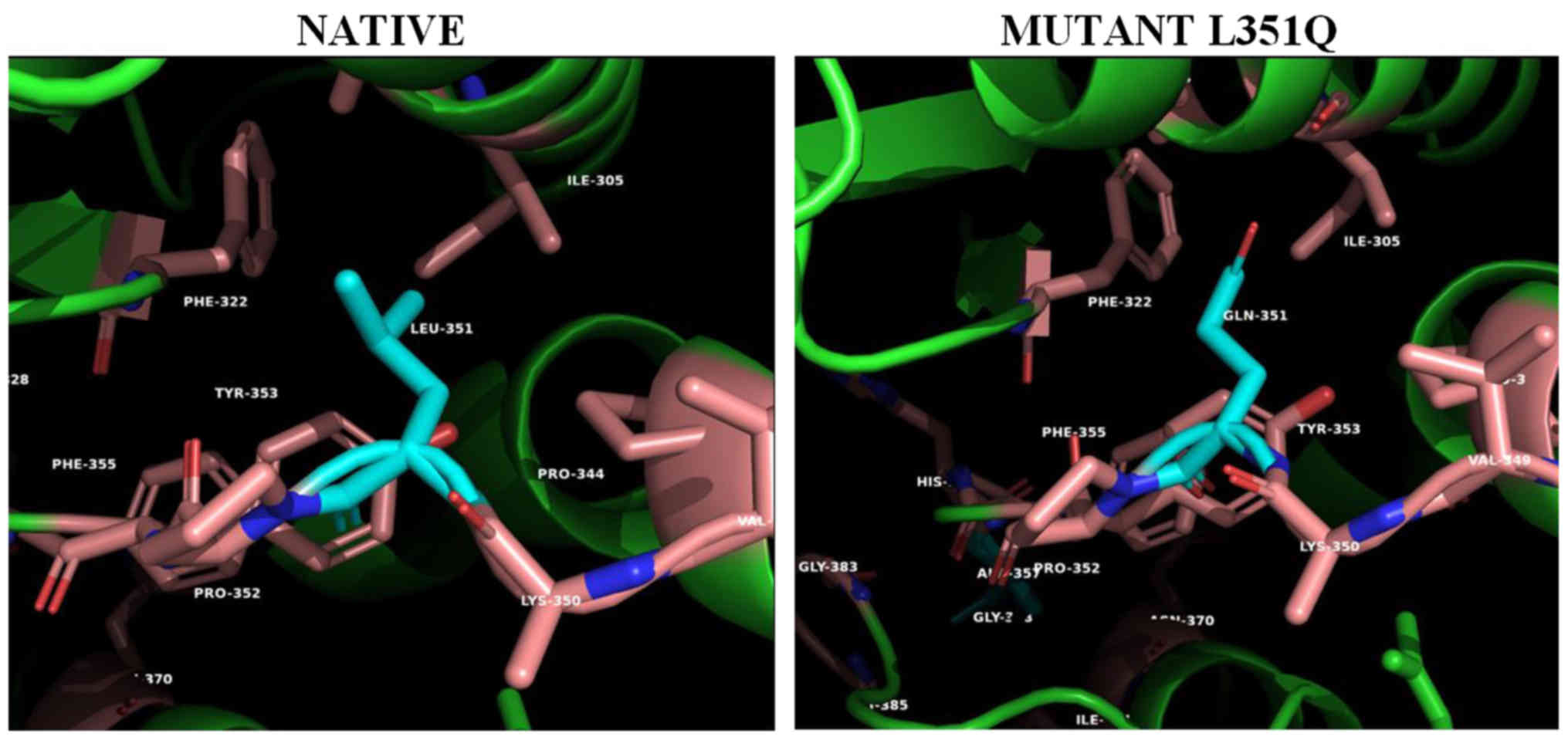
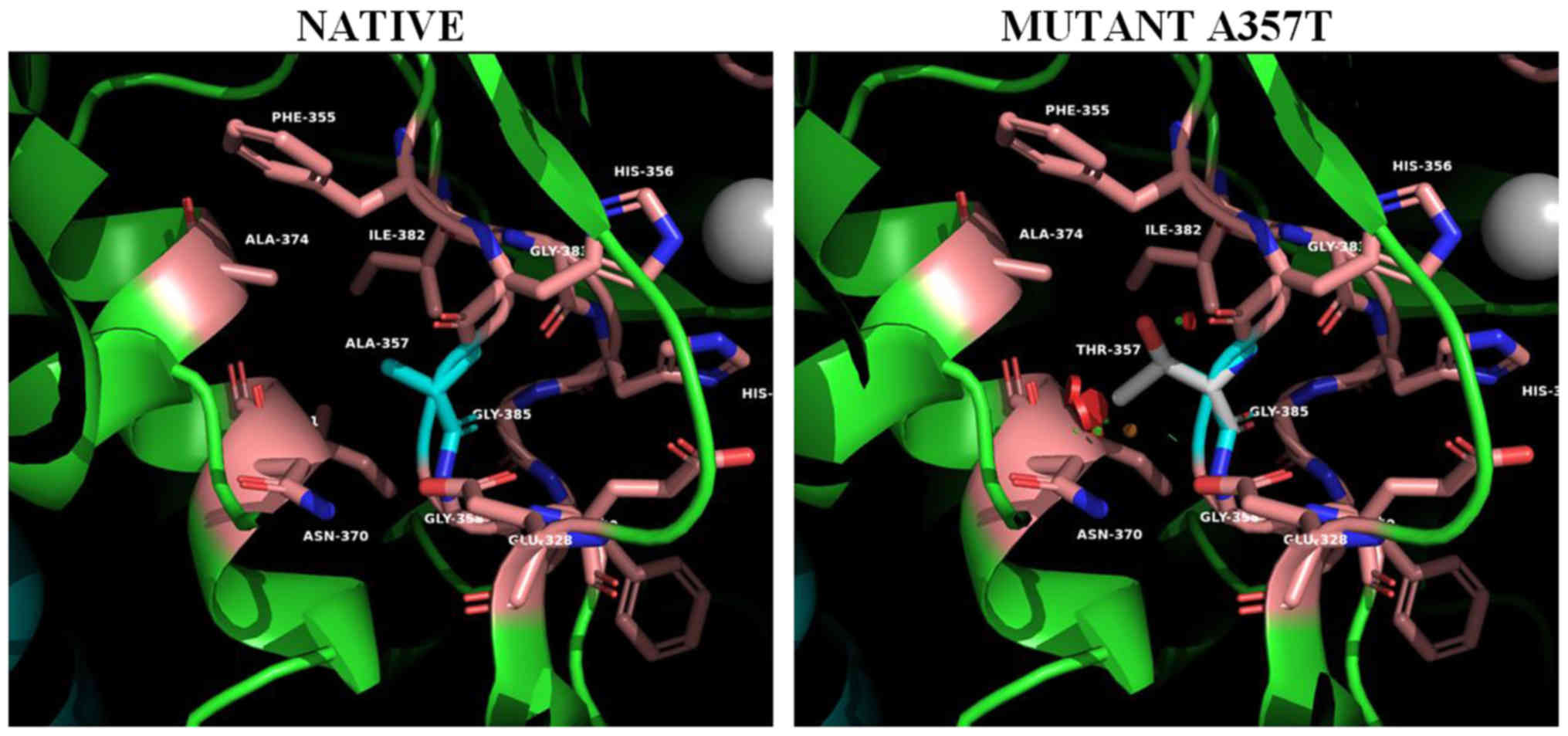
|
1
|
Navon Elkan P, Pierce SB, Segel R, Walsh
T, Barash J, Padeh S, Zlotogorski A, Berkun Y, Press JJ, Mukamel M,
et al: Mutant adenosine deaminase 2 in a polyarteritis nodosa
vasculopathy. N Engl J Med. 370:921–931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou Q, Yang D, Ombrello AK, Zavialov AV,
Toro C, Zavialov AV, Stone DL, Chae JJ, Rosenzweig SD, Bishop K, et
al: Early-onset stroke and vasculopathy associated with mutations
in ADA2. N Engl J Med. 370:911–920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caorsi R, Penco F, Schena F and Gattorno
M: Monogenic polyarteritis: The lesson of ADA2 deficiency. Pediatr
Rheumatol Online J. 14:512016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Francès C, Papo T, Wechsler B, Laporte JL,
Biousse V and Piette JC: Sneddon syndrome with or without
antiphospholipid antibodies. A comparative study in 46 patients.
Medicine (Baltimore). 78:209–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Eyck L Jr, Hershfield MS, Pombal D,
Kelly SJ, Ganson NJ, Moens L, Frans G, Schaballie H, De Hertogh G,
Dooley J, et al: Hematopoietic stem cell transplantation rescues
the immunologic phenotype and prevents vasculopathy in patients
with adenosine deaminase 2 deficiency. J Allergy Clin Immunol.
135:283–287.e5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben-Ami T, Revel-Vilk S, Brooks R, Shaag
A, Hershfield MS, Kelly SJ, Ganson NJ, Kfir-Erenfeld S, Weintraub
M, Elpeleg O, et al: Extending the Clinical Phenotype of Adenosine
Deaminase 2 Deficiency. J Pediatr. 177:316–320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cipe FE, Aydogmus C, Serwas NK,
Keskindemirci G and Boztuğ K: Novel mutation in CECR1 leads to
deficiency of ADA2 with associated neutropenia. J Clin Immunol.
38:273–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Westendorp WF, Nederkoorn PJ,
Aksentijevich I, Hak AE, Lichtenbelt KD and Braun KP: Unexplained
early-onset lacunar stroke and inflammatory skin lesions: Consider
ADA2 deficiency. Neurology. 84:2092–2093. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elbracht M, Mull M, Wagner N, Kuhl C,
Abicht A, Kurth I, Tenbrock K and Häusler M: Stroke as initial
manifestation of adenosinedeaminase 2 deficiency. Neuropediatrics.
48:111–114. 2017.PubMed/NCBI
|
|
10
|
Sahin S, Adrovic A, Barut K, Ugurlu S,
Turanli ET, Ozdogan H and Kasapcopur O: Clinical, imaging and
genotypical features of three deceased and five surviving cases
with ADA2 deficiency. Rheumatol Int. 38:129–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bulut E, Erden A, Karadag O, Oguz KK and
Ozen S: Deficiency of adenosine deaminase 2; special focus on
central nervous system imaging. J Neuroradiol. 46:193–198. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zavialov AV, Gracia E, Glaichenhaus N,
Franco R, Zavialov AV and Lauvau G: Human adenosine deaminase 2
induces differentiation of monocytes into macrophages and
stimulates proliferation of T helper cells and macrophages. J
Leukoc Biol. 88:279–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zavialov AV and Engström A: Human ADA2
belongs to a new family of growth factors with adenosine deaminase
activity. Biochem J. 391:51–57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haskó G, Linden J, Cronstein B and Pacher
P: Adenosine receptors: Therapeutic aspects for inflammatory and
immune diseases. Nat Rev Drug Discov. 7:759–770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meyts I and Aksentijevich I: Deficiency of
Adenosine Deaminase 2 (DADA2): Updates on the Phenotype, Genetics,
Pathogenesis, and Treatment. J Clin Immunol. 38:569–578. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson DK, Rudolph FB and Quiocho FA:
Atomic structure of adenosine deaminase complexed with a
transition-state analog: Understanding catalysis and
immunodeficiency mutations. Science. 252:1278–1284. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charlab R, Valenzuela JG, Andersen J and
Ribeiro JM: The invertebrate growth factor/CECR1 subfamily of
adenosine deaminase proteins. Gene. 267:13–22. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gibson KM, Morishita KA, Dancey P,
Moorehead P, Drögemöller B, Han X, Graham J, Hancock REW, Foell D,
Benseler S, et al PedVas Investigators Network, : Identification of
novel adenosine deaminase 2 gene variants and varied clinical
phenotype in pediatric vasculitis. Arthritis Rheumatol.
71:1747–1755. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schrödinger LLC: The PyMOL Molecular
Graphics System 2016 version 2.2. simplepymol.org/2/support.htmlMarch 5–2019
|
|
20
|
Kinoshita T, Nakanishi I, Terasaka T, Kuno
M, Seki N, Warizaya M, Matsumura H, Inoue T, Takano K, Adachi H, et
al: Structural basis of compound recognition by adenosine
deaminase. Biochemistry. 44:10562–10569. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meyts I, Bosch B, Bolze A, Boisson B, Itan
Y, Belkadi A, Pedergnana V, Moens L, Picard C, Cobat A, et al:
Exome and genome sequencing for inborn errors of immunity. J
Allergy Clin Immunol. 138:957–969. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caorsi R, Penco F, Grossi A, Insalaco A,
Omenetti A, Alessio M, Conti G, Marchetti F, Picco P, Tommasini A,
et al: ADA2 deficiency (DADA2) as an unrecognised cause of early
onset polyarteritis nodosa and stroke: A multicentre national
study. Ann Rheum Dis. 76:1648–1656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Özen S, Batu ED, Taskiran EZ, Özkara HA,
Ünal S, Güleray N, Erden A, Karadağ Ö, Gümrük F, et al: A monogenic
disease with a variety of phenotypes: deficiency of adenosine
deaminase 2. J Rheum. May 1–2019.(Epub ahead of print). View Article : Google Scholar
|
|
24
|
Van Eyck L, Liston A and Wouters C: Mutant
ADA2 in vasculopathies. N Engl J Med. 371:4802014.PubMed/NCBI
|
|
25
|
Van Montfrans JM, Hartman EA, Braun KP,
Hennekam EA, Hak EA, Nederkoorn PJ, Westendorp WF, Bredius RG,
Kollen WJ, Schölvinck EH, et al: Phenotypic variability in patients
with ADA2 deficiency due to identical homozygous R169Q mutations.
Rheumatology (Oxford). 55:902–910. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fellmann F, Angelini F, Wassenberg J,
Perreau M, Arenas Ramirez N, Simon G, Boyman O, Demaria O,
Christen-Zaech S, Hohl D, et al: IL-17 receptor A and adenosine
deaminase 2 deficiency in siblings with recurrent infections and
chronic inflammation. J Allergy Clin Immunol. 137:1189–1196.e2.
2016. View Article : Google Scholar : PubMed/NCBI
|