregs and Th17 cells are the most important subtypes
of CD4+ effector T cells associated with AIH and
ConA-induced hepatitis. The balance between these cell types is the
key to immune tolerance, and an impaired Treg/Th17 cell ratio
participates in the pathogenesis of many autoimmune diseases.
Considering that IL-10 is the key cytokine for Treg differentiation
and IL-6 induces the differentiation of naïve CD4+ T
cells into Th17 cells, and that according to our results, the IL-10
and IL-6 levels were altered following tofacitinib treatment, MNCs
were isolated from the livers of the experimental mice and the
Treg/Th17 cell ratios were analyzed. Herein, it was first
demonstrated that the number of both Tregs and Th17 cells increased
during immune-mediated liver injury. However, the ratio of
Tregs/Th17 cells decreased after the ConA injection and was notably
restored in the tofacitinib treatment group, thereby strongly
confirming our previous hypothesis. Tregs maintain immunological
tolerance and prevent autoimmunity by inhibiting the activation and
proliferation of effector T cells. In the mouse model of
ConA-induced immune-mediated liver injury, Tregs played an
important immunosuppressive role against severe liver inflammation
and finally contributed to immune tolerance. The inflammation in
the mouse liver was severe at 24 h, and according to the present
results, the number of both Th17 cells and Tregs increased.
However, the ratio of Tregs/Th17 cells among CD4+ T
cells decreased at 24 h, suggesting that the number of Th17 cells
increased more rapidly than that of Tregs at this time-point.
However, the present results indicated that tofacitinib improved
this situation and significantly restored the ratio of Treg/Th17
cells, resulting in the development of immune tolerance in the
mouse livers in a much shorter time.
Although the mouse model of ConA-induced hepatitis
is a well-recognized and typical tool to study T-cell-dependent
liver injury, hepatitis in this model is acute and disappears after
several days. Therefore, it was further confirmed that tofacitinib
could suppress liver fibrosis in an AIH mouse model, which provides
additional evidence for the protective effect of tofacitinib
against immune-mediated liver injury. In summary, the
aforementioned results indicated that tofacitinib can attenuate
immune-mediated liver injury in mice, and this treatment effect was
associated with the regulation of the Treg/Th17 cell ratio and the
inhibition of the expression of inflammatory cytokines. The present
study indicated that tofacitinib is very likely to serve as a new
treatment for immune-mediated liver diseases, providing a
prospective therapeutic strategy for liver disease-related clinical
challenges.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81600448, 81572419
and 81700515).
Availability of data and materials
The datasets used and/or analyzed are available from
the corresponding author on reasonable request.
Authors' contributions
HW, YL and YX performed the experiments and
contributed to the manuscript writing. XF and PH analyzed the data.
DT and WY designed the experiments and contributed to data
analysis. All authors read and approved the final manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal studies were approved by the Ethics
Committee of Animal Experiments of Tongji Medical College (Wuhan,
China) and monitored by the Department of Experimental Animals of
Tongji Medical College (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eksteen B, Afford SC, Wigmore SJ, Holt AP
and Adams DH: Immune-mediated liver injury. Semin Liver Dis.
27:351–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Webb GJ, Hirschfield GM, Krawitt EL and
Gershwin ME: Cellular and molecular mechanisms of autoimmune
hepatitis. Annu Rev Pathol. 13:247–292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manns MP, Czaja AJ, Gorham JD, Krawitt EL,
Mieli-Vergani G, Vergani D and Vierling JM; American Association
for the Study of Liver Diseases, : Diagnosis and management of
autoimmune hepatitis. Hepatology. 51:2193–2213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liberal R, Grant CR, Yuksel M, Graham J,
Kalbasi A, Ma Y, Heneghan MA, Mieli-Vergani G, Vergani D and Longhi
MS: Regulatory T-cell conditioning endows activated effector T
cells with suppressor function in autoimmune hepatitis/autoimmune
sclerosing cholangitis. Hepatology. 66:1570–1584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Romano M, Fanelli G, Tan N, Nova-Lamperti
E, McGregor R, Lechler RI, Lombardi G and Scottà C: Expanded
regulatory T cells induce alternatively activated monocytes with a
reduced capacity to expand T helper-17 cells. Front Immunol.
