Introduction
There are an increasing number of cases of type II
diabetes worldwide, including in Eastern and Western countries.
Type II diabetes accounts for ~90% of all patients with diabetes
and is becoming a global public health challenge (1). As a common complication of diabetes,
diabetic peripheral neuropathy (DPN) is characterized by swelling,
degeneration, necrosis of dorsal root ganglia (DRG) neurons
accompanying axonal degeneration and atrophy, leading to a high
rate of disability and mortality in patients with type II diabetic
(2). Western medicines, such as
methylcobalamin and neurotrophin, are usually used in clinical
treatment; however, the therapeutic effects are poor in patients
with DPN (3). Additionally, the
high cost also increases patient burden, for example, the annual
cost of DPN and its complications was 4.6–13.7 billion dollars in
the US in 2001 (4). Traditional
Chinese medicine, such as Chinese herbs, acupuncture and massage,
is now attracting attention for the treatment of DPN due to its
lower cost, accessibility and efficacy (5).
Cortex Mori Radicis extract (CMR), collected from
the root bark of some Morus species, including M. alba, M.
mongolica, M. cathayana, and M. australis, has been used
as an anti-diabetic agent in traditional Chinese medicine for years
(6). A previous study reported
that hot water extracts from CMR (Morus alba L.) possessed
hypoglycemic activity in streptozotocin (STZ)-induced diabetic mice
(7). CMR was also reported to
attenuate myocardial damage in diabetic rats, such as cardiac
hypertrophy and fibrosis (8). Our
previous study indicated that CMR induced the neurite outgrowth in
pheochromocytoma PC12 cells and the primary cortical neuron of rats
(9). These previous findings
suggested that CMR possesses anti-diabetic and neuroprotective
potential, and the roles of CMR have been demonstrated using
different methods, including in vivo vs. in vitro
experiments. However, few previous studies have focused on the
effects of CMR on neuroregeneration of DPN in Type II diabetics and
a detailed mechanism remains unclear.
Therefore, the present study was designed to
investigate the effects of CMR on DPN in diabetic rats using DRG
neurons and explore the underlying mechanisms. The results of the
present study showed that CMR induced and promoted the neurite
outgrowth of DRG in diabetic rats, which was associated with the
activation of PI3K/AKT signaling and inhibition of Ca2+
influx by upregulating transient receptor potential canonical 1
(TRPC1).
Materials and methods
Preparation of CMR extract
CMR was purchased from Anhui Tienho Herbal Source
Company. CMR (100 g) was thinly sliced with scissors soaked in 500
ml distilled water at 50°C for 3 h, and concentrated using a rotary
evaporator (BUCHI B-480; BUCHI, Ltd.) at 60 rpm and 70°C for 2
days. The concentrated extracts were lyophilized using a freeze
dryer (FDU-540; EYELA) for 24 h. After the lyophilization, a
yellow-brownish active powder was obtained (yield=11.1 g).
Animals
A total of 45 Male Sprague-Dawley rats (5–6 weeks
old) weighing 200±20 g were obtained from Hubei Research Center of
Laboratory Animals. They were maintained at room temperature
(24±2°C) and relative humidity 45–55% under a 12-h light/dark
cycle. Food and water were provided ad libitum throughout
the experiments.
Groups
The rats were randomly divided into 5 groups (n=8
each group): i) Control group (control), fed with a standard diet,
gavaged with normal saline; ii) type II diabetic model group
(model), induced with high-fat diet/low-dose STZ and gavaged with
normal saline; iii) CMR treatment group (CMR), diabetic model
followed by gastrointestinal treatment with CMR; iv) CMR plus
TRPC1-small interfering (si-)RNA treatment group (CMR+si-TRPC1),
diabetic model treated with CMR and a tail vein injection of
TRPC1-si-RNA; and v) CMR plus control-si-RNA treatment group
(CMR+si-Control), diabetic model treated with CMR and a tail vein
injection of Control-siRNA.
High-fat diet/low-dose STZ-induced
diabetic model
The control group was fed with a normal diet, which
contained 4.25 gm% fat. The other four groups were fed with a
high-fat diet for 8 consecutive weeks (1–8 weeks), which contained
24 gm% fat, 24 gm% protein and 41 gm% carbohydrate. Following this,
the high-fat-diet rats were treated with STZ (30 mg/kg in 0.9%
NaCl, i.p.; Sigma-Aldrich) in weeks 5–8 (once per week; a total of
4 times). Diabetes was verified by evaluating the fasting blood
glucose levels using glucose oxidase reagent strips (Aviva
Accu-Chek; Roche Diagnostics GmbH), a glucose level >13.9 mM was
considered to indicate diabetes (10).
Treatment with CMR and injection via
tail vein delivery of siRNA
Diabetic rats were subjected to 100 mg/kg CMR (lot
no. 14015121; Beijing Tcmages Pharmaceutical Co., Ltd.) via
gastrointestinal treatment for 4 consecutive weeks (once a day in
weeks 9–12) in CMR group (11).
Another two groups of diabetic rats were treated with 100 mg/kg CMR
together with the tail vein delivery of TRPC1-si-RNA (0.1 mg/kg;
5′-GAACAUAAAUUGCGUAGAU-3′; OriGene Technologies, Inc.) or
Control-si-RNA (0.1 mg/kg; 5′-UAGCGACUAAACACAUCAA-3′; OriGene
Technologies, Inc.) in 2 ml PBS via rapid tail vein delivery (5–10
sec), once every 4 days during weeks 9–12 (a total of 6 times), as
shown in Fig. 1A (12,13).
During the experiments, the following data was collected about rats
in the different groups: Weight, food intake, drink and
excrement.
 | Figure 1.Effect of CMR treatment on the blood
glucose levels of diabetic rats. (A) Schematic diagram of the
experimental procedure. Rats were fed a high-fat diet for 8 weeks.
During weeks 5–8, STZ (30 mg/kg) was injected intraperitoneally
once a week. The rats were then subjected to 4 continuous weeks of
CMR (once a day) with or without the tail vein delivery of si-TRPC1
of si-control (once every 4 days). The rats were sacrificed and the
samples were collected 24 h after the last CMR treatment. (B) The
blood glucose levels between the diabetic model and CMR treatment
groups, including low (1 mg/kg), moderate (10 mg/kg) and high (100
mg/kg) doses of CMR, were measured through the tail vein using a
blood glucose meter. (C) At the end of the experiment, the blood
glucose levels of rats from the different groups were measured and
statistically analyzed. The red line indicates a blood glucose
level of 13.9 mM. (D) Weight, (E) food intake, (F) drinking water
and (G) excrement were monitored. The parameters refer to the
average value per rat in the different groups (n=5 per group).
*P<0.05, **P<0.01, ***P<0.001 vs. control;
#P<0.05 and ##P<0.01 vs. model;
ΔP<0.05 vs. CMR. STZ, streptozotocin; CMR, Cortex
Mori Radicis extract; TRPC1, transient receptor potential canonical
channel 1; si-, small interfering RNA. |
Nociceptive behavioral tests
Thermal nociceptive threshold was assessed by
measuring the withdrawal latency on hot plate as previous described
(14). The temperature of the
hot-plate was maintained at 50°C. The withdrawal latency started
from putting the mouse on the plate and terminated when a brisk
withdrawal or paw flinching was observed. A cut-off time of 30 sec
was set to avoid lesions on the paw. The mechanical nociceptive
threshold was quantified using the Randall-Selitto paw withdrawal
test (15) using an analgesy meter
(Ugo Basile S.R.L.) that generates a linearly increasing mechanical
force. Results represents the maximal pressure (g) tolerated by the
animal. The test was repeated three times with each rat, and the
mean value was calculated for evaluation.
Hematoxylin and eosin (H&E)
staining for the evaluation of Nissl bodies
The rats were deeply anesthetized using sodium
pentobarbital (50 mg/kg, i.p.) and no contraction response was
observed when rat paws were clamped with tweezers. Subsequently,
rats were transcardially perfused with 100 ml ice-cold PBS (pH 7.4)
followed by 500 ml 4% paraformaldehyde. After perfusion, the spine
was surgically isolated followed by a longitudinal incision, the
spinal cord was carefully removed and the intervertebral foramen
was exposed. DRGs (T8-L5) were isolated and obtained. DRGs were
post-fixed in the same fixatives for 90 min at room temperature and
cryoprotected overnight at 4°C in PBS containing 30% sucrose. The
DRGs were embedded in optimal cutting temperature compound (Bayer
Corporation), frozen and then cut into 15 µm sections. The sections
were mounted on gelatin coated slides for H&E staining to
observe changes in Nissl bodies. According to the manufacturers'
protocol (C0105, Beyotime Institute of Biotechnology), the slides
were stained in hematoxylin for 5 min and washed with
H2O for 10 min at room temperature. After dipping in 80%
EtOH, the slides were stained with Eosin for 30 sec, and dipped
sequentially in 95% EtOH and 100% EtOH. Then, the slides were
immersed in xylene for 5 min, and images were captured using a
Nikon Eclipse Ti-S microscope (Nikon Corporation) at 400×
magnification. All sections were randomized and evaluated by two
trained observers who were blinded to the treatment groups.
DRG neuron culture
After deep anesthesia induced by intraperitoneal
injection of sodium pentobarbital (50 mg/kg), rats were
unconsciousness with a slow respiratory and heart rate. DRGs
(T8-L5) were isolated following the aforementioned protocol without
intracardiac perfusion. The DRGs were digested with 0.5% trypsin
and 1% collagenase (type IA; Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C for 1 h to obtain a single cell suspension. Following
this, 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.) was
added to stop digestion and the cells were centrifuged for 10 min
at 1,000 rpm. The cells were resuspended and cultured in the
neurobasal medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 0.5 mM glutamine, 100 U/ml penicillin and 100
µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in a 5% CO2 incubator.
Assessment of total neurite
outgrowth
DRG neurons from different groups were loaded with
fura-2/AM (2 µM; S1052, Beyotime Institute of Biotechnology) at
room temperature for 30 min to assay neurite outgrowth as
previously described (16). The
cells were cultured on round coverslips, and then treated with
fura-2/AM. Images were captured using a Leica DMI 6000B
fluorescence microscope (Leica Microsystems GmbH) controlled using
SlideBook software 4.2 (Intelligent Imaging Innovations, Inc). The
length of neurite outgrowth was assessed by measuring the total
length from the cell body to the end of all neuritis. The final
length was the sum of all neurites that were measured from one cell
body.
