Introduction
Orthodontic treatment alters the location of
abnormally positioned teeth by applying mechanical force, which in
turn affects the attachment apparatus, including the periodontal
ligament, alveolar bone, cementum and gingiva (1). Tooth movement is achieved following
alveolar bone remodeling and response of the periodontal ligament
to mechanical force (2). In recent
decades, alveolar bone and periodontal membrane remodeling under
mechanical force have been widely studied (3). However, the mechanisms underlying
gingival tissue reconstruction remain largely unexplored.
Gingival tissues are constantly exposed to the
effect of physical forces (4).
Mechanical stimuli are regulators of connective tissue homeostasis,
and sustained mechanical stimulation may lead to modifications in
cell activity and extracellular matrix (ECM) composition (5). Human gingival fibroblasts (HGFs) are
the major cell type that constitutes human gingival connective
tissue (6), which are responsible
for the synthesis and degradation of the ECM and bone resorption,
as well as the secretion of proteolytic enzymes (7–9).
Accordingly, they are crucial for regulating the homeostasis of
periodontal tissues under healthy and diseased states (10). Nevertheless, strategies to improve
correction efficiency and promote HGFs to maintain the stability of
adaptive remodeling following orthodontic treatment remain to be
elucidated.
Transforming growth factor (TGF)-β is an important
regulator of gingival tissue regeneration (11–13).
Guo et al (14)
demonstrated that mechanical shear force promotes the proliferation
of gingival fibroblasts through activation of the TGF-β signaling
pathway. In addition, Jeon et al (15) revealed that mechanical force
induces the synthesis of type I collagen (COL-1) and osteopontin in
HGFs through TGF-β signaling. Therefore, it was hypothesized that
TGF-β may have an important role in converting mechanical force
into biochemical signals in HGFs, thus promoting cell proliferation
and extracellular ECM synthesis.
Since other cells and ECM surround almost all cells
in the in vivo environment in a 3-dimensional (3D) fashion,
2-dimensional (2D) cell culture does not adequately take into
account the natural 3D environment of cells (16). The development of biological
scaffold material provides a structural basis for 3D cell culture
(17). Cells cultured in a 3D
cell-based system more realistically mimic in vivo cell
behaviors and provide more predictable results for subsequent in
vivo experiments. Biodegradable poly(lactide-co-glycolide)
(PLGA) is a biocompatible material that is widely used in clinical
settings (18). In the present
study, a 3D HGFs culture model was established, using PLGA
scaffolds, in order to investigate the effects of mechanical force
on the proliferation of HGFs, and to explore the functions of TGF-β
in HGF proliferation, as well as COL-1 and matrix metallopeptidase
(MMP)-1 expression. The results may provide a theoretical basis for
the understanding of gingival remodeling under mechanical
force.
Materials and methods
3D culture of HGFs
HGFs were 3D cultured as previously reported
(19). Gingival tissues were
collected from healthy teeth extracted from 17 males and 19 females
(age range, 10–14 years) during orthodontic extraction or gingival
resection. Subsequently, the tissues were dissociated using 0.25%
trypsin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing
100 ng/ml DNase (Roche Diagnostics, Basel, Switzerland) at 37°C for
35 min. The digestion was terminated by adding Dulbecco's modified
Eagle's medium (GE Healthcare Life Sciences, Logan, UT, USA) with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The dissociated HGFs were cultured in Eagle's
minimum essential medium (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 2 mM L-glutamine and 100 U/ml antibiotics
(Gibco; Thermo Fisher Scientific, Inc.) in plastic culture flasks.
Cells were maintained at 37°C with 5% CO2. The PLGA
scaffold (size, 2 cm × 2 cm × 300 µm; porosity, 85%; average pore
size, 80–120 µm) was synthesized using the solvent
casting/particulate leaching technique (20). After 4–6 passages, 1×105
HGFs were seeded onto a single sheet of PLGA scaffold. The present
study was approved by the Hospital Institutional Review Board
(approval no. 20150304-22) of Guangxi Medical University (Nanning,
China). All donors and their guardians signed an informed consent
form.