9:16252018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beringer A and Miossec P: IL-17 and
IL-17-producing cells and liver diseases, with focus on autoimmune
liver diseases. Autoimmun Rev. 17:1176–1185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Longhi MS, Liberal R, Holder B, Robson SC,
Ma Y, Mieli-Vergani G and Vergani D: Inhibition of interleukin-17
promotes differentiation of CD25− cells into stable T
regulatory cells in patients with autoimmune hepatitis.
Gastroenterology. 142:1526–1535.e6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun XF, Gu L, Deng WS and Xu Q: Impaired
balance of T helper 17/T regulatory cells in carbon
tetrachloride-induced liver fibrosis in mice. World J
Gastroenterol. 20:2062–2070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noack M and Miossec P: Th17 and regulatory
T cell balance in autoimmune and inflammatory diseases. Autoimmun
Rev. 13:668–677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tiegs G, Hentschel J and Wendel A: A T
cell-dependent experimental liver injury in mice inducible by
concanavalin A. J Clin Invest. 90:196–203. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji YR, Kim HJ, Bae KB, Lee S, Kim MO and
Ryoo ZY: Hepatic serum amyloid A1 aggravates T cell-mediated
hepatitis by inducing chemokines via Toll-like receptor 2 in mice.
J Biol Chem. 290:12804–12811. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Erhardt A, Biburger M, Papadopoulos T and
Tiegs G: IL-10, regulatory T cells, and Kupffer cells mediate
tolerance in concanavalin A-induced liver injury in mice.
Hepatology. 45:475–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shinohara Y and Tsukimoto M: Adenine
nucleotides attenuate murine T cell activation induced by
concanavalin A or T cell receptor stimulation. Front Pharmacol.
8:9862018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang HX, Liu M, Weng SY, Li JJ, Xie C, He
HL, Guan W, Yuan YS and Gao J: Immune mechanisms of Concanavalin A
model of autoimmune hepatitis. World J Gastroenterol. 18:119–125.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Küsters S, Gantner F, Künstle G and Tiegs
G: Interferon gamma plays a critical role in T cell-dependent liver
injury in mice initiated by concanavalin A. Gastroenterology.
111:462–471. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saitis A, Gatselis N, Zachou K and Dalekos
GN: Use of TNFα antagonists in refractory AIH: Revealing the
unforeseen. J Hepatol. 59:197–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ehser J, Holdener M, Christen S, Bayer M,
Pfeilschifter JM, Hintermann E, Bogdanos D and Christen U:
Molecular mimicry rather than identity breaks T-cell tolerance in
the CYP2D6 mouse model for human autoimmune hepatitis. J Autoimmun.
42:39–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hintermann E, Ehser J, Bayer M,
Pfeilschifter JM and Christen U: Mechanism of autoimmune hepatic
fibrogenesis induced by an adenovirus encoding the human liver
autoantigen cytochrome P450 2D6. J Autoimmun. 44:49–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hardtke-Wolenski M, Dywicki J, Fischer K,
Hapke M, Sievers M, Schlue J, Anderson MS, Taubert R, Noyan F,
Manns MP and Jaeckel E: The influence of genetic predisposition and
autoimmune hepatitis inducing antigens in disease development. J
Autoimmun. 78:39–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holdener M, Hintermann E, Bayer M, Rhode
A, Rodrigo E, Hintereder G, Johnson EF, Gonzalez FJ, Pfeilschifter
J, Manns MP, et al: Breaking tolerance to the natural human liver
autoantigen cytochrome P450 2D6 by virus infection. J Exp Med.
