Introduction
Abuse of opioids, particularly heroin, causes
substantial medical and economic harm. The United Nations Office on
Drugs and Crime indicated that, globally, opioids accounted for 66%
(110,000) of the 167,000 cases of drug-associated mortality in 2017
(1). Among users of opioids, there
were 29.2 million past-year users of opiates (heroin and opium),
accounting for 0.6% of the global population aged 15–64 in 2017. In
China, a national survey on drug use showed that there were 889,000
past-year users of heroin, accounting for 37% of the global number
of drug abuse in 2018 (2). Heroin
addiction is a relapsing brain disorder characterized by
continuous, compulsive drug-taking, uncontrollable drug-seeking
behavior and high recurrence rates (3–5).
Heroin addiction interferes with central nervous system plasticity
and adaptability by triggering the brain reward centers, which are
prompted to initiate and maintain the drug-taking habit (6). In the context of addictive disorders,
the mesocorticolimbic circuitry underlying drug addiction comprises
of the ventral tegmental area (VTA) and the regions of the brain
that are innervated by projections from the VTA, including the
nucleus accumbens (NAc), the prefrontal cortex (PFC), the
hippocampus and the prelimbic areas (7,8).
Acting as the main component of the brain reward circuit, the PFC
and NAc code for various aspects of reward-related behavior
(9,10). The PFC exerts a strong influence on
relapse to opiate seeking behavior during periods of drug
abstinence, especially the ventral and medial parts of the PFC
(11,12), with the medial PFC (mPFC)
contributing to drug-, stress- and cue-induced drug seeking
(13–16). The NAc serves an important role in
drug self-administration through mediating Pavlovian influences on
instrumental seeking behavior (17).
Neuronal activation leads to epigenetic changes in
gene expression, such as those modulated by histone modifications
(18). In particular, histone
acetylation serves a crucial role in drug addiction, memory
formation and transcriptional regulation by relaxing the chromatin
structure, thus allowing better access to transcriptional
activators (19,20). A previous study has reported that
histone H3 ac (H3ac) may serve an essential role in the long-term
neural and behavioral response to addictive drugs (21). For example, hyperacetylation of H3
lysine 27 (H3K27ac) increases chromatin accessibility at
hyperacetylated regions in a rat model of heroin
self-administration and in humans with heroin addiction behavior
(22). Furthermore, one study
revealed that methamphetamine injection decreases the level of
H3K9ac in nuclear extracts (23).
Another study reported that long-term administration of cocaine can
significantly upregulate H3ac in the NAc of rats (24). Additionally, class IIa histone
deacetylases (HDACs), such as HDAC5 in the NAc, were previously
demonstrated to reduce the cocaine reward behavior (25,26).
Our previous study has revealed that the HDAC inhibitor sodium
butyrate increases the reinstatement of heroin seeking (27). Another HDAC inhibitor, valproic
acid is essential for the reduction of behavioral and
reinforcement-related effects of alcohol (28).
Brahma/SWI2-related gene-1 (BRG1) encodes the
ATPase subunit of the switch/sucrose non-fermentable (SWI/SNF)
chromatin remodeling complex (CRC), which contributes to chromatin
remodeling and transcriptional regulation of target genes by using
energy from ATP hydrolysis (29).
Emerging evidence suggests that BRG1 is required to regulate
transcription of neuronal genes concurrent with histone
modifications. Rodent models of drug self-administration provide a
way to simulate the process of heroin addiction in humans and to
systematically evaluate brain plasticity and adaptability following
heroin self-administration.
The present study aimed to elucidate the possible
relationship between heroin self-administration and H3K9ac
associated with BRG1 in the specific brain subregions
involved in the reward circuitry, thus gaining insight into the
potential function of the epigenetic regulation of addictive
behaviors.
Materials and methods
Heroin preparation
Heroin was provided by the National Institute of
Forensic Science and dissolved in physiological saline at a final
concentration of 0.2 mg/ml, as previously described (30). Heroin was administered at a dose of
50 µg/kg per infusion based on a previous report (31).
Animals
Male Sprague-Dawley (SD) rats (weight, 250–300 g;
age, 7–8 weeks) were provided by the Experimental Animal Center of
Zhejiang Province. A total of 20 SD rats were housed in cages
controlled for temperature (22-25°C) and humidity (40–60%) under a
standard 12-h light/dark cycle (lights on between 7:00 p.m. and
7:00 a.m.) with free access to water and food. Rats were randomly
divided into two groups (n=10 rats/group): i) Heroin addiction
group, which received self-administered heroin for 14 days, and ii)
Control group, which received saline injections for 14 days.
