Introduction
Cervical carcinoma, as one of the most common types
of malignancies in women, caused >300,000 cases of mortality
annually in 2008 (1). The majority
of cases of cervical carcinoma are cervical squamous cell
carcinomas (CSCC), which originate from precursor squamous
intraepithelial lesions (2). It is
generally believed that human papillomavirus (HPV) infection is the
main cause of CSCC and >90% patients with CSCC are HPV-positive
(3,4). With the popularization of the HPV
vaccination and HPV infection screening, incidence of CSCC
experienced continuous decrease during 20th century (5). However, has been observed that the
incidence rate of HPV-negative CSCC has had an increasing trend in
previous years (6). Compared with
HPV-positive CSCC, the survival rate of patients with HPV-negative
CSCC is even worse (6).
Hypoxia-inducible factor 1α (HIF-1α) serves pivotal
functions in the pathogenesis of various types of human
malignancies (7). Cellular oxygen
balance is highly impaired in cancer and HIF-1α expression induced
by hypoxic conditions promotes cancer cell proliferation, migration
and invasion through multiple different pathways (8). Previous studies have demonstrated
that HIF-1α, in certain cases, achieves its biological functions
through the interactions with long non-coding RNAs (lncRNAs)
(9), which are a subgroup of
non-coding RNAs that serve pivotal functions in different human
diseases (10). LncRNA
anti-differentiation ncRNA (ANCR), a lncRNA identified to be
involved in cell differentiation, serves different functions in
different types of malignancies (11,12).
In breast cancer, the downregulation of lncRNA ANCR promotes tumor
growth factor-β-induced epithelial-mesenchymal transition and
metastasis, indicating its potential function as a tumor suppressor
gene in this disease (11). In
contrast, the downregulation of lncRNA ANCR inhibits the invasion
and migration of colorectal cancer cells, suggesting that it may
have oncogenic functions (12).
The present study investigated the role of lncRNA ANCR in CSCC.
Patients and methods
Specimen collection
The present study included a total of 38
HPV-negative patients with CSCC, 22 HPV-16 positive patients with
CSCC and 28 HPV-18 positive patients with CSCC. Those patients were
diagnosed and treated at The Second Hospital of Lanzhou University
(Lanzhou, China) between August 2015 and October 2017. The
inclusion criteria were as follows: i) Pathologically diagnosed as
CSCC; ii) diagnosed and treated for the first time; and iii) were
not treated prior to admission. The exclusion criteria were as
follows: i) Possessing any other cervical diseases or malignancies;
and ii) having received any other type of treatment prior to
admission. At the same time, 38 healthy women were also included to
function as healthy controls. The age of the 38 HPV-negative
patients with CSCC ranged from 28 to 56 years, with a mean age of
42.8±4.3 years. The age of the 22 HPV-16 positive patients with
CSCC ranged from 25 to 58 years, with a mean age of 41.5±4.9 years.
The age of the 28 HPV-18 positive patients with CSCC ranged from 29
to 53 years, with a mean age of 43.1±5.1 years. The age of the 38
healthy women (HPV-negative) ranged from 29 to 60 years, with a
mean age of 44.3±4.4 years. No significant differences in age, body
mass index, drinking and smoking habits and other basic clinical
data were observed amongst the 4 groups. The study was ethically
approved by the Ethics Committee of The Second Hospital of Lanzhou
University. All patients provided written informed consent prior to
the study.
Tissue collection
Cervical biopsies (100–200 mg) were collected from
patients with CSCC and healthy controls. Cervical biopsies were
performed on healthy controls for the detection of potential
cervical lesions and those disease conditions were finally
excluded. Blood (5 ml) was extracted from each participant in the
morning on the day of admission and 3 and 6 days following
admission. Blood was maintained at 37°C for 2 h in order to achieve
hemolysis, followed by centrifugation at 1,200 × g for 15 min at
room temperature to collect serum. Serum samples derived from 3
separate time points were mixed. All samples were stored in liquid
nitrogen prior to use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to extract total RNA
from biopsies, serum and in vitro cultivated cells according
to the manufacturer's protocol. Genomic DNA was removed from RNA
samples by DNase I (Thermo Fisher Scientific, Inc.) digestion.