205:1409–1422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Christen U, Holdener M and Hintermann E:
Cytochrome P450 2D6 as a model antigen. Dig Dis. 28:80–85. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghoreschi K, Laurence A and O'Shea JJ:
Janus kinases in immune cell signaling. Immunol Rev. 228:273–287.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shuai K and Liu B: Regulation of JAK-STAT
signalling in the immune system. Nat Rev Immunol. 3:900–911. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perner F, Schnoder TM, Ranjan S,
Wolleschak D, Ebert C, Pils MC, Frey S, Polanetzki A, Fahldieck C,
Schönborn U, et al: Specificity of JAK-kinase inhibition determines
impact on human and murine T-cell function. Leukemia. 30:991–995.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vainchenker W and Constantinescu SN:
JAK/STAT signaling in hematological malignancies. Oncogene.
32:2601–2613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dhillon S: Tofacitinib: A review in
rheumatoid arthritis. Drugs. 77:1987–2001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boyle DL, Soma K, Hodge J, Kavanaugh A,
Mandel D, Mease P, Shurmur R, Singhal AK, Wei N, Rosengren S, et
al: The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT
signalling in rheumatoid arthritis. Ann Rheum Dis. 74:1311–1316.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mease P, Hall S, FitzGerald O, van der
Heijde D, Merola JF, Avila-Zapata F, Cieślak D, Graham D, Wang C,
Menon S, et al: Tofacitinib or Adalimumab versus placebo for
psoriatic arthritis. N Engl J Med. 377:1537–1550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pouillon L, Bossuyt P and Peyrin-Biroulet
L: Tofacitinib is the right OCTAVE for ulcerative colitis.
Gastroenterology. 153:862–864. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bayart CB, DeNiro KL, Brichta L, Craiglow
BG and Sidbury R: Topical Janus kinase inhibitors for the treatment
of pediatric alopecia areata. J Am Acad Dermatol. 77:167–170. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghoreschi K, Jesson MI, Li X, Lee JL,
Ghosh S, Alsup JW, Warner JD, Tanaka M, Steward-Tharp SM, Gadina M,
et al: Modulation of innate and adaptive immune responses by
tofacitinib (CP-690,550). J Immunol. 186:4234–4243. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakamura S, Maehara T, Watanabe S,
Ishihara M and Sato M: Improvement of hydrodynamics-based gene
transfer of nonviral DNA targeted to murine hepatocytes. Biomed Res
Int. 2013:9287902013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vidal B, Cascao R, Finnila MA, Lopes IP,
da Glória VG, Saarakkala S, Zioupos P, Canhão H and Fonseca JE:
Effects of tofacitinib in early arthritis-induced bone loss in an
adjuvant-induced arthritis rat model. Rheumatology (Oxford).
57:1461–1471. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng XX, Chi G, Wang H, Gao Y, Chen Q, Ru
YX, Luo ZL, Yan W, Li PY, Liu M, et al: IL-37 suppresses the
sustained hepatic IFN-γ/TNF-α production and T cell-dependent liver
injury. Int Immunopharmacol. 69:184–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chi G, Feng XX, Ru YX, Xiong T, Gao Y,
Wang H, Luo ZL, Mo R, Guo F, He YP, et al: TLR2/4 ligand-amplified
liver inflammation promotes initiation of autoimmune hepatitis due
to sustained IL-6/IL-12/IL-4/IL-25 expression. Mol Immunol.
99:171–181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Tan Q, Wang Y, Lv H, Wang Z, Lin
Z, Du Z, Xiong S, Han J, Tian D and Wang B: Overexpression of KLF14
protects against immune-mediated hepatic injury in mice. Lab
Invest. 99:37–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ye T, Wang T, Yang X, Fan X, Wen M, Shen
Y, Xi X, Men R and Yang L: Comparison of concanavalin a-induced
murine autoimmune hepatitis models. Cell Physiol Biochem.
46:1241–1251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Wang FP, She WM, Yang CQ, Li L, Tu
CT, Wang JY and Jiang W: Enhanced high-mobility group box 1 (HMGB1)
modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via
toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients
with chronic hepatitis B. J viral hepatitis. 21:129–140. 2014.