Application of mechanical force
Prior to the application of mechanical force, HGFs
were allowed to stabilize for 48 h. HGFs were continuously
compressed using the uniform compression method, as presented in
Fig. 1. Briefly, the PLGA sheet
was placed into the well with a wire stool to prevent it from
floating. A HGF suspension was dripped into the well. After 24 h of
incubation at 37°C, the PLGA/HGF construct was moved to another
well. After cell implantation for 6 h, glass bottles containing
lead granules were placed on top of the 3D models. The bottles
provided compressive stress of four magnitudes (5, 15, 25 and 35
g/cm2) for 6, 24 and 72 h. Control cells (without
application of mechanical force) were cultured under the same 3D
conditions. Cells were also cultured at 37°C in the presence of
SB431542 (cat. no. HY-10431; MedChemExpress Monmouth Junction, NJ,
USA) for 24 h, a TGF-β inhibitor, at 20 µM.
Immunocytochemistry
HGFs from the PLGA scaffold were plated on
coverslips and incubated for 6 h at 37°C in an atmosphere
containing 5% CO2, in order to allow cells to adhere and
proliferate. Cells were harvested and fixed with 4%
paraformaldehyde for 30 min at room temperature. Peroxidase
activity was blocked using 3% hydrogen peroxide for 30 min at 37°C.
After blocking in normal rabbit serum (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), the cells were incubated with monoclonal
antibodies against vimentin (cat. no. MAB3404; 1:100) and
cytokeratin (cat. no. MAB3400; 1:100) (both from Sigma-Aldrich;
Merck KGaA) at 4°C overnight. Following three washes with PBS for 5
min at room temperature, sheep anti-rat immunoglobulin G secondary
antibody (1:5,000; cat. no. 5647; Abcam, Cambridge, UK) was added
and incubated at 37°C for 1 h. Subsequently, the membrane was
analyzed using the UltraSensitive™ S-P Immunohistochemistry kit
(cat. no. 40398a; Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China)
according to the manufacturer's protocol. Finally, cells were
counterstained with 3% hematoxylin for 60 sec at room temperature
and examined using a fluorescence microscope (Olympus Corporation,
Tokyo, Japan).
Immunofluorescence
Sections of the scaffolds (300 µm-thick) containing
the HGFs were deparaffinized with xylene. Following rehydration
with descending ethanol series, the samples were incubated for
antigen retrieval in a microwave oven with EDTA buffer at pH 8.0
for 30 min at 95°C, followed by fixation with 4% paraformaldehyde
for 30 min at room temperature. Immunofluorescence analysis was
performed to detect fibrillar actin (F-actin) and nuclei.
Structures of F-actin were detected using
tetramethylrhodamine-phalloidin (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) staining solution at a
concentration of 100 nM and incubated for 30 min at room
temperature. Nuclei were stained with DAPI at 10 µg/ml for 30 sec
at room temperature (Sigma-Aldrich; Merck KGaA), according to the
manufacturer's protocol. After washing with PBS, samples were
examined by confocal microscopy (fv-500; Olympus Corporation).
Cell viability
Following the application of mechanical force,
HGFs-PLGA constructs were incubated with MTT reagent
(Sigma-Aldrich; Merck KGaA) for 4 h at 37°C, at a concentration of
20 µM. Subsequently, 500 µl DMSO (Sigma-Aldrich; Merck KGaA) was
added into each well to dissolve the formazan and the optical
density (OD) values were determined at 570 nm.
Flow cytometry
Trypsin was used to detach and collect the cells
from the PLGA scaffold, and cells were washed twice with PBS at
4°C. The cells were collected by centrifugation at 300 × g for 5
min and were fixed in 1 ml cold 70% ethanol for 30 min at 4°C.