Growth cone turning assay
To assess growth cone turning, a micropipette was
placed 15 mm away from the growth cone center at an angle of 45°
with respect to the initial direction of the neurite extension.
Axons were positioned with their growth cones 100 µm away from a
glass micropipette containing nerve growth factor (NGF, 50 ng/ml)
with their direction of growth at 45° to the pipette tip. NGF was
expelled at 2 Hz using 3 psi to create gradients with a 10–15%
change in concentration across 10 µm. Phase contrast images were
acquired at a magnification of ×20 for 1 h at 1 min intervals with
a Zeiss Axio Observer (Zeiss GmbH) at room temperature.
Reverse transcription-quantitative
(RT-q)PCR
Neurons were collected and total RNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
RT was performed using a Reverse Transcription kit (Takara
Biotechnology Co., Ltd.). The reaction conditions were as follows:
37°C for 15 min, 85°C for 5 sec and a 4°C hold. The expression
levels of TRPC1 were determined using qPCR with the Real-Time PCR
System 7500 Fast (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following primers were synthesized by Sangon Biotech
Co., Ltd.: TRPC1 forward, 5′-GCAGAACAGCTTGAAGGAGTG-3′ and reverse,
5′-CACTAGGCAGCACATCACCT-3′; and GAPDH forward,
5′-TCTCTGCTCCTCCCTGTTCTA-3′ and reverse, 5′-GCCAAATCCGTTCACACCG-3′.
The amplification conditions were as follows: 50°C for 2 min and
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, 60°C for
30 sec and 72°C for 30 sec. The fold change in mRNA expression was
quantified using the 2−ΔΔCq method (17) and GAPDH was used for
normalization.
Western blotting
Neurons were collected and lysed with RIPA buffer
(Beyotime Institute of Biotechnology) and the protein concentration
was determined using a BCA Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Proteins (25 µg) were resolved using 10%
SDS-PAGE, transferred to PVDF membranes and probed with antibodies
against TRPC1 (cat. no. sc-133076; Santa Cruz Biotechnology, Inc.),
PI3K (p110α, 1:1,000; cat. no. 4255; Cell Signaling Technology,
Inc.), AKT (1:1,000, cat. no. 4691; Cell Signaling Technology,
Inc.), phospho-Ser473 AKT (1:2,000, p-AKT; cat. no. 4060; Cell
Signaling Technology, Inc.) and GAPDH (1:1,000, cat. no. 5174; Cell
Signaling Technology, Inc.). The membranes were then incubated with
horseradish peroxidase-conjugated secondary antibody [horse
anti-mouse IgG (1:2,000, cat. no. 7076) or goat anti-rabbit IgG
(1:2,000, cat. no. 7074)] at room temperature for 1 h.
Immunoreactive bands were visualized using ECL (SignalFire™ Plus
ECL Reagent, cat. no. 12630, Cell Signaling Technology, Inc.) and
bands were scanned using a scanner (HP Scanjet 7400C; Hewlett
Packard). Optical density for each band was assessed using ImageJ
analysis software (version 1.60, National Institutes of Health).
Sample loading was normalized by quantities of GAPDH detected in
parallel.
Calcium imaging
Ratiometric imaging of intracellular Ca2+
using cells loaded with Fura-2/AM. DRG neurons were seeded in a
6-well plate (5×105 cells/well) and were cultured for 24
h. Coverslips with cells were placed in a cation-safe solution
composed of (107 mM NaCl, 7.2 mM KCl, 1.2 mM MgCl2, 11.5
mM glucose, 20 mM HEPES-NaOH, pH 7.3) and loaded with Fura-2/AM (2
µM final concentration) for 30 min at 37°C. Cells were washed and
Ca2+ measurements were performed using a Leica DMI 6000B
fluorescence microscope controlled using SlideBook software.
Intracellular Ca2+ measurements are shown as the 340/380
nm ratio obtained from groups of single cell (~50 cells).
Statistical analysis
Data are presented as the mean ± SD from at least 3
independent experiments. Comparisons between two groups were
conducted using a one-way ANOVA and Bonferroni-Dunn test for
multiple comparisons using Prism 5.0 software (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
CMR treatment reduces the blood
glucose levels of diabetic rats
As shown in Fig.
1B, a high dose of CMR (100 mg/kg) decreased the blood glucose
levels of diabetic rats. Compared with the model group, the blood
glucose levels in diabetic rats following CMR treatment were
significantly decreased (30.3±1.96 vs. 8.92±1.02 mM in the model
and CMR groups, respectively; P<0.01; Fig. 1C). Additionally, CMR treatment
restored the weight of diabetic rats and showed an inhibitive
effect on food intake, drink and excrement in rats who received an
injection of STZ (Fig. 1D-G).
siRNA-TRPC1 treatment abrogated the suppressive effect of CMR in
diabetic rats (8.92±1.02 vs 18.3±2.14 mM in the CMR vs. CMR +
si-TRPC1; P<0.05), suggesting that TRPC1 is involved in the
anti-diabetic effects of CMR (Fig.
1C-G).
CMR improved nerve functions in rats
with STZ-induced DPN
To evaluate the effects of CMR on nerve functions in
rats with DPN, thermal hypoalgesia and the mechanical threshold was
assessed. CMR significantly inhibited the increase of thermal
latency (Fig. 2A). Compared with
the model group, CMR prevented a decrease in the mechanical
threshold (Fig. 2B). Consistent
with the aforementioned results, si-TRPC1 treatment reversed the
protective effects of CMR on nerve functions in diabetic rats.
CMR reverses the loss of Nissl bodies
in the DRG neurons of diabetic rats
Pathological changes in the DRG neurons were
investigated by assessing the morphology of Nissl bodies using
H&E staining. As shown in Fig.
3, DRG neurons in diabetic rats showed a loss of Nissl bodies.
However, Nissl body dissolution was distinctly decreased with CMR
treatment. Compared with the CMR group without TRPC1-siRNA
treatment, Nissl bodies showed more pathological changes following
si-TRPC1 transfection.
CMR induces the neurite outgrowth of
DRG neurons in diabetic rats
To analyze neurite outgrowth, the total lengths of
neurites were measured. Compared with the control group, the total
neurite outgrowth in the DRG neurons of diabetic rats were
significantly decreased (119.8±3.7 vs. 43.4±5.86 µm in the control
and model groups, respectively; P<0.01; Fig. 4). Conversely, CMR treatment induced
the neurite outgrowth of DRG neurons (43.4±5.86 vs. 111.2±6.76 µm
in the model and CMR groups, respectively; P<0.01). si-TRPC1
transfection reduced the effect of CMR on the neurite outgrowth of
DRG neurons in diabetic rats (111.2±6.76 vs. 57.0±6.5 µm in the CMR
and CMR + si-TRPC1groups, respectively; P<0.05; Fig. 4).
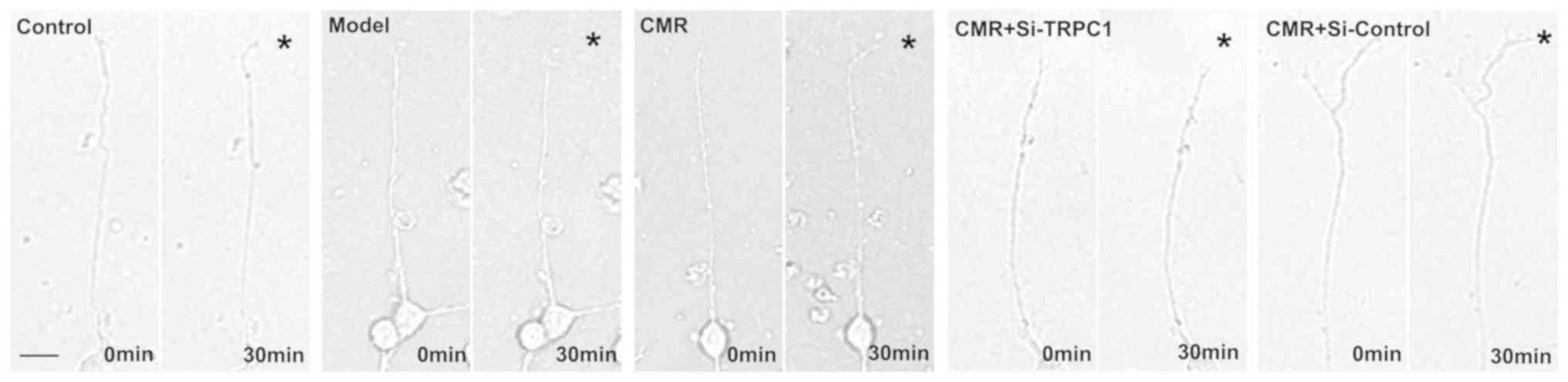
CMR restores the response of the
growth cone to NGF in diabetic rats
The chemotropic responses of DRG neurons as assessed
by exposing growth cones to gradients of NGF. The growth cones in
the model group exhibited no apparent bias in the direction of
extension. CMR treatment evoked a marked chemotropic turning
response toward the source of the NGF. Transfection with si-TRPC1
in the CMR group showed little effect on growth cone turning in
response to NGF (Fig. 5).
CMR upregulates TRPC1 and inhibits
Ca2+ influx in the DRG neurons of diabetic rats
As shown in Fig.
6A, TRPC1 was successfully knocked down in si-TRPC1 rats. CMR
treatment upregulated the expression of TRPC1 compared with the
model group at the mRNA and protein levels (Fig. 6B-D). si-TRPC1 transfection
suppressed the increase in the expression level of TRPC1 expression
at protein level following CMR administration. Additionally, the
Ca2+ influx in the DRG neurons from diabetic rats was
significantly increased compared with the control group. CMR
treatment inhibited extracellular Ca2+ influx in the DRG
neurons of diabetic rats. Moreover, the suppressive effect of CMR
on Ca2+ influx was partially reversed by si-TRPC1
transfection (Fig. 6E and F).
Activation of PI3K/AKT signaling
participates in the neurite outgrowth-inducing effects of CMR in
diabetic rats
To investigate the possible molecular mechanisms of
CMR-induced neurite outgrowth in diabetic rats, the effect of CMR
on the activation of PI3K/AKT signaling was assessed. As shown in
Fig. 7, CMR treatment increased
the expression of PI3K and enhanced the phosphorylation of AKT. On
the contrary, si-TRPC1 transfection in the CMR group reduced the
activation of the PI3K/AKT signaling associated with CMR treatment,
suggesting that the PI3K/AKT signaling pathway may be involved in
the neurite outgrowth-promoting effects of CMR in DPN.
Discussion
STZ-induced diabetic rats are one of the most
frequently used animal models to study diabetes. A combination of a
high-fat diet and a low dose of STZ can be used to induce a DPN
model in Sprague-Dawley rats and C57Bl/6J mice (18,19).
Based on the achievable treatment strategies, such as controlling
hyperglycemia, encouraging neurite elongation, increasing the
supply of angiogenic and neurotrophic factors, previous studies
have indicated that traditional Chinese medicine can be considered
and used as a promising treatment for DPN (20–23).
Jiaweibugan decoction was reported to significantly ameliorate
motor nerve conduction velocity in diabetic rats and to play a
protective role in peripheral nerve injury (24). Bogijetong decoction had the
potential to induce neurite outgrowth of DRG neurons in
STZ-diabetic animals (25).
Consistent with these previous findings, in the present study CMR
was found to lower the blood glucose levels of diabetic rats,
improve nerve functions, reverse the loss of Nissl bodies, induce
neurite outgrowth in DRG neurons and restore the response of growth
cones to NGF, suggesting the potential of CMR on neuroregeneration
in diabetic rats.
Neurite outgrowth is a critical step during neuronal
differentiation and regeneration (26). Ca2+ has been shown to
participate in the process of neurite outgrowth (27). In diabetes, neurons experience
metabolic stress and mitochondrial dysfunction, which results in
the deregulation of Ca2+ homeostasis (28). In turn, Ca2+ homeostasis
disequilibrium aggravated the pathological cellular reactions
contributing to development of diabetic neuropathies (29). Diabetic neuropathy potentiated the
activity of T-type and high voltage-activated Ca2+
channels in primary sensory neurons (30). A recent study showed that
mesenchymal stem cells improved DPN by ameliorating intracellular
Ca2+ homeostasis (31).
Ca2+ signaling-associated factors in DRG neurons include
several types of Ca2+-permeable membrane channels
(32). In the brain,
Ca2+ influx is related to the opening of TRPC1, and is
frequently associated with the regulation of adult neural
progenitor cells (33). Including
the change of extracellular calcium influx, a previous study
reported that resting intracellular Ca2+ rose
progressively in the neurons (DRG and dorsal horn) with the
duration of diabetes and that calcium mobilization from the
endoplasmic reticulum decreased during diabetes (34), suggesting the involvement of
calcium in the intracellular calcium pool. In the present study,
Ca2+ influx was reduced in the CMR group compared with
the diabetic model group, which suggested that Ca2+
influx was related to the biological activation of CMR in diabetic
rats. More studies are required to investigate whether calcium in
intracellular calcium pools participates in the progress of
DPN.
siRNA delivery via tail vein injection in rats has
been reported to have a prolonged, but not permanent, effect on
target mRNA and the potential inflammatory sequelae (13). However, this technique can also be
used to introduce siRNAs into the tissues of the whole animal
through a rapid and large volume tail-vein injection by increasing
its hydrostatic pressure (35). A
previous study reported that in vivo tail vein delivery of
siRNA to block caspase-8 or Fas could attenuate the onset of
morbidity and mortality in polymicrobial sepsis (13). Similarly, this strategy was used in
the present study to deliver si-TRPC1 into rats (2 ml was injected
into the tail vein within 10 sec a total of six times) to knockdown
TRPC1 in DRG neurons. As expected, si-TRPC1 treatment induced a
decrease in TRPC1 expression at the mRNA and protein levels, which
confirmed the effectiveness of siRNA delivery via tail vein
injection. It was notable that si-TRPC1 treatment had no obvious
effect on the blood glucose in diabetic rats but showed the
antagonistic effect during CMR application. The result suggested
that TRPC1 only involved in the hypoglycemic effect of CMR but not
fundamentally contributed to the development of diabetes although
reduction in STZ-treated rats.
TRPCs belong to a superfamily of
Ca2+-permeable receptor-operated channels that have been
reported to participate in axon regeneration of peripheral nerves
(36). TRPC1 is closely related to
neuron viability and growth cone sensitivity (37,38).
TRPC1 promoted neurite outgrowth in PC12 cells, while TRPC5 had a
suppressive effect on neurite outgrowth (39). Although TRPC1 expression was
reduced in patients with diabetes and diabetic db/db mice (40), TRPC1 played an important role in
the regulation of adiposity associated with type II diabetes, which
functioned as a Ca2+ entry channel and were activated
upon depletion of intracellular Ca2+ stores (41). Based on these previous studies, it
was speculated that the regulation of TRPC1 and Ca2+ may
participate in the neurite outgrowth in diabetic nephropathy.
Consistent with this hypothesis, CMR upregulated TRPC1 expression,
which was accompanied by reduced Ca2+ influx in the DRG
neurons of rats with DPN, and the anti-diabetic and neuroprotective
effects were reversed by the depletion of TRPC1.