View Article : Google Scholar
|
|
40
|
Li J, Qiu SJ, She WM, Wang FP, Gao H, Li
L, Tu CT, Wang JY, Shen XZ and Jiang W: Significance of the balance
between regulatory T (Treg) and T helper 17 (Th17) cells during
hepatitis B virus related liver fibrosis. PLoS One. 7:e393072012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang F, Wang Q, Bian Z, Ren LL, Jia J and
Ma X: Autoimmune hepatitis: East meets west. J Gastroenterol
Hepatol. 30:1230–1236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liberal R, Krawitt EL, Vierling JM, Manns
MP, Mieli-Vergani G and Vergani D: Cutting edge issues in
autoimmune hepatitis. J Autoimmun. 75:6–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Czaja AJ: Emerging opportunities for
site-specific molecular and cellular interventions in autoimmune
hepatitis. Dig Dis Sci. 55:2712–2726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lopez-Sanz L, Bernal S, Recio C, Lazaro I,
Oguiza A, Melgar A, Jimenez-Castilla L, Egido J and Gomez-Guerrero
C: SOCS1-targeted therapy ameliorates renal and vascular oxidative
stress in diabetes via STAT1 and PI3K inhibition. Lab Invest.
98:1276–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fleischmann R, Mysler E, Hall S, Kivitz
AJ, Moots RJ, Luo Z, DeMasi R, Soma K, Zhang R, Takiya L, et al:
Efficacy and safety of tofacitinib monotherapy, tofacitinib with
methotrexate, and adalimumab with methotrexate in patients with
rheumatoid arthritis (ORAL Strategy): A phase 3b/4, double-blind,
head-to-head, randomised controlled trial. Lancet. 390:457–468.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Uttley L, Bermejo I, Ren S, Martyn-St
James M, Wong R, Scott DL, Young A and Stevenson M: Tofacitinib for
treating rheumatoid arthritis after the failure of
disease-modifying anti-rheumatic drugs: An evidence review group
perspective of a NICE single technology appraisal.
Pharmacoeconomics. 36:1063–1072. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
White JR, Phillips F, Monaghan T, Fateen
W, Samuel S, Ghosh S and Moran GW: Review article: Novel
oral-targeted therapies in inflammatory bowel disease. Aliment
Pharmacol Ther. 47:1610–1622. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Patel NU, Oussedik E, Grammenos A and
Pichardo-Geisinger R: A case report highlighting the effective
treatment of alopecia universalis with tofacitinib in an adolescent
and adult patient. J Cutan Med Surg. 22:439–442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Silberstein SD, Yeung PP and Aycardi E:
Preventive therapies for chronic migraine. N Engl J Med.
378:7742018.PubMed/NCBI
|
|
50
|
Longhi MS, Hussain MJ, Mitry RR, Arora SK,
Mieli-Vergani G, Vergani D and Ma Y: Functional study of CD4+CD25+
regulatory T cells in health and autoimmune hepatitis. J Immunol.
176:4484–4491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nakaya M, Hashimoto M, Nakagawa R,
Wakabayashi Y, Ishizaki T, Takada I, Komai K, Yoshida H and
Yoshimura A: SOCS3 in T and NKT cells negatively regulates cytokine
production and ameliorates ConA-induced hepatitis. J Immunol.
183:7047–7053. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kato J, Okamoto T, Motoyama H, Uchiyama R,
Kirchhofer D, Van Rooijen N, Enomoto H, Nishiguchi S, Kawada N,
Fujimoto J and Tsutsui H: Interferon-gamma-mediated tissue factor
expression contributes to T-cell-mediated hepatitis through
induction of hypercoagulation in mice. Hepatology. 57:362–372.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Iwamoto S, Kido M, Aoki N, Nishiura H,
Maruoka R, Ikeda A, Okazaki T, Chiba T and Watanabe N: TNF-α is
essential in the induction of fatal autoimmune hepatitis in mice
through upregulation of hepatic CCL20 expression. Clin Immunol.
146:15–25. 2013. View Article : Google Scholar : PubMed/NCBI
|