Cells were resuspended in 1 ml propidium iodide staining solution
(cat. no. CCS012; Multisciences Lianke Biotech Co., Ltd., Hangzhou,
China) and incubated for 30 min at room temperature. The stained
cells were analyzed for DNA content by flow cytometry (BD Accuri™
C6; BD Biosciences, Franklin Lakes, NJ, USA). The number of cells
in the S and G2/M phases was divided by the number of cells in the
G0/G1 phase, in order to calculate the proliferation index (ModFit
LT software; version 4.0; Verity Software House, Inc., Topsham, ME,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HGFs on the PLGA
scaffold using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
An additional round of purification was performed with
deoxyribonuclease I (ribonuclease-free; Takara Bio, Inc., Otsu,
Japan) to remove genomic DNA. RNA quality was assessed using RNA
6000 Nano-Chips on an Agilent 2100 Bioanalyzer (Agilent
Technologies, Inc., Santa Clara, CA, USA). All samples showed
intact 18S/28S bands. Total RNA (1 µg) underwent cDNA synthesis
using an ExScript RT reagent kit (Takara Bio, Inc.), according to
the manufacturer's protocol. RT-qPCR was performed using a T100™
Thermal Cycler system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), according to the manufacturer's protocol. The following
primers were used: TGF-β1, forward, 5′-CGCATCCTAGACCCTTTCTCCTC-3′
and reverse, 5′-GGTGTCTCAGTATCCCACGGAAAT-3′; COL-1, forward,
5′-GAGGGCAACAGCAGGTTCACTTA-3′ and reverse,
5′-TCAGCACCACCGATGTCCA-3′; MMP-1, forward,
5′-ACAACTGCCAAATGGGCTTGA-3′ and reverse,
5′-CTGTCCCTGAACAGCCCAGTACTTA-3′; and GAPDH, forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
The RT-qPCR reactions were performed on an ABI 7300 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with
SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd., Dalian, China)
at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and
60°C for 31 sec, after which a melt curve analysis was performed at
95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. All reactions
were performed in triplicate and average of 2−ΔΔCt
(21). GAPDH was used as an
internal control. Each experiment was repeated at least three
times.
ELISA
ELISA kits were used to quantify TGF-β1 (cat. no.
JL10706), COL-1 (cat. no. CSB-E08082h) and MMP-1 (cat. no.
CSB-E08082h) levels (Quantikine; R&D Systems, Inc.,
Minneapolis, MI, USA) according to the manufacturer's protocol. A
standard curve was created with the serially diluted TGF-β1
standard provided in the kit. Samples were measured at 450 nm using
a microplate reader (Model 3550; Thermo Fisher Scientific, Inc.).
Data were linearized by plotting the log of the TGF-β1
concentrations vs. the log of the OD; TGF-β1 concentrations were
determined by linear regression. COL-1 and MMP-1 protein levels
were detected using the same method. Each sample was assessed in
triplicate.
Statistical analysis
All data are presented as the means ± standard
deviation of three independent experiments. One-way analysis of
variance (ANOVA) with Dunnett's post hoc test or two-way ANOVA with
Bonferroni post hoc test was used for multiple comparisons.
Statistical analyses were performed using SPSS 21.0 (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Mechanical force induces HGF
proliferation
As presented in Fig.
2A, HGFs had a long, fusiform shape, with radial and swirling
growth. In addition, HGFs were positive for vimentin staining
(Fig. 2B) and negative for keratin
staining (Fig. 2C). Based on the
sample origin combined with the mesodermal nature of the cells
(rather than epithelial), the cells were considered to be HGFs.
Histological sections revealed 2–3 layers of HGFs overlying the
surface of the 3D scaffold. Throughout the scaffold, the cells
exhibited an elongated shape and were arranged in multiple layers
(Fig. 2D). In addition,
microfilaments, stained in red, were arranged along the
longitudinal axis of the HGFs, as detected by F-actin staining
(Fig. 2E).