Following cDNA synthesis through reverse transcription, the PCR
reaction system was prepared using SYBR® Green Real-Time
PCR Master Mixes (Thermo Fisher Scientific, Inc.). Sequences of
primers used in PCR reactions were as follows: Human ANCR forward,
5′-GCCACTATGTAGCGGGTTTC-3′ and reverse, 5′-ACCTGCGCTAAGAACTGAGG-3′;
human HIF-1α forward, 5′-CATAAAGTCTGCAACATGGAAGGT-3′ and reverse,
5′-ATTTGATGGGTGAGGAATGGGTT-3′; human β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
The thermocycling conditions were as follows: 95°C for 1 min,
followed by 40 cycles of 15 sec at 95°C and 33 sec at 56°C. The
expression levels of ANCR and HIF-1α were normalized to β-actin
using the 2−ΔΔCq method (13).
Cell lines, cell culture and
transfection
Two human CSCC cell lines C33A (HPV-negative) and
SiHa (HPV-positive) were purchased from American Type Culture
Collection (ATCC; Manassas, VA, USA). Cells of these two cell lines
were cultured with ATCC-formulated Eagle's Minimum Essential Medium
(cat. no. 30-2003) containing 10% fetal bovine serum in an
incubator (37°C, 5% CO2). PCR reactions were performed
using Pwo DNA Polymerase (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) to obtain full length ANCR and HIF-1α cDNA surrounded by
EcoRI-EcoRI cutting sites. Primers were: forward,
GAATTCCCCGCCCCGCGCCGCCTCTC and reverse,
AATTCTATTTCTGAATATACAGCCAAG. The thermocycling conditions were:
95°C for 2 min, followed by 30 cycles of 30 sec at 95°C, 30 sec at
60°C and 1 min at 72°C. The two DNA fragments were inserted into an
EcoRI linearized pIRSE2-EGFP vector (Clontech Laboratories, Inc.,
Mountainview, CA, USA) to produce ANCR and HIF-1α expression
vectors, respectively. Lipofectamine 2000 reagent (cat. no.
11668-019; Invitrogen; Thermo Fisher Scientific, Inc.) was used to
transfect 10 nM vectors into 6×105 cells according to
the manufacturer's protocol. The incubation of cells with vectors
was performed for 6 h at 37°C. Empty pIRSE2-EGFP vectors were used
as the negative control. ANCR and HIF-1α overexpression was
confirmed by RT-qPCR.
Cell proliferation assay
Subsequent to transfection, the cells of C33A and
SiHa cell lines were collected during the logarithmic growth phase
to prepare a cell suspension at a density of 6×104
cells/ml. Then, a 0.1 ml cell suspension containing
6×103 cells was transferred into each well of a 96-well
plate. The plate was cultured under hypoxic conditions (8%
O2 and 92% N2). A total of 10 µl Cell
Counting kit-8 (CCK-8) solution (Sigma-Aldrich; Merck KGaA) was
added into each well at the following time points: 24, 48, 72 and
96 h according to the manufacturer's protocol and subsequent to the
initiation of cell culture. Cells were cultured for another 4 h at
37°C following the addition of CCK-8, and the SpectraMax iD5
Multi-Mode Microplate Reader was used to measure optical density
values (450 nm). Data was analyzed using Excel 2016 (Microsoft
Corporation, Redmond, WA, USA).
Western blot analysis
RIPA buffer solution (Thermo Fisher Scientific,
Inc.) was used to extract total protein from in vitro
cultivated cells according to the manufacturer's protocol, and
protein concentrations were measured using a BCA assay. SDS-PAGE
(12%) gel electrophoresis was then performed with 35 µg protein per
lane to separate the proteins with different molecular weights. Gel
transfer to polyvinylidene difluoride (PVDF) membranes was
performed, followed by incubating the PVDF membranes with 5%
skimmed milk at room temperature for 2 h. Subsequent to washing
with TBST (0.2% Tween) 3 times, 15 min each time, membranes were
incubated with primary antibodies for HIF-1α (rabbit anti human,
1:1,400; cat. no. ab51608; Abcam, Cambridge, UK) and GAPDH (rabbit
anti human, 1:1,200; cat. no. ab37168; Abcam) overnight at 4°C. The
next day, membranes were incubated with goat anti-rabbit
immunoglobulin G horseradish peroxidase-conjugated secondary
antibody (1:1,000; cat. no. MBS435036; MyBioSource, Inc., San
Diego, CA, USA) for 4 h at room temperature. Signal development was
performed using Pierce enhanced chemiluminescent Western Blotting
Substrate (Thermo Fisher Scientific, Inc.). Gray scale of HIF-1α
was normalized to that of the GAPDH band using Image J software
version 1.46 (National Institutes of Health, Bethesda, MD, USA) to
represent the relative expression level of HIF-1α.