The PI3K/AKT pathway is an important intracellular
signaling pathway in regulating cellular proliferation (42). PI3K activation leads to the
phosphorylation and activation of AKT, leading to downstream
reactions involved in nerve regeneration in diabetic rats (43). A previous study reported that
plasmacytoma variant translocation 1 regulated the occurrence and
progression of DPN by activating the PI3K/AKT pathway (44). Proanthocyanidin B2 attenuated the
high-glucose-induced neurotoxicity of DRG neurons through the
PI3K/AKT signaling pathway (45).
These previous studies suggested that there may be a link between
diabetic enteric neuropathy and PI3K/AKT signaling. In the present
study, CMR activated PI3K and increased the phosphorylation of AKT,
resulting in the neurite outgrowth of DRG neurons. Further studies
are required to determine if other signaling cascade responses
participate in the biological effect of CMR in DPN, such as the p38
mitogen-activated protein kinase signaling pathway, the ERK1/2
signaling pathway, the nuclear factor-κB signaling pathway or the
nuclear factor erthyroid-2 related factor/heme oxygenase-1
signaling pathway.
Limitations of the present study should also be
mentioned. Firstly, si-TRPC1 cannot completely mimic DPN conditions
in vivo. A diabetic model with a TRPC1 knockout would mimic
the in vivo scenario more closely and provide further
support to the conclusions reached in the present study.
Furthermore, the relationship between Ca2+ influx, the
increase in TRPC1 expression and the activation of PI3K/AKT
signaling remains unclear. Therefore, future studies should be
conducted using inhibitors of Ca2+ influx and the
PI3K/AKT pathway to further confirm the efficacy of CMR on neurite
outgrowth and neural regeneration in DRG neurons in diabetic
rats.
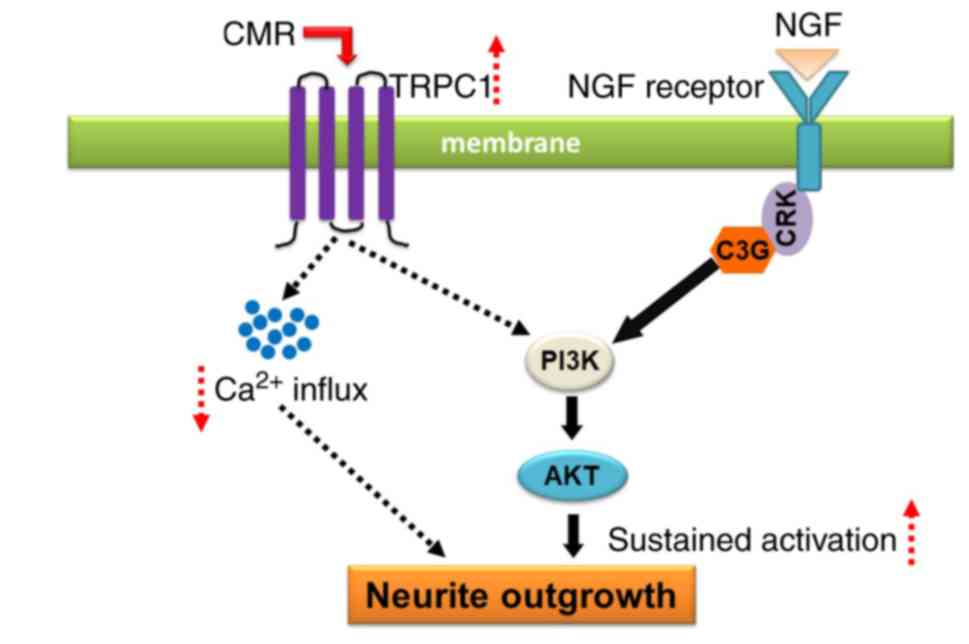
In conclusion, CMR treatment increased the
expression of TRPC1, resulting in the reduced Ca2+
influx. The upregulation of TRPC1 was associated with the
activation of PI3K/AKT signaling, which contributed, a least
partially, to the neurite outgrowth-prompting effects of CMR in
diabetic rats (Fig. 8). The
findings of the present study provided a theoretical basis and
indicated a potential use for CMR in the clinical treatment of
nerve injury linked to diabetic neuropathy.
Acknowledgements
The authors would like to thank Dr Zhigang Wang
(Department of Pathogen Biology, School of Basic Medical Sciences,
Hubei University of Chinese Medicine, Wuhan, China) for providing
advice on the experimental design and critical comments on the
present manuscript.
Funding
The present study was supported by the National
Natural Science Foundation for Young Scientists of China (grant no.
81600651) and the Natural Science Foundation of Hubei Province,
China (grant no. 2018CFB728).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and NY designed the study, performed the
experiments, analyzed the data and wrote the manuscript. TY and YX
contributed to feeding and handling of mice. QW helped with the
detection of calcium imaging.
Ethics approval and consent to
participate
The study was approved by the Animal Care and Use
Committee of Hubei University of Chinese Medicine (approval no.
SYXK2012-0068).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiang Y, Zhou Z, Deng C and Leslie RD:
Latent autoimmune diabetes in adults in Asians: Similarities and
differences between east and west. J Diabetes. 5:118–126. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Becker M, Benromano T, Shahar A, Nevo Z
and Pick CG: Changes in the basal membrane of dorsal root ganglia
schwann cells explain the biphasic pattern of the peripheral
neuropathy in streptozotocin-induced diabetic rats. J Mol Neurosci.
54:704–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Q, Pan J, Yu J, Liu X, Liu L, Zuo X, Wu
P, Deng H, Zhang J and Ji A: Meta-analysis of methylcobalamin alone
and in combination with lipoic acid in patients with diabetic
peripheral neuropathy. Diabetes Res Clin Pract. 101:99–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gordois A, Scuffham P, Shearer A, Oglesby
A and Tobian JA: The health care costs of diabetic peripheral
neuropathy in the US. Diabetes Care. 26:1790–1795. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
WEI ZX: Experiences in treating diabetic
peripheral neuropathy with traditional Chinese medicine. Chin J
Integr Med. 14:248–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi HJ, Kim NJ and Kim DH: Inhibitory
effects of crude drugs on alpha-glucosidase. Arch Pharm Res.
23:261–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen F, Nakashima N, Kimura I and Kimura
M: Hypoglycemic activity and mechanisms of extracts from mulberry
leaves (folium mori) and cortex mori radicis in
streptozotocin-induced diabetic mice. Yakugaku Zasshi. 115:476–482.
1995.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lian J, Chen J, Yuan Y, Chen J, Daud M,
Sayed M, Luo L, Zhu Y, Li S and Bu S: Cortex mori radicis extract
attenuates myocardial damages in diabetic rats by regulating ERS.
Biomed Pharmacother. 90:777–785. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin N, Hong X, Han Y, Duan Y, Zhang Y and
Chen Z: Cortex mori radicis extract induces neurite outgrowth in
PC12 cells activating ERK signaling pathway via inhibiting Ca(2+)
influx. Int J Clin Exp Med. 8:5022–5032. 2015.PubMed/NCBI
|
|
10
|
Hwang SH, Kang IJ and Lim SS: Antidiabetic
effect of fresh nopal (opuntia ficus-indica) in low-dose
streptozotocin-induced diabetic rats fed a high-fat diet. Evid
Based Complement Alternat Med. 2017:43807212017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee MS, Park WS, Kim YH, Kwon SH, Jang YJ,
Han D, Morita K and Her S: Antidepressant-like effects of Cortex
Mori Radicis extract via bidirectional phosphorylation of
glucocorticoid receptors in the hippocampus. Behav Brain Res.
236:56–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yue HY, Yin C, Hou JL, Zeng X, Chen YX,
Zhong W, Hu PF, Deng X, Tan YX, Zhang JP, et al: Hepatocyte nuclear
factor 4alpha attenuates hepatic fibrosis in rats. Gut. 59:236–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wesche-Soldato DE, Chung CS, Lomas-Neira
J, Doughty LA, Gregory SH and Ayala A: In vivo delivery of
caspase-8 or Fas siRNA improves the survival of septic mice. Blood.
106:2295–2301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taiana MM, Lombardi R, Porretta-Serapiglia
C, Ciusani E, Oggioni N, Sassone J, Bianchi R and Lauria G:
Neutralization of schwann cell-secreted VEGF is protective to in
vitro and in vivo experimental diabetic neuropathy. PLoS One.
9:e1084032014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao F, Xiang HC, Li HP, Jia M, Pan XL, Pan
HL and Li M: Electroacupuncture inhibits NLRP3 inflammasome
activation through CB2 receptors in inflammatory pain. Brain Behav
Immun. 67:91–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gasperini R, Choi-Lundberg D, Thompson MJ,
Mitchell CB and Foa L: Homer regulates calcium signaling in growth
cone turning. Neural Dev. 4:292009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coppey LJ, Shevalye H, Obrosov A, Davidson
EP and Yorek MA: Determination of peripheral neuropathy in high-fat
fed low-dose streptozotocin treated female C57BI/6J mice and
Sprague-Dawley rats. J Diabetes Investig. 9:1033–1040. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmidt RE, Parvin CA and Green KG:
Synaptic ultrastructural alterations anticipate the development of
neuroaxonal dystrophy in sympathetic ganglia of aged and diabetic
mice. J Neuropathol Exp Neurol. 67:1166–1186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang X, Yao W, Liu H, Gao Y, Liu R and Xu
L: Tangluoning, a traditional Chinese medicine, attenuates in vivo
and in vitro diabetic peripheral neuropathy through modulation of
PERK/Nrf2 pathway. Sci Rep. 7:10142017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang TH, Moon E, Hong BN, Choi SZ, Son M,
Park JH and Kim SY: Diosgenin from Dioscorea nipponica ameliorates
diabetic neuropathy by inducing nerve growth factor. Biol Pharm
Bull. 34:1493–1498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HJ, Lee HJ, Jeong SJ, Lee HJ, Kim SH
and Park EJ: Cortex mori radicis extract exerts antiasthmatic
effects via enhancement of CD4(+)CD25(+)Foxp3(+) regulatory T cells
and inhibition of Th2 cytokines in a mouse asthma model. J
Ethnopharmacol. 138:40–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
You S and Kim GH: Protective effect of
Mori Cortex radicis extract against high glucose-induced oxidative
stress in PC12 cells. Biosci Biotechnol Biochem. 83:1893–1900.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Chen Z, Ye R, He Y, Li Y and Qiu
X: Protective effect of jiaweibugan decoction against diabetic
peripheral neuropathy. Neural Regen Res. 8:1113–1121.
2013.PubMed/NCBI
|
|
25
|
Kim KJ, Namgung U and Cho CS: Protective
effects of bogijetong decoction and its selected formula on
neuropathic insults in streptozotocin-induced diabetic animals.
Evid Based Complement Alternat Med. 2017:42963182017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sarina, Yagi Y, Nakano O, Hashimoto T,
Kimura K, Asakawa Y, Zhong M, Narimatsu S and Gohda E: Induction of
neurite outgrowth in PC12 cells by artemisinin through activation
of ERK and p38 MAPK signaling pathways. Brain Res. 1490:61–71.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang D, Chan JD, Nogi T and Marchant JS:
Opposing roles of voltage-gated Ca2+ channels in neuronal control
of regenerative patterning. J Neurosci. 31:15983–15995. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balasubramanyam M, Balaji RA, Subashini B
and Mohan V: Evidence for mechanistic alterations of Ca2+
homeostasis in type 2 diabetes mellitus. Int J Exp Diabetes Res.
1:275–287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Verkhratsky A and Fernyhough P:
Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal
pathology in diabetes. Cell Calcium. 44:112–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao XH, Byun HS, Chen SR and Pan HL:
Diabetic neuropathy enhances voltage-activated Ca2+ channel
activity and its control by M4 muscarinic receptors in primary
sensory neurons. J Neurochem. 119:594–603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chandramoorthy HC, Bin-Jaliah I, Karari H,
Rajagopalan P, Ahmed Shariff ME, Al-Hakami A, Al-Humayad SM,
Baptain FA, Ahmed HS, Yassin HZ and Haidara MA: MSCs ameliorates
DPN induced cellular pathology via [Ca2+]i homeostasis and
scavenging the pro-inflammatory cytokines. J Cell Physiol.
233:1330–1341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Louhivuori LM: Calcium a key player in
early neural development and migration: TRPCs and VGCCs.
Argumentation. 1:201–207. 2015.
|
|
33
|
Li M, Chen C, Zhou Z, Xu S and Yu Z: A
TRPC1-mediated increase in store-operated Ca2+ entry is required
for the proliferation of adult hippocampal neural progenitor cells.
Cell Calcium. 51:486–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kruglikov I, Gryshchenko O, Shutov L,
Kostyuk E, Kostyuk P and Voitenko N: Diabetes-induced abnormalities
in ER calcium mobilization in primary and secondary nociceptive
neurons. Pflugers Arch. 448:395–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song E, Lee SK, Wang J, Ince N, Ouyang N,
Min J, Chen J, Shankar P and Lieberman J: RNA interference
targeting Fas protects mice from fulminant hepatitis. Nat Med.
9:347–351. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vigont V, Kolobkova Y, Skopin A, Zimina O,
Zenin V, Glushankova L and Kaznacheyeva E: Both Orai1 and TRPC1 are
involved in excessive store-operated calcium entry in striatal
neurons expressing mutant huntingtin Exon 1. Front Physiol.
6:3372015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen HC, Wang CH, Shih CP, Chueh SH, Liu
SF, Chen HK and Lin YC: TRPC1 is required for survival and
proliferation of cochlear spiral ganglion stem/progenitor cells.
Int J Pediatr Otorhinolaryngol. 79:2290–2294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shim S, Yuan JP, Kim JY, Zeng W, Huang G,
Milshteyn A, Kern D, Muallem S, Ming GL and Worley PF:
Peptidyl-prolyl isomerase FKBP52 controls chemotropic guidance of
neuronal growth cones via regulation of TRPC1 channel opening.
Neuron. 64:471–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heo DK, Chung WY, Park HW, Yuan JP, Min GL
and Kim JY: Opposite regulatory effects of TRPC1 and TRPC5 on
neurite outgrowth in PC12 cells. Cell Signal. 24:899–906. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang D, Freedman BI, Flekac M, Santos E,
Hicks PJ, Bowden DW, Efendic S, Brismar K and Gu HF: Evaluation of
genetic association and expression reduction of TRPC1 in the
development of diabetic nephropathy. Am J Nephrol. 29:244–251.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Krout D, Schaar A, Sun Y, Sukumaran P,
Roemmich JN, Singh BB and Claycombe-Larson KJ: The TRPC1
Ca2+-permeable channel inhibits exercise-induced
protection against high-fat diet-induced obesity and type II
diabetes. J Biol Chem. 292:20799–20807. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
17:3050–3060. 2016. View Article : Google Scholar
|
|
43
|
Li R, Li Y, Wu Y, Zhao Y, Chen H, Yuan Y,
Xu K, Zhang H, Lu Y, Wang J, et al: Heparin-poloxamer
thermosensitive hydrogel loaded with bFGF and NGF enhances
peripheral nerve regeneration in diabetic rats. Biomaterials.
168:24–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen L, Gong HY and Xu L: PVT1 protects
diabetic peripheral neuropathy via PI3K/AKT pathway. Eur Rev Med
Pharmacol Sci. 22:6905–6911. 2018.PubMed/NCBI
|
|
45
|
Zhang YP, Liu SY, Sun QY, Ren J, Liu HX
and Li H: Proanthocyanidin B2 attenuates high-glucose-induced
neurotoxicity of dorsal root ganglion neurons through the PI3K/Akt
signaling pathway. Neural Regen Res. 13:1628–1636. 2018. View Article : Google Scholar : PubMed/NCBI
|