Forces of 5, 15, 25 and 35 g/cm2 were
applied in the present study. Compared with in the control group
(no mechanical force), HGF proliferation increased under the action
of 5 and 15 g/cm2 for 24 h, and cell proliferation
activity reached its peak in response to 25 g/cm2
(Fig. 3A). At 35 g/cm2,
HGF proliferation was inhibited and cell death increased, as
measured by flow cytometry (Fig.
3A).
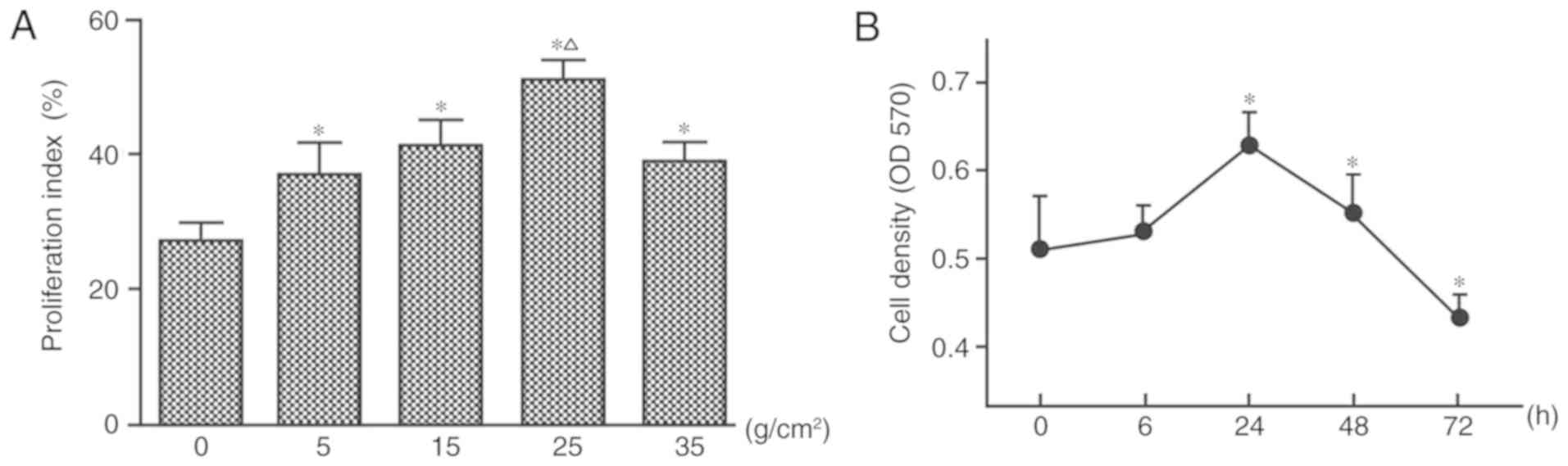 | Figure 3.Mechanical force induces the
proliferation of HGFs. (A) Proliferation index was calculated by
flow cytometry following the application of four different force
magnitudes (0, 5, 15, 25 and 35 g/cm2) for 24 h. (B) HGF
proliferation under 25 g/cm2 for 0, 6, 24, 48 and 72 h,
as measured by MTT assay. *P<0.05 vs. the control group;
ΔP<0.05 vs. 5, 15 and 35 g/cm2. One-way
analysis of variance followed by Dunnett's post hoc test was used
to analyze data. HGFs, human gingival fibroblasts; OD, optical
density. |
When applying 25 g/cm2, no significant
difference in cell proliferation was observed after 6 h of
stimulation, whereas cell proliferation was significantly increased
after 24 (peak) and 48 h (P<0.05; Fig. 3B). However, with the gradual
increase of application time, the number of cells gradually
decreased, and cell proliferation was inhibited after 72 h
(Fig. 3B).