Statistical analysis
GraphPad Prism 6 statistical software (GraphPad
Software, Inc., La Jolla, CA, USA) was used for all data analyses.
Data were presented at mean ± standard deviation. Cell
proliferation in addition to ANCR and HIF-1α expression data were
compared using one way analysis of variance followed by a
Least-Significant-Difference test. HPV-negative patients were
divided in to high-expression (n=12) and low-expression (n=8)
groups (cutoff value=1.72) according to Youdens index. Associations
between the expression levels of ANCR and patients'
clinicopathological data were analyzed using a χ2 test.
Receiver operating characteristic (ROC) curve analysis was
performed to evaluate the diagnostic values of ANCR expression for
different types of CSCC with patients with different types of CSCC
as true positive cases and healthy controls as true negative cases.
P<0.05 was considered to indicate a statistically significant
difference.
Results
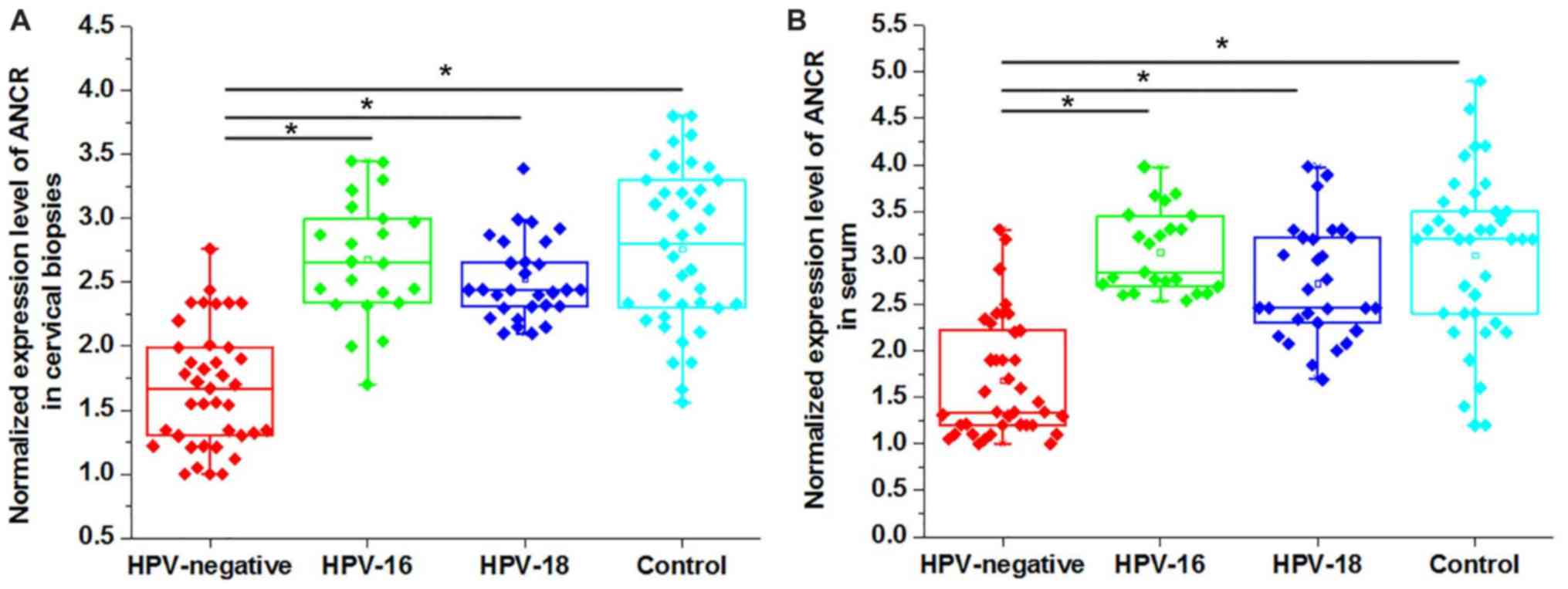
Comparison of expression levels of
ANCR in patients with CSCC and healthy controls
The differential expression of a gene in patients
and healthy people indicate the involvement of the certain gene in
a disease. Therefore, the expression levels of ANCR in patients
with CSCC and healthy controls were detected. As presented in
Fig. 1, the expression levels of
ANCR in cervical biopsies (Fig.
1A) and serum (Fig. 1B) were
significantly lower in HPV-negative patients with CSCC compared
with in HPV-16 positive patients, HPV-18 positive patients in
addition to healthy controls (P<0.05). Although a slight
decrease in the expression level of ANCR was also observed in
HPV-16 positive patients and HPV-18 positive patients compared with
the healthy controls, the differences were not significant.
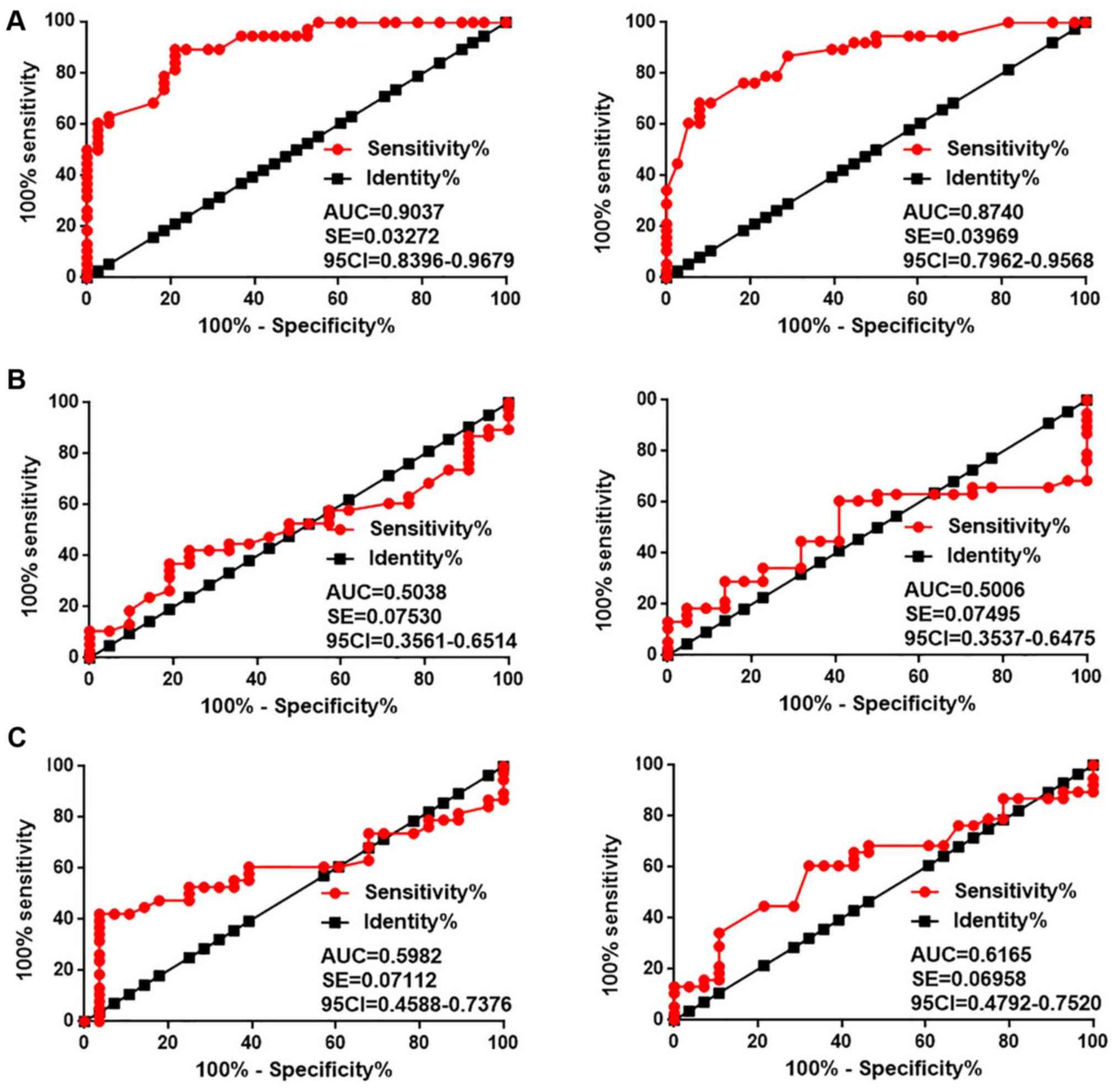
Diagnostic values of ANCR expression
for different types of CSCC
ROC curve analysis was performed to evaluate the
diagnostic values of ANCR expression for different types of CSCC.
As presented in Fig. 2A, the area
under the curve (AUC) of the use of ANCR expression in cervical
tissues (left) for the diagnosis of HPV-negative CSCC was 0.9073
with a standard error (SE) of 0.03272 and 95% confidence interval
(95% CI) of 0.8396–0.9679 (P<0.001). As for ANCR expression in
serum (right), the AUC was 0.8740 with a SE of 0.03696 and 95% CI
of 0.7962–0.9568 (P<0.001). Therefore, ANCR expression may serve
as a potential diagnostic biomarker for HPV-negative CSCC. In
contrast, ANCR expression in cervical tissues and serum failed to
effectively distinguish HPV-16 (Fig.
2B) or HPV-18 (Fig. 2C)
positive patients with CSCC from the heathy controls (all AUCs
<0.65, all P>0.05).
Association between the expression
levels of ANCR and the clinicopathological data of HPV-negative
patients
χ2 analysis was performed to analyze the
association between the expression levels of ANCR and the
clinicopathological data of HPV-negative patients. The results
revealed that ANCR expression in cervical tissues (Table I) and serum (Table II) were not significantly
associated with the patients' age, living habits (smoking and
drinking) in addition to distant tumor metastasis (P>0.05).
However, ANCR expression (in the high expression group compared
with the low expression group) was significantly associated with
tumor size (P<0.05).
 | Table I.Association between the expression
levels of anti-differentiation non-coding RNA in cervical biopsies
and the clinicopathological data of human papillomavirus-negative
patients. |
Table I.
Association between the expression
levels of anti-differentiation non-coding RNA in cervical biopsies
and the clinicopathological data of human papillomavirus-negative
patients.
| Variables | Groups | Cases | High-expression | Low-expression | χ2 | P-value |
|---|
| Age | >40 (years) | 20 | 12 | 8 | 1.69 | 0.19 |
|
| <40 (years) | 18 | 7 | 11 |
|
|
| Smoking | Yes | 9 | 4 | 5 | 0.15 | 0.70 |
|
| No | 29 | 15 | 14 |
|
|
| Drinking | Yes | 12 | 7 | 5 | 0.49 | 0.49 |
|
| No | 26 | 12 | 14 |
|
|
| Primary tumor
diameter | >5 cm | 14 | 3 | 11 | 7.90 | 0.02 |
|
| 3–5 cm | 12 | 7 | 5 |
|
|
|
| 1–3 cm | 12 | 9 | 3 |
|
|
| Tumor distant
metastasis | Yes | 16 | 7 | 9 | 0.43 | 0.51 |
|
| No | 22 | 12 | 10 |
|
|
 | Table II.Association between the expression
levels of anti-differentiation non-coding RNA in the serum and
clinicopathological data of human papillomavirus-negative
patients. |
Table II.
Association between the expression
levels of anti-differentiation non-coding RNA in the serum and
clinicopathological data of human papillomavirus-negative
patients.
| Variables | Groups | Cases | High-expression | Low-expression | χ2 | P-value |
|---|
| Age | >40 (years) | 20 | 11 | 9 | 0.42 | 0.52 |
|
| <40 (years) | 18 | 8 | 10 |
|
|
| Smoking | Yes | 9 | 3 | 6 | 1.31 | 0.25 |
|
| No | 29 | 16 | 13 |
|
|
| Drinking | Yes | 12 | 5 | 7 | 0.49 | 0.49 |
|
| No | 26 | 14 | 12 |
|
|
| Primary tumor
diameter | >5 cm | 14 | 3 | 11 | 7.90 | 0.02 |
|
| 3–5 cm | 12 | 7 | 5 |
|
|
|
| 1–3 cm | 12 | 9 | 3 |
|
|
| Tumor distant
metastasis | Yes | 16 | 6 | 10 | 1.72 | 0.19 |
|
| No | 22 | 13 | 9 |
|
|
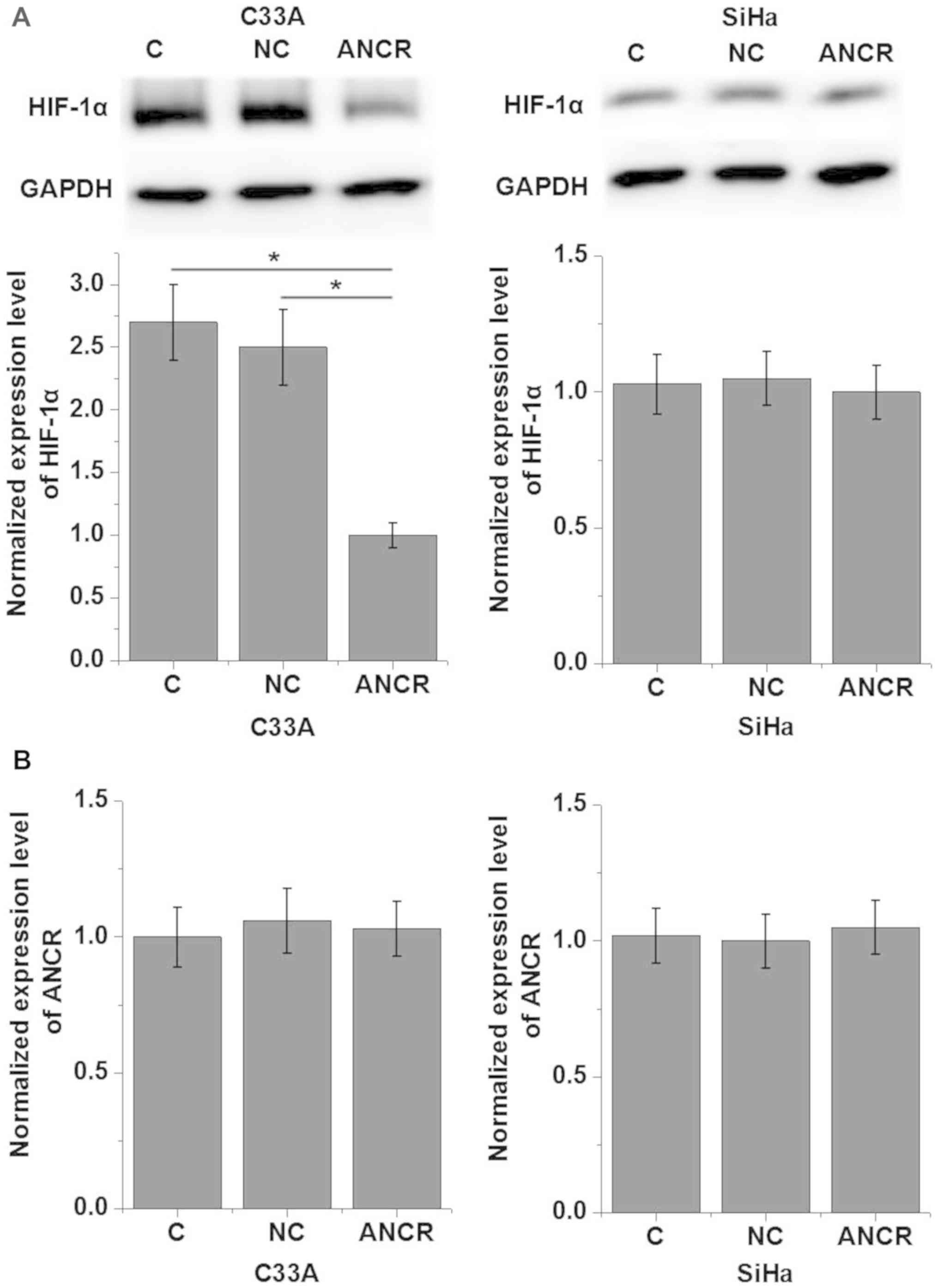
LncRNA ANCR regulates HIF-1α in the
cells of HPV-positive and negative human CSCC cell lines
Based on the data in Tables I and II, it was hypothesized that ANCR is
involved in the regulation of the growth of HPV-negative CSCC.
HIF-1α serves pivotal functions in the tumor growth of various
types of cancer (14). Therefore,
potential interactions between ANCR and HIF-1α in CSCC cells were
investigated. As presented in Fig.
3, ANCR overexpression resulted in the significantly
downregulated expression of HIF-1α in C33A (HPV-negative) cells
compared with the negative control cells (P<0.05), but not in
SiHa (HPV-positive) cells (P>0.05). In addition, HIF-1α
overexpression exerted no significant effects on ANCR expression in
either of the two cell lines (P>0.05).
Effects of ANCR and HIF-1α
overexpression on the proliferation of cells of HPV-positive and
negative human CSCC cell lines
To further examine the involvement of ANCR in the
regulation of tumor growth in CSCC, a ANCR expression vector was
transfected into CSCC cells and cell proliferation was detected
using a CCK-8 assay. As presented in Fig. 4A, ANCR overexpression significantly
inhibited the cell proliferation of cells of the HPV-negative cell
line C33A under hypoxic conditions compared with the empty vector
or untransfected cells (P<0.05). However, ANCR overexpression
exerted no significant effect on the proliferation of the cells of
the HPV-positive cell line SiHa under hypoxic conditions compared
with the empty vector or untransfected cells (P<0.05; Fig. 4B). In addition to that, HIF-1α
overexpression reversed the effects of ANCR overexpression on the
proliferation of the cells of the HPV-negative cell line C33A
(P<0.05; Fig. 4A). Furthermore,
HIF-1α overexpression also promoted the proliferation of the cells
of the HPV-positive cell line SiHa under hypoxic conditions
(P<0.05; Fig. 4B).
Discussion
To the best of our knowledge, the present study is
the first to report the involvement of lncRNA ANCR in HPV-negative
CSCC but not in HPV-positive CSCC. As far as we know, this lncRNA
is the first reported HPV-negative CSCC-specific lncRNA. It should
also be noted that ANCR is likely to be involved in the regulation
of tumor growth in HPV-negative CSCC and the functions of ANCR in
this disease are likely achieved through interaction with
HIF-1α.
LncRNA ANCR serves different functions in different
types of malignances. It has been reported that lncRNA ANCR is
substantially upregulated in colorectal cancer tissues and cells
compared with in paired adjacent normal tissues and normal cells,
and that the downregulation of ANCR inhibits the invasion and
migration of cancer cells (12).
In contrast, in breast cancer it is reported that lncRNA ANCR
mediates the degradation of enhancer of zeste 2 polycomb repressive
complex 2 subunit and attenuates the invasion and metastasis of
breast cancer (15), indicating
the function of ANCR as a tumor suppressor gene in this disease. In
the present study, the expression of ANCR was revealed to be
significantly downregulated in HPV-negative patients with CSCC but
not in HPV-positive patients with CSCC. The specific downregulation
of ANCR in HPV-negative CSCC indicates its potential function as a
tumor suppressor gene achieved through a HPV-independent pathway in
CSCC.
Survival of HPV-negative CSCC is usually poor and
early diagnosis remains critical for survival (6). Pathological examination through
biopsy is still the gold standard for the diagnosis of cancer.
However, the application of this examination in certain cases is
limited by its invasive nature (16). In previous years, using circulating
biomarkers as a non-invasive technique has been increasingly used
to assist disease diagnosis (17).
In the present study, circulating ANCR has been detected in all
participants. Diagnostic values evaluated by ROC curve analysis
also revealed that the expression of ANCR in cervical biopsies and
serum may be used to effectively distinguish HPV-negative but not
HPV-positive patients from healthy controls. The performance of
serum circulating ANCR is comparable to that of ANCR expression in
cervical biopsies. Therefore, ACNR may function as a potential
biomarker for HPV-negative CSCC. ANCR expression is altered in
several types of malignancies (11,12),
therefore multiple biomarkers may be combined during diagnosis to
exclude the potential of other cancer types. The present study also
demonstrated that the expression of ANCR is not affected by age or
smoking and drinking habits, which are known factors to affect the
expression of certain lncRNAs (18–20),
indicating the high stability of ANCR as a biomarker.
ANCR is involved in the regulation of tumor
metastasis in several types of malignancies (11,12,17),
but may not be involved in HPV-negative CSCC as no significant
association was observed between ANCR expression and the existence
of distant tumor metastasis. In contrast, a significant association
between ANCR expression and tumor size was identified, indicating
its function in tumor growth. HIF-1α serves pivotal functions in
the tumor growth of various types of cancer (14), and the overexpression of HIF-1α is
common in CSCC (21). The present
study demonstrated that ANCR is likely to be an upstream inhibitor
of HIF-1α, and it may be concluded from the facts that ANCR
overexpression downregulates HIF-1α but HIF-1α overexpression does
not significantly affect ANCR expression. An in vitro cell
proliferation assay further confirmed this conclusion, as HIF-1α
overexpression reversed the inhibitory effects of ANCR
overexpression on the proliferation of HPV-negative cells. It is
also worth noting that HIF-1α overexpression additionally promoted
the proliferation of HPV-positive CSCC cells, in which ANCR is
unlikely to be involved. Therefore, HIF-1α may interact with
different factors to participate in different types of
malignancies. In addition, the present data also suggests that ANCR
overexpression may function as a potential diagnostic target for
HPV-negative CSCC cells.
However, the present study only included one
HPV-positive cell line and one HPV-negative CSCC cell line, which
may be insufficient to make substantial conclusions. Future studies
will include a greater number of cell lines to further confirm the
conclusions in the present study. The present study suggests that
HPV-associated factors may reverse the tumor suppression effects of
IncRNA ANCRs. These HPV-associated factors remain to be identified.
In addition to HPV-16 and HPV-18, CSCC may also be caused by other
HPV strains including HPV-11 (4).
Therefore, future studies will focus on patients with CSCC infected
with other HPV strains.
In conclusion, ANCR is a tumor suppressor gene in
HPV-negative CSCC but not in HPV-positive CSCC. The function of
ANCR in HPV-negative CSCC is likely to be achieved by
downregulating HIF-1α.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WT designed the study and guaranteed the integrity
of the entire study, and defined the intellectual content. YZ
performed the literature research, collected the data, assisted
with the experiments, and reviewed and edited the manuscript. SZ
performed the experiments and analyzed the data. PS performed the
statistical analysis and completed the manuscript.
Ethics approval and consent to
participate
The study was ethically approved by the Ethics
Committee of The Second Hospital of Lanzhou University. All
patients provided written informed consent prior to the study.
Patient consent for publication
All patients provided consent for possible
publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forman D, de Martel C, Lacey CJ,
Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J,
Bray F, Plummer M and Franceschi S: Global burden of human
papillomavirus and related diseases. Vaccine. 5 (Suppl 30):F12–F23.
2012. View Article : Google Scholar
|
|
2
|
Groves IJ and Coleman N: Pathogenesis of
human papillomavirus-associated mucosal disease. J Pathol.
235:527–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiffman M, Castle PE, Jeronimo J,
Rodriguez AC and Wacholder S: Human papillomavirus and cervical
cancer. Lancet. 370:890–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galic V, Herzog TJ, Lewin SN, Neugut AI,
Burke WM, Lu YS, Hershman DL and Wright JD: Prognostic significance
of adenocarcinoma histology in women with cervical cancer. Gynecol
Oncol. 125:287–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Semenza GL: HIF-1 and human disease: One
highly involved factor. Genes Dev. 14:1983–1991. 2000.PubMed/NCBI
|
|
9
|
Yang F, Zhang H, Mei Y and Wu M:
Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the
warburg effect. Mol Cell. 53:88–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Z, Dong M, Fan D, Hou P, Li H, Liu L,
Lin C, Liu J, Su L, Wu L, et al: LncRNA ANCR down-regulation
promotes TGF-β-induced EMT and metastasis in breast cancer.
Oncotarget. 8:67329–67343. 2017.PubMed/NCBI
|
|
12
|
Yang ZY, Yang F, Zhang YL, Liu B, Wang M,
Hong X, Yu Y, Zhou YH and Zeng H: LncRNA-ANCR down-regulation
suppresses invasion and migration of colorectal cancer cells by
regulating EZH2 expression. Cancer Biomark. 18:95–104. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Hou P, Fan D, Dong M, Ma M, Li H,
Yao R, Li Y, Wang G, Geng P, et al: The degradation of EZH2
mediated by lncRNA ANCR attenuated the invasion and metastasis of
breast cancer. Cell Death Differ. 24:59–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saslow D, Solomon D, Lawson HW, Killackey
M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur
DC, et al: American Cancer Society, American Society for Colposcopy
and Cervical Pathology, and American Society for Clinical Pathology
screening guidelines for the prevention and early detection of
cervical cancer. CA Cancer J Clin. 62:147–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Appierto V, Di Cosimo S, Reduzzi C, Pala
V, Cappelletti V and Daidone MG: How to study and overcome tumor
heterogeneity with circulating biomarkers: The breast cancer case.
Semin Cancer Biol. 44:106–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayfield RD: Emerging roles for ncRNAs in
alcohol use disorders. Alcohol. 60:31–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neppl RL, Wu CL and Walsh K: lncRNA
Chronos is an aging-induced inhibitor of muscle hypertrophy. J Cell
Biol. 216:3497–3507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Qiu M, Xu Y, Li M, Dong G, Mao Q,
Yin R and Xu L: Long noncoding RNA CCAT2 correlates with smoking in
esophageal squamous cell carcinoma. Tumour Biol. 36:5523–5528.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G and Oberhuber G: Overexpression of hypoxia-inducible
factor 1alpha is a marker for an unfavorable prognosis in
early-stage invasive cervical cancer. Cancer Res. 60:4693–4696.
2000.PubMed/NCBI
|