Self-administration apparatus
Plexiglas custom-made operant boxes (working area
29×29×29 cm) were enclosed inside a sound-attenuating plywood
chamber. Two nose-poke response devices were located 5 cm from the
floor in each operant box, an active nose-poke hole and an inactive
one. A yellow LED cue light was placed inside each nose-poke hole,
and a white LED (10 cm in diameter) was placed on the wall above
the holes. The drug solution was delivered at a constant flow rate
(1.1 ml/min) through Tygon tubing, which was attached to an
infusion pump (PHM-100; Med Associates, Inc.). The tubing was
protected by a leash spring and hung from the ceiling with a
plastic fluid swivel (PHM-115; Med Associates, Inc.). The leash
spring was fitted to a connector on the animal's jacket. The
experimental procedure was controlled by an IBM-compatible PC,
using input/output interfaces and computer software (Super State
Version 1.0; Ningbo Institute of Microcirculation and Henbane)
(32).
Surgery
Chronically indwelling intravenous catheters were
implanted in the rats after sodium pentobarbital anesthesia (50
mg/kg, intramuscular; Sigma-Aldrich; Merck KGaA). A silicon
catheter (length, 3.5 cm; inner diameter, 0.5 mm; outer diameter,
0.94 mm) was placed into the right external jugular vein, passing
through the back of the body to the right atrium.
Catheters were irrigated daily with 0.2 ml of saline
containing sterile benzylpenicillin sodium (60,000 units) and
heparin (5 units) in a syringe to prevent bacterial infection and
blockage. All rats were allowed to recover for at least 1 week
after surgery. All animal procedures were conducted in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals.
Heroin self-administration
Four-hour training sessions of heroin
self-administration were performed daily for 14 consecutive days
under a fixed-ratio-1 paradigm after rats had recovered from
surgery. Rats were placed in the operant chambers, and their
connectors were attached to the infusion lines. The trial began
with illumination of the yellow light. Each response in the active
hole was immediately reinforced with an infusion of heroin.
Responding in the inactive nose-poke resulted in no heroin
delivery. Rats received a single heroin infusion at a dose of 50
µg/kg, paired with 2 sec illumination of the white light and in
combination with the noise of the infusion pump, followed by a
20-sec refractory period with no injection. During the training
cycle, there were no heroin infusions on the right side of the
operant box (inactive nose-poke). After each refractory period, the
yellow light was turned on again, and another trial began. Rats
were put back into their individual home cages after the training
sessions ended. Rats in the control group received the same
treatment schedule as above but with an injection of saline instead
of heroin.
At 24 h after the last behavioral session, all rats
were anesthetized with sodium pentobarbital (50 mg/kg,
intramuscular) and rapidly decapitated. Brain tissues were
dissected from the bregma [2.2 to 0.8 mm according to coordinates
from a rat brain atlas of Paxinos and Watson (33)] using a rat brain matrix, and were
sectioned (1.4±0.02 mm). The NAc and mPFC were harvested using a
biopsy punch (2 mm in diameter).
Chromatin immunoprecipitation (ChIP)
assay
Brain tissues were minced into small pieces and
homogenized in 1 ml 10X PBS. Next, they were centrifuged at 800 × g
(4°C, 5 min) and resuspended in 1 ml cold PBS according to the
manufacturer's instructions (Magna ChIP™ A kit; Merck KGaA), and
crosslinked with 0.1% formaldehyde for 8 min at room temperature.
Crosslinking was stopped with 100 µl glycine (10X), then
precipitates were collected by centrifugation (800 × g, 4°C, 5
min). Precipitates were washed with 1 ml of cold PBS [containing 5
µl Protease Inhibitor Cocktail II (Magna ChIP™ A kit; Merck KGaA)],
resuspended with 500 µl cell lysis buffer, centrifuged at 800 × g
(4°C, 5 min) and resuspended with 300 µl nuclear lysis buffer
containing Protease Inhibitor Cocktail II (Magna ChIP™ A kit; Merck
KGaA).
Nuclear lysates were sonicated using a Bioruptor
UCD-200 (Diagenode SA) with optimal conditions determined to shear
crosslinked DNA to 100–500 bp. Briefly, samples were sonicated at
60 KHz for 30 cycles for 30 sec on and 30 sec off at 4°C and
briefly spun down (800 × g, 4°C, 10 sec) for every 5 cycles. To
evaluate the quality of sonicated chromatin, a 20 µl aliquot was
removed, reverse-crosslinked and purified with the Magna ChIP A kit
for agarose gel electrophoresis (2% agarose gels were prepared,
ethidium bromide was added to the gel matrix to enable fluorescent
visualization of the DNA bands, and electrophoresis was performed
at 150 mA for 30 min at room temperature).
Chromatin extracts were diluted 10-fold with ChIP
buffer H from the Auto Histone ChIP-seq kit (Diagenode, SA) to a
final volume of 200 µl. A 2 µl aliquot of pre-immunoprecipitated
lysates (1%) was saved as ‘input’ for normalization.
The Diagenode SX-8G IP-Star® Automated
System (Diagenode SA) was used for immunoprecipitation.
Immunoprecipitations were performed with 1.5 µl (2 µg) rabbit
anti-H3K9ac (cat. no. C15410004; Diagenode SA) and 2 µl (2 µg)
normal rabbit immunoglobulin G (IgG; cat. no. C15410206; Diagenode
SA), which was used as a negative IP control. Then 10 µl of protein
A coated with magnetic beads were incubated at 4°C with the
anti-H3K9ac or the IgG for 2 h before immunoprecipitation of
chromatin extracts for 13 h, and washing for 5 min at 4°C. The
immunoprecipitated material and the input were de-crosslinked at
65°C for 4 h under high-salt conditions (4 µl; 0.585 g/ml NaCl).
Immunoprecipitated DNA was extracted with Auto IPure kit
(Diagenode, SA) and resuspended in 25 µl Buffer C.
Quantitative PCR (qPCR)
Two different regions of the BRG1 gene,
namely the promoter (BRG1+) and the last exon
(BRG1−), were amplified with primers shown in
Table I. Primers were selected
with Primer Premier 5.0 software (Premier Biosoft International;
Fig. 1). All oligomers were
synthesized by Sangon Biotech Co., Ltd.
 | Table I.Primers for ChIP and RT-qPCR. |
Table I.
Primers for ChIP and RT-qPCR.
| Reaction | Gene | Primer sequence
(5′→3′) | Length (bp) | Tm (°C) |
|---|
| ChIP |
BRG1+ | F:
ACAGAGCCTTGCAGAGCA | 18 | 60 |
|
|
| R:
GAGGAAAGTGAAGCCGAGA | 19 |
|
|
|
BRG1− | F:
CTTAGCAGTGATGGGTAGCA | 20 | 60 |
|
|
| R:
CAGACCAGGGCCAACAGA | 18 |
|
| RT-qPCR | BRG1 | F:
CCTGATGACCCACGATACAACC | 22 | 60 |
|
|
| R:
GGAGGCATGTTCAGAGCCACC | 21 |
|
|
| GAPDH | F:
ACCCAGAAGACTGTGGATGG | 20 | 60 |
|
|
| R:
TTCTAGACGGCAGGTCAGGT | 20 |
|
The level of specific histone modification at
BRG1 was determined by qPCR with LightCycler® 480
(Roche Applied Science). qPCR was performed with 5 µl
immunoprecipitated or input DNA, 1 µl primer pairs (10 µM each),
12.5 µl SYBR Green Master mix (Roche Corporation) and 6.5 µl
DNase/RNase-free water in a final volume of 25 µl. Amplification
reactions were run in triplicate for every sample; the
thermocycling conditions were: Pre-incubation at 95°C for 5 min,
followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec, 72°C for
30 sec for primer extension, and then a melting curve period (90°C
for 10 sec, 60°C for 30 sec and 90°C for 10 sec), followed by final
cooling step of 40°C for 5 min. The differential quantitation cycle
(ΔCq) value (34) was used to
quantify the relative amount of the target regions, and melting
curves were generated to evaluate the specificity of the
amplification products.
The efficiency of ChIP and DNA recovery of a
particular genomic locus was normalized to the input DNA calculated
from qPCR data and reported as a percentage of starting material: %
(ChIP/total input)=2[(Cq(1% input)-log(1%) log2)-Cq
(ChIP)] ×100. Fold enrichment over IgG was calculated by the
recovery of immunoprecipitated genomic loci.
Reverse transcription-qPCR
(RT-qPCR)
Total RNA was extracted from 15–20 mg of brain
tissues by using TRIzol® Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and trichloromethane (Jiangsu Tongsheng
Chemical Reagent Co., Ltd.). RT was performed with the miScript II
RT kit (Qiagen GmbH), and cDNA was synthesized from 1 µg total RNA.
The nucleotide sequences of the qPCR primers are shown in Table I. GAPDH was used for
normalization. All reactions were performed in a
LightCycler® 480 (Roche Applied Science). The reaction
mixture contained 5 µl SYBR Green premix, 2 µl primer pairs (10 µM
each), 1.5 µl cDNA, and 1.5 µl water. The thermocycling conditions
were: 95°C for 10 min, 45 cycles of 95°C for 10 sec, 60°C for 10
sec and 72°C for 10 sec. The 2−ΔΔCq method was used to
analyze the RT-qPCR data and to report the relative change in
BRG1 mRNA expression (33).
Statistical analysis
One-way ANOVA for repeated measures was used to
compare the number of infusions or active and inactive nose-poke
responses in the two groups during the training sessions. Student's
t-test was used to evaluate the statistical differences in the mPFC
and NAc between the heroin self-administration and control group.
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc.) and
GraphPad Prism 5.0 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Establishment of a rat model of heroin
self-administration
The number of nose-pokes (active or inactive
responses) in rats are presented as mean ± SEM during 14
consecutive days of training (Fig.
2A). The nose pokes in the active holes and the number of
infusions reached a stable level within 14 days of
self-administration training (Fig. 2A
and B). Notably, the number of inactive pokes remained stable
at baseline during the training program. The criterion of stable
heroin self-administration was defined as <10% variation in the
number of active pokes that rats touched on the last 3 days
(27). A total of 18 rats were
included in the final analysis (n=9 rats/group); the remaining 2
rats were excluded from further analysis as they did not meet the
criteria for stable model.
Quality control of ChIP assay
To evaluate chromatin quality after sonication, the
length of sheared DNA was analyzed by agarose gel electrophoresis.
The results showed that sheared DNA fragments were 100–500 bp long
(Fig. 3A). H3K9ac of BRG1
was evaluated by qPCR. Amplification curves for one qPCR product
are presented in Fig. 3B. The
curves demonstrated good reproducibility. Moreover, the qPCR
results of the H3K9ac antibody-immunoprecipitated DNA over rabbit
IgG (negative control) showed that the IgG antibody was not
amplified by qPCR, which indicated that the experimental system was
reliable (data not shown). To avoid errors introduced by
differences in the amount loaded, BRG1− was
considered as the internal negative reference for qPCR; the results
demonstrated that 0.08–0.16% of the BRG1− with
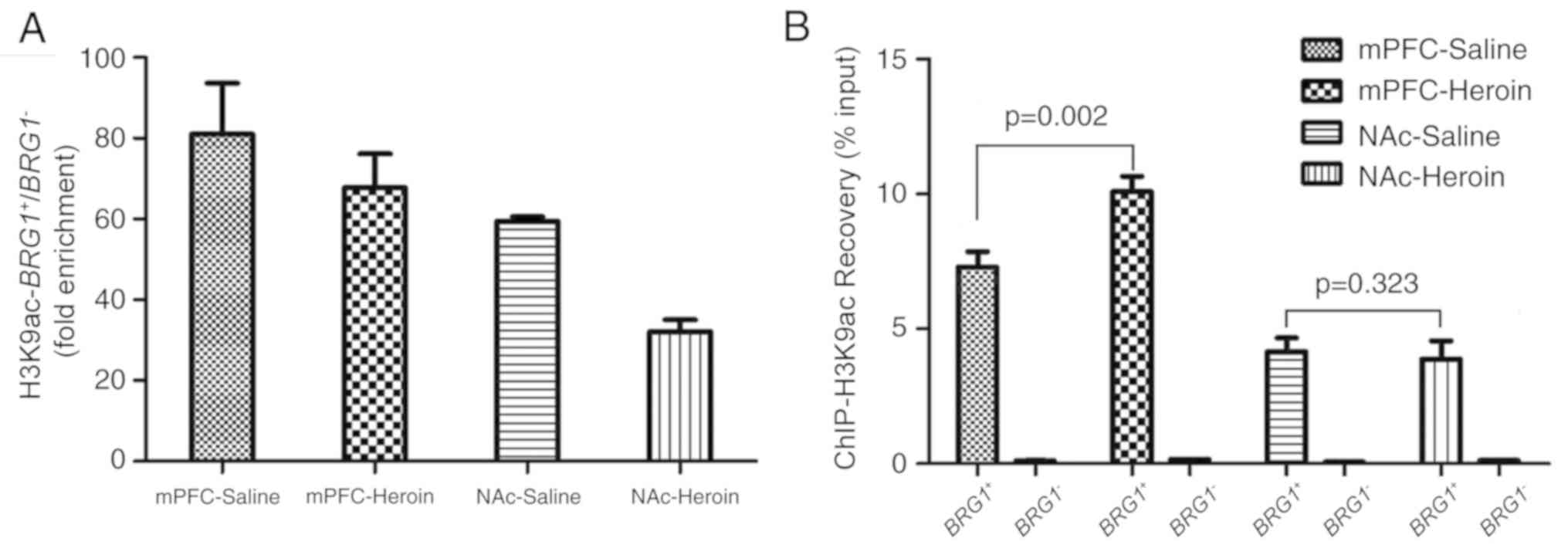
H3K9ac was recovered in the mPFC and NAc (Fig. 4B). Highly sensitive and specific
ChIP enabled >10-fold enrichment; this value depended not only
on the antibody but also on the target. In the present, study the
fold enrichment over H3K9ac was calculated by the recovery of the
target BRG1+/BRG1−. It was
revealed the fold enrichment ≥30-fold, which indicated that the
signal-to-noise ratio was acceptable (Fig. 4A). Therefore, the sensitivity and
specificity of the H3K9ac antibody was satisfactory.
H3K9ac recovery rate
Recovery is a measure of the association of an
antibody with a specific locus. As the target and negative regions,
the promoter region and the final exon region of BRG1 were
tested. Results showed the quantity of BRG1+
combined with H3K9ac differed among the four groups (Fig. 4B). H3K9ac increased in response to
heroin self-administration in the mPFC; H3K9ac enrichment was
significantly higher in the heroin self-administration group
compared with the control group (recovery % input, 7.79±0.81 vs.
10.09±0.81, respectively; P=0.002; Fig. 4B). However, no significant
differences were identified between the two groups in the NAc
(recovery % input, 4.15±0.74 vs. 3.88±0.95, respectively; Fig. 4B; P=0.323). Additionally, there
were significant differences within treatment groups between the
mPFC and the NAc (P<0.05).
Expression of BRG1
BRG1 mRNA expression was elevated after
heroin self-administration, corresponding to 1.47-fold of the
saline control (P<0.05) in the mPFC. Whereas BRG1 mRNA
expression was reduced in the NAc, corresponding to 0.72-fold of
the saline control (P>0.05; Fig.
5). The relative change in the mRNA expression of BRG1
following heroin self-administration between the mPFC and NAc was
2.04-fold (data not shown).
Discussion
Heroin addiction is a debilitating psychiatric
disorder characterized by compulsive heroin craving behavior and
relapse, despite prolonged periods of abstinence (35), posing a significant threat to human
health. Heroin addicts often exhibit long-lasting and complicated
cognitive impairment and neural deficits (36). Neurobiological adaptations or
drug-induced synaptic neuroplasticity, such as epigenetic
alterations in the brain reward circuitry, are thought to
contribute to this chronic disease (37).
H3K9ac is a chromatin mark that is increased
significantly following nerve injury and is closely related to gene
transcription (38,39). Previous studies have shown that
BRG1 expression is enriched in neurons (40), and BRG1 contributes to
transcription factor-DNA interactions (39). For instance, BRG1 forms a complex
with SLIT-ROBO Rho GTPase Activating Protein 3, which is related to
GTPase activator activity, subsequently disrupting the BRG1-related
SWI/SNF CRC and affecting transcription of neuron-specific target
genes in cortical neurons or Neuro2a cells, which are essential
during neural development (41).
Furthermore, BRG1 combined with calcium-responsive transactivator
regulates promoter activation downstream of calcium-dependent
signaling in resting neurons (42)
and our previous study demonstrated that H4K5ac of BRG1 in
the VTA may be related to heroin administration, but not addiction
(43).
The present study assessed a possible relationship
between H3K9ac and the BRG1 locus after heroin
self-administration. The data demonstrated that in contrast to a
saline control, there was a significant increase in the level of
H3K9 acetylation at the BRG1 promoter in the mPFC after
heroin self-administration, and an increased expression of
BRG1 mRNA. However, heroin self-administration did not
induce any significant alteration in H3K9ac at the BRG1
locus in the NAc and the mRNA expression of BRG1 was
decreased.
These results suggested an important and novel role
for BRG1 in heroin-mediated neural adaptation. This is
consistent with the recent publication from Martin et al
(44), which demonstrated that
BRG1 expression in the PFC increased after heroin
self-administration, but remained unchanged in the NAc. In
addition, genes regulated by BRG1, such as the sex determining
region Y-box 10 (Sox10), which is crucial for neural crest
and peripheral nervous system development (45,46),
have been seen to change during heroin self-administration in the
PFC. Upon further investigation, it was demonstrated that the
overexpression of Sox10 or BRG1 decreased the
motivation to obtain heroin infusions (44). These results demonstrated the
crucial role of BRG1 in regulating motivation for heroin. A
study on cocaine demonstrated that BRG1 expression increased
in the NAc, and BRG1 can be incorporated into the transcriptional
complex with SMAD family members, which are signal transducers that
mediate multiple signaling pathways and modulate gene expression
and behavioral changes following cocaine exposure (47). Interestingly, the overexpression of
BRG1 can exacerbate the cocaine-reinstatement behavior. In
conclusion, BRG1 contributes to addiction phenotypes through
association with other genes, modulators and epigenetic mechanisms
that regulate histone lysine acetylation. The current study
provided evidence that the direct relationship of
BRG1-H3K9ac might be implicated in heroin
self-administration. Notably, the relationship between H3K9ac and
BRG1 has been previously reported in human cancer (48). A previous study showed that
BRG1 remodels chromatin to facilitate the action of HDAC2 by
binding to the transcription start site of the telomerase reverse
transcriptase (hTERT) promoter, which catalyzes the de
novo synthesis of telomeric repeats using a telomere-specific
RNA template, leading to H3K9 deacetylation and suppression of
hTERT transcription. Thus, BRG1 protein regulates H3K9ac
through HDAC and possibly by regulating hTERT expression.
Overall, these data suggested that the BRG1 gene is
regulated by H3K9ac at its promoter and increased H3K9ac might
correlate with increased BRG1 expression, which in turn may
downregulate neuron-addictive behavior through promoting the
accessibility of HDACs.
In the present study, there were significant
differences in H3K9ac at the BRG1 promoter between the
heroin self-administration group and the control group in the mPFC,
but not in the NAc, which suggested region-specific regulation. The
NAc, which serves as a critical node within the brain's reward
circuitry, directs attention and behavior toward multiple stimuli,
including drug abuse and natural rewards such as food and sex, in
addition to other secondary reinforcers (49). In addition, it integrates mnemonic
and emotional signals from cortical and limbic structures to
mediate goal-directed behaviors (49,50).
The PFC has long been suspected to be involved in cognitive
control; the ability to orchestrate thought and action in
accordance with internal states or intentions (51), and other aspects of the addiction
phenotype (52). Activity in the
dorsal mPFC is thought to initiate drug-seeking behavior and is
crucial for cue-induced nicotine or morphine relapse (15,53),
whilst lesions in the mPFC may lead to insensitivity to changes in
compulsive behavior (17) and
marked inability to make choices (52). Results from the present study
demonstrated the specificity of the H3K9ac-BRG1 association
in response to heroin self-administration for the mPFC, which may
inform further research in specific brain regions. Nevertheless,
this study has several limitations. Firstly, the influence of
heroin on H3K9ac was only examined in the mPFC and NAc; other
tissues related with addiction, including the VTA and hippocampus,
should be studied in the future. Secondly, the mechanisms by which
BRG1 affects heroin self-administration through association with
H3K9ac remain largely elusive. Further investigations are required
to reveal how the increased expression of BRG1 and its association
with H3K9ac affects heroin self-administration behavior.
In conclusion, the present study demonstrated an
elevated acetylation of H3K9 in the BRG1 promoter in the
mPFC after heroin self-administration, indicating a potential novel
role for BRG1 and its relationship with H3K9ac in a
heroin-mediated addicted phenotype and neuroadaptations.
Specifically, BRG1 may be an essential component of transcriptional
complexes mediating gene expression after heroin
self-administration. This may provide a novel biomarker for
detecting and monitoring heroin addiction, or even a novel
therapeutic target for addiction treatment.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Key R&D
Program of China (grant no. 2017YFC1310400), The Natural Science
Foundation of Zhejiang (grant no. LY18H090008) and China (grant no.
81671321), The National Basic Research Program of China (grant no.
2015CB553504), The Ningbo Natural Science Foundation (grant nos.
2017A610217 and 2016A610187), and The Ningbo City Medical Science
and Technology Project (grant no. 2017A04).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ and HL designed the study. QH and JL contributed
equally, reviewed the publication and drafted this manuscript. QH,
JL and ZL contributed to data interpretation and revised the
manuscript critically. DZ, WX and ZX performed laboratory work. ML
and HZ participated in data acquisition and data analysis. All
authors contributed to finalize the manuscript and approved the
final version of the manuscript. All authors agree to be
accountable for all aspects of the research.
Ethics approval and consent to
participate
All procedures involving animals were approved by
the Laboratory of Behavioral Neuroscience, Animal Care and Use
Committee in Ningbo Institute of Microcirculation and Henbane
(Ningbo, China) and conformed to guidelines for the Care and Use of
Laboratory Animals of the Ministry of Health, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global overview of drug demand and supply.
UNODC 2019. Retrieved from, . https://wdr.unodc.org/wdr2019/prelaunch/WDR19_Booklet_2_DRUG_DEMAND.pdfSeptember
26–2019
|
|
2
|
Report on China's drug situation in 2018,
. http://www.nncc626.com/2019-06/17/c_1210161797.htmJune
17–2019
|
|
3
|
Clark L, Robbins TW, Ersche KD and
Sahakian BJ: Reflection impulsivity in current and former substance
users. Biol Psychiatry. 60:515–522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giuliano C, Robbins TW, Wille DR, Bullmore
ET and Everitt BJ: Attenuation of cocaine and heroin seeking by
µ-opioid receptor antagonism. Psychopharmacology (Berl).
227:137–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schippers MC, Binnekade R, Schoffelmeer
AN, Pattij T and De Vries TJ: Unidirectional relationship between
heroin self-administration and impulsive decision-making in rats.
Psychopharmacology (Berl). 219:443–452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Souza MS: Glutamatergic transmission in
drug reward: Implications for drug addiction. Front Neurosci.
9:4042015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang YY, Kandel DB, Kandel ER and Levine
A: Nicotine primes the effect of cocaine on the induction of LTP in
the amygdala. Neuropharmacology. 74:126–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Huijstee AN and Mansvelder HD:
Glutamatergic synaptic plasticity in the mesocorticolimbic system
in addiction. Front Cell Neurosci. 8:4662015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cooper DC: The significance of action
potential bursting in the brain reward circuit. Neurochem Int.
41:333–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scobie KN, Damez-Werno D, Sun H, Shao N,
Gancarz A, Panganiban CH, Dias C, Koo J, Caiafa P, Kaufman L, et
al: Essential role of poly(ADP-ribosyl)ation in cocaine action.
Proc Natl Acad Sci USA. 111:2005–2010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rogers JL, Ghee S and See RE: The neural
circuitry underlying reinstatement of heroin-seeking behavior in an
animal model of relapse. Neuroscience. 151:579–588. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmidt ED, Voorn P, Binnekade R,
Schoffelmeer AN and De Vries TJ: Differential involvement of the
prelimbic cortex and striatum in conditioned heroin and sucrose
seeking following long-term extinction. Eur J Neurosci.
22:2347–2356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Archer T, Beninger RJ, Palomo T and
Kostrzewa RM: Epigenetics and biomarkers in the staging of
neuropsychiatric disorders. Neurotox Res. 18:347–366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caffino L, Cassina C, Giannotti G, Orru A,
Moro F, Di Clemente A, Racagni G, Fumagalli F and Cervo L:
Short-term abstinence from cocaine self-administration, but not
passive cocaine infusion, elevates αCaMKII autophosphorylation in
the rat nucleus accumbens and medial prefrontal cortex. Int J
Neuropsychopharmacol. 17:323–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gipson CD, Reissner KJ, Kupchik YM, Smith
AC, Stankeviciute N, Hensley-Simon ME and Kalivas PW: Reinstatement
of nicotine seeking is mediated by glutamatergic plasticity. Proc
Natl Acad Sci USA. 110:9124–9129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van den Oever MC, Spijker S, Smit AB and
De Vries TJ: Prefrontal cortex plasticity mechanisms in drug
seeking and relapse. Neurosci Biobehav Rev. 35:276–284. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Liu WJ, Lu B, Wang YH and Liu JG:
Differential expression of Arc in the mesocorticolimbic system is
involved in drug and natural rewarding behavior in rats. Acta
Pharmacol Sin. 34:1013–1024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su Y, Shin J, Zhong C, Wang S,
Roychowdhury P, Lim J, Kim D, Ming GL and Song H: Neuronal activity
modifies the chromatin accessibility landscape in the adult brain.
Nat Neurosci. 20:476–483. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peixoto L and Abel T: The role of histone
acetylation in memory formation and cognitive impairments.
Neuropsychopharmacology. 38:62–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Appelbaum PC: Reduced glycopeptide
susceptibility in methicillin-resistant Staphylococcus aureus
(MRSA). Int J Antimicrob Agents. 30:398–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Lai J, Cui H, Zhu Y, Zhao B, Wang
W and Wei S: Inhibition of histone deacetylase in the basolateral
amygdala facilitates morphine context-associated memory formation
in rats. J Mol Neurosci. 55:269–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Egervari G, Landry J, Callens J, Fullard
JF, Roussos P, Keller E and Hurd YL: Striatal H3K27 acetylation
linked to glutamatergic gene dysregulation in human heroin abusers
holds promise as therapeutic target. Biol Psychiatry. 81:585–594.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin TA, Jayanthi S, McCoy MT, Brannock
C, Ladenheim B, Garrett T, Lehrmann E, Becker KG and Cadet JL:
Methamphetamine causes differential alterations in gene expression
and patterns of histone acetylation/hypoacetylation in the rat
nucleus accumbens. PLoS One. 7:e342362012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Biliński P, Wojtyła A, Kapka-Skrzypczak L,
Chwedorowicz R, Cyranka M and Studziński T: Epigenetic regulation
in drug addiction. Ann Agric Environ Med. 19:491–496.
2012.PubMed/NCBI
|
|
25
|
Taniguchi M, Carreira MB, Cooper YA,
Bobadilla AC, Heinsbroek JA, Koike N, Larson EB, Balmuth EA, Hughes
BW, Penrod RD, et al: HDAC5 and its target gene, Npas4, function in
the nucleus accumbens to regulate cocaine-conditioned behaviors.
Neuron. 96:130–144.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taniguchi M, Carreira MB, Smith LN, Zirlin
BC, Neve RL and Cowan CW: Histone deacetylase 5 limits cocaine
reward through cAMP-induced nuclear import. Neuron. 73:108–120.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen WS, Xu WJ, Zhu HQ, Gao L, Lai MJ,
Zhang FQ, Zhou WH and Liu HF: Effects of histone deacetylase
inhibitor sodium butyrate on heroin seeking behavior in the nucleus
accumbens in rats. Brain Res. 1652:151–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al Ameri M, Al Mansouri S, Al Maamari A
and Bahi A: The histone deacetylase (HDAC) inhibitor valproic acid
reduces ethanol consumption and ethanol-conditioned place
preference in rats. Brain Res. 1583:122–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Sansam CG, Thom CS, Metzger D,
Evans JA, Nguyen PT and Roberts CW: Oncogenesis caused by loss of
the SNF5 tumor suppressor is dependent on activity of BRG1, the
ATPase of the SWI/SNF chromatin remodeling complex. Cancer Res.
69:8094–8101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Zhang F, Tang S, Lai M, Hao W,
Zhang Y, Yang J and Zhou W: Lack of effect of habenula lesion on
heroin self-administration in rats. Neurosci Lett. 461:167–171.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang F, Zhou W, Tang S, Lai M, Liu H and
Yang G: Motivation of heroin-seeking elicited by drug-associated
cues is related to total amount of heroin exposure during
self-administration in rats. Pharmacol Biochem Behav. 79:291–298.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang F: SuperState: A computer program
for the control of operant behavioral experimentation. J Neurosci
Methods. 155:194–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. 4th. Academic Press; San Diego: 1998,
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang HC, Chu SK, Huang CL, Kuo HW, Wang
SC, Liu SW, Ho IK and Liu YL: Genome-wide pharmacogenomic study on
methadone maintenance treatment identifies SNP rs17180299 and
multiple haplotypes on CYP2B6, SPON1, and GSG1L associated with
plasma concentrations of methadone R- and S-enantiomers in
heroin-dependent patients. PLoS Genet. 12:e10059102016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu Y, Wang Y, Lai J, Wei S, Zhang H, Yan
P, Li Y, Qiao X and Yin F: Dopamine D1 and D3 receptors modulate
heroin-induced cognitive impairment through opponent actions in
mice. Int J Neuropsychopharmacol. 20:257–268. 2017.PubMed/NCBI
|
|
37
|
Volkow ND and Morales M: The brain on
drugs: From reward to addiction. Cell. 162:712–725. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Laumet G, Chen SR, Hittelman WN
and Pan HL: Pannexin-1 Up-regulation in the dorsal root ganglion
contributes to neuropathic pain development. J Biol Chem.
290:14647–14655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu M, Zhang Y, Wu NH and Shen YF: Histone
marks and chromatin remodelers on the regulation of neurogenin1
gene in RA induced neuronal differentiation of P19 cells. J Cell
Biochem. 107:264–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seo S, Richardson GA and Kroll KL: The
SWI/SNF chromatin remodeling protein Brg1 is required for
vertebrate neurogenesis and mediates transactivation of Ngn and
NeuroD. Development. 132:105–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dai YK, Ma Y, Chen K, Mi YJ, Fu HL, Cui DX
and Jin WL: A link between the nuclear-localized srGAP3 and the
SWI/SNF chromatin remodeler Brg1. Mol Cell Neurosci. 60:10–25.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qiu Z and Ghosh A: A calcium-dependent
switch in a CREST-BRG1 complex regulates activity-dependent gene
expression. Neuron. 60:775–787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu L, Hong Q, Chen X, Xu X, Liu H, Zhou W
and Duan S: H4K5 histone acetylation of BRG1 is associated with
heroin administration rather than addiction. Exp Ther Med.
12:1929–1933. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martin JA, Caccamise A, Werner CT,
Viswanathan R, Polanco JJ, Stewart AF, Thomas SA, Sim FJ and Dietz
DM: A novel role for oligodendrocyte precursor cells (OPCs) and
Sox10 in mediating cellular and behavioral responses to heroin.
Neuropsychopharmacology. 43:1385–1394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bondurand N, Kuhlbrodt K, Pingault V,
Enderich J, Sajus M, Tommerup N, Warburg M, Hennekam RC, Read AP,
Wegner M and Goossens M: A molecular analysis of the yemenite
deaf-blind hypopigmentation syndrome: SOX10 dysfunction causes
different neurocristopathies. Hum Mol Genet. 8:1785–1789. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maeno N, Takahashi N, Saito S, Ji X,
Ishihara R, Aoyama N, Branko A, Miura H, Ikeda M, Suzuki T, et al:
Association of SOX10 with schizophrenia in the Japanese population.
Psychiatr Genet. 17:227–231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang ZJ, Martin JA, Mueller LE, Caccamise
A, Werner CT, Neve RL, Gancarz AM, Li JX and Dietz DM: BRG1 in the
nucleus accumbens regulates cocaine-seeking behavior. Biol
Psychiatry. 80:652–660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu S, Ge Y, Huang L, Liu H, Xue Y and Zhao
Y: BRG1, the ATPase subunit of SWI/SNF chromatin remodeling
complex, interacts with HDAC2 to modulate telomerase expression in
human cancer cells. Cell Cycle. 13:2869–2878. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Floresco SB: The nucleus accumbens: An
interface between cognition, emotion, and action. Annu Rev Psychol.
66:25–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Scofield MD, Heinsbroek JA, Gipson CD,
Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D and Kalivas PW:
The nucleus accumbens: Mechanisms of addiction across drug classes
reflect the importance of glutamate homeostasis. Pharmacol Rev.
68:816–871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Koechlin E, Ody C and Kouneiher F: The
architecture of cognitive control in the human prefrontal cortex.
Science. 302:1181–1185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kennerley SW and Walton ME: Decision
making and reward in frontal cortex: Complementary evidence from
neurophysiological and neuropsychological studies. Behav Neurosci.
125:297–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhao Y, Zhang J, Yang H, Cui D, Song J, Ma
Q, Luan W, Lai B, Ma L, Chen M and Zheng P: Memory retrieval in
addiction: A role for miR-105-mediated regulation of D1 receptors
in mPFC neurons projecting to the basolateral amygdala. BMC Biol.
15:1282017. View Article : Google Scholar : PubMed/NCBI
|