COL-1 and MMP-1 mRNA and protein
expression is altered in response to mechanical force in HGFs
Under 25 g/cm2, the mRNA and protein
expression levels of COL-1 were upregulated compared with in the
control group; the highest expression was detected at 24 h, whereas
the expression level was decreased at 48 and 72 h (Fig. 4A and B). Conversely, the mRNA and
protein expression levels of MMP-1 were lowest at 24 h, and were
consequently increased with time, although they remained lower than
the control group (Fig. 4C and
D).
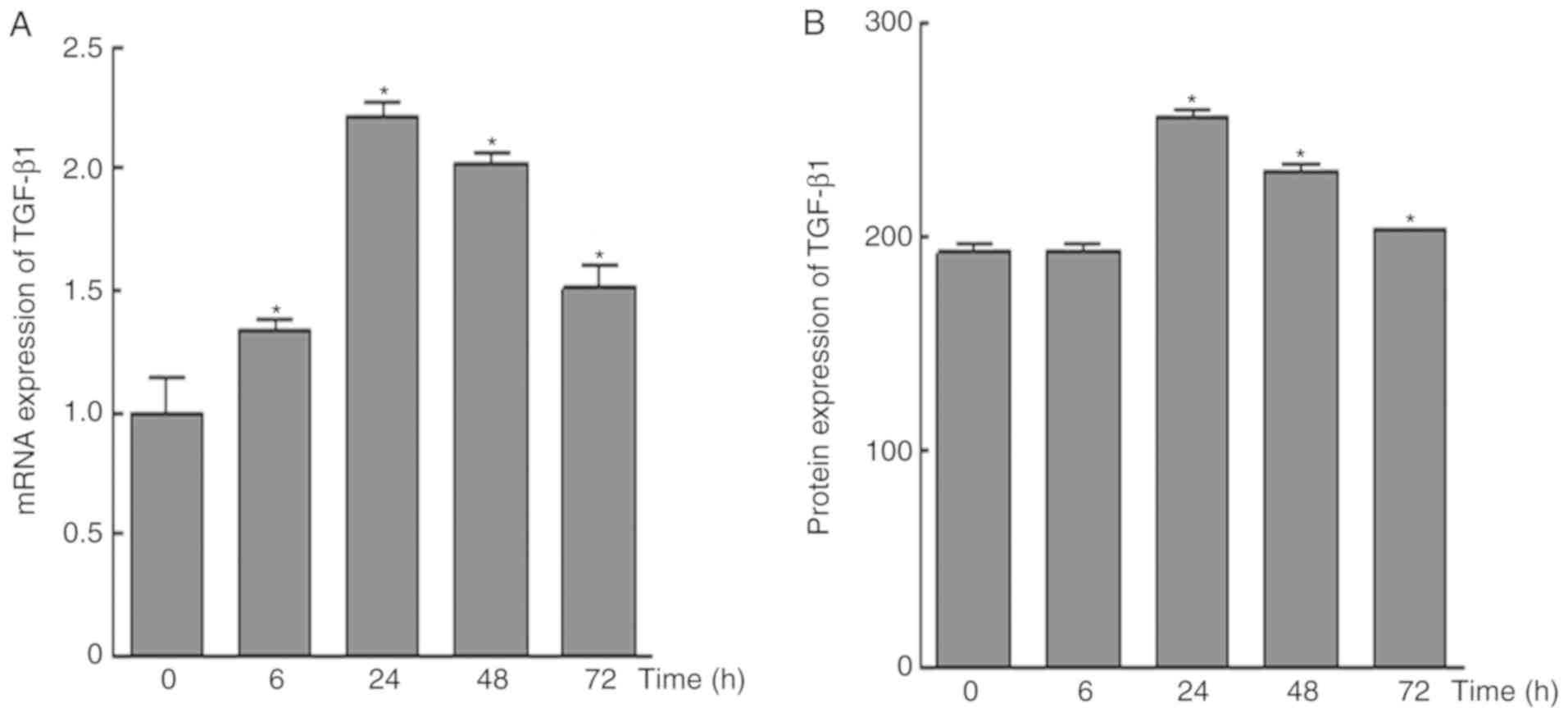
Mechanical force increases TGF-β1
expression in HGFs
The results of RT-qPCR analysis demonstrated that
the mRNA expression levels of TGF-β1 were upregulated in response
to mechanical force; the highest expression was detected at 24 h,
whereas the expression level was decreased at 48 and 72 h
(P<0.05; Fig. 5A). In addition,
compared with in the control group, no significant alterations in
TGF-β1 protein expression were observed at 6 h; however, protein
expression was significantly increased after 24 h, and was
decreased over time after this point.
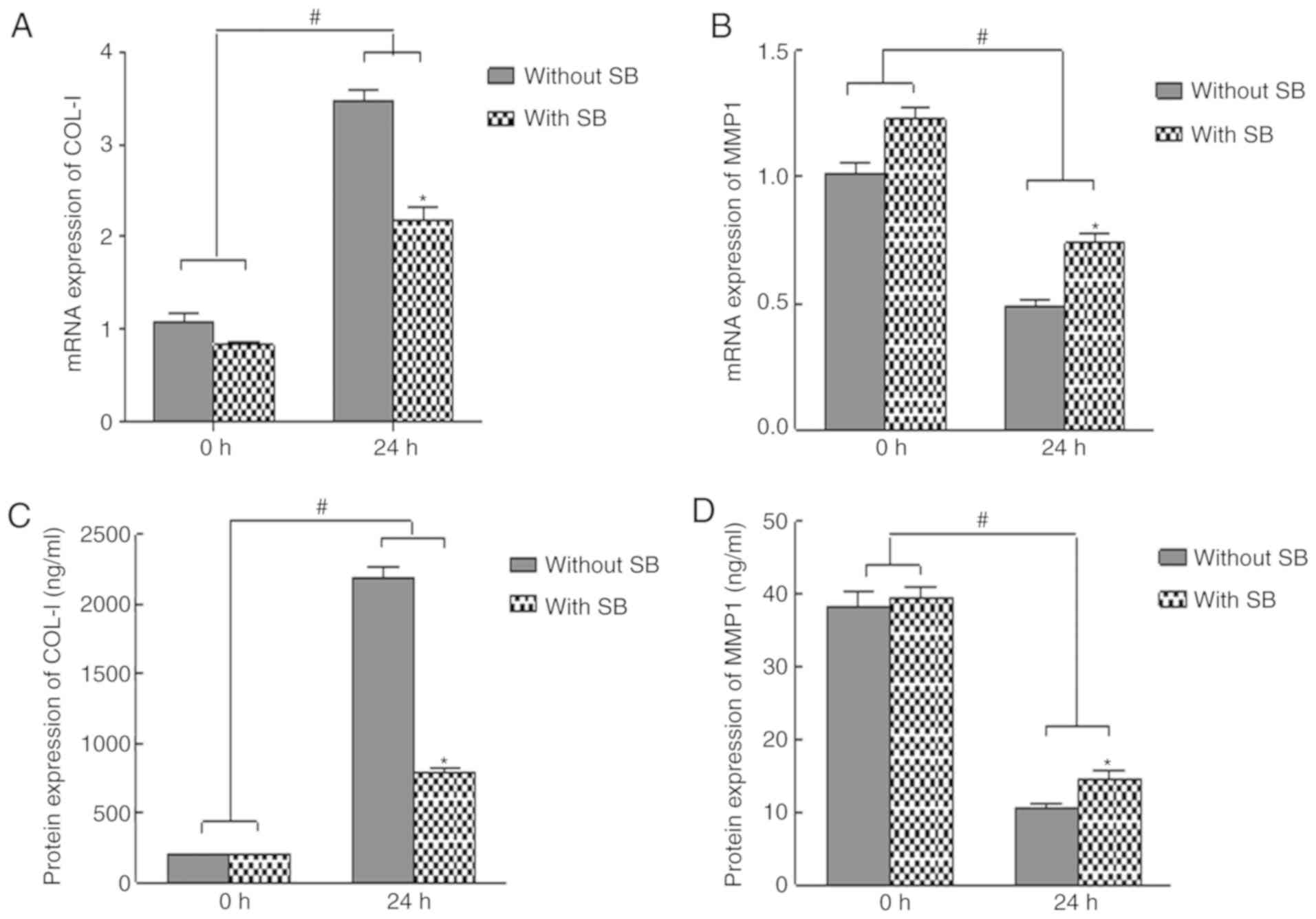
TGF-β1 inhibitor reverses the effects
of mechanical force on cell proliferation, and COL-1 and MMP-1
expression in HGFs
To determine whether TGF-β1 mediated the effects of
mechanical force on HGF proliferation, as well as COL-1 and MMP-1
expression, cells were treated with the TGF-β1 inhibitor SB431542
at 20 µM. The results indicated that mechanical force-induced
upregulation of COL-1 expression and downregulation of MMP-1
expression were reversed in the presence of SB431542 (Fig. 6). These data suggested that TGF-β1
may be a key regulator in the of HGF biological function induced by
mechanical force. As shown in Fig.
7, presents the effects of the TGF-β1 inhibitor SB431542 were
also determined on cell proliferation. Compared with in the control
group, cells only treated with SB431542 for 24 h did not exhibit a
significant decrease in cell proliferation (P>0.05). However,
continuous stimulation with SB431542 for 24 h significantly
decreased mechanical stress-induced cell proliferation compared
with in the mechanical stress group (P<0.05).
Discussion
The present study strongly indicated that the
application of mechanical force on HGFs cultured on 3D PLGA
scaffolds led to a significant increase in cell proliferation and
in COL-1 gene expression, as well as a decrease in MMP-1
expression. Furthermore, these effects were mediated, at least
partially, via the TGF-β signaling pathway. These findings further
elucidated the mechanisms underlying mechanical force-induced HGF
proliferation and ECM synthesis, and may aid in the development of
targeted therapeutics, in a pre-clinical and clinical setting.
A growing body of evidence has suggested that 3D
cell culture systems, in contrast to 2D culture systems, more
accurately represent the microenvironment in which cells reside in
tissues (16). Therefore, the
behavior of 3D-cultured cells is more reflective of the actual
in vivo cellular responses. Kang et al (22) attempted to use collagen gel to
establish a 3D culture model of periodontal ligament cells;
however, the porosity and pressure resistance, as well as other
characteristics of the periodontal ligament are significantly
different to those of collagen gel (22). The PLGA used in the present study
is a copolymer of polyglycolic acid (PGA) and polylactic acid
(PLA), which has the advantages of PGA in terms of cell adhesion,
and PLA in terms of mechanical strength (18,23).
In addition, it is more consistent with periodontal ligament
tissue, regarding its physical characteristics. Furthermore,
hypoxia must be considered. In previous studies, four-point bending
and Flexcell loading models were used to expose the cells to
aerobic conditions (24). When
applying mechanical forces to the teeth, due to vascular atresia,
the periodontal ligament cells on the pressure side suffer
simultaneously from mechanical and hypoxic stimuli, which interact
and promote osteoclast formation, facilitating the absorption of
alveolar bone on the pressure side. The combination of these
stimuli were demonstrated to be beneficial to orthodontic tooth
movement (25,26). In the present study, cells in the
central part of the complex were under hypoxic and nutrient
deprivation conditions due to limited diffusion following the
application of mechanical force. Therefore, the PLGA 3D model
simultaneously simulated the effects of mechanical force and the
hypoxic environment, providing a solid foundation for subsequent
experiments.
Mechanical stimulation has an important function in
the improvement of orthodontic tooth movement. Kook et al
(27,28) demonstrated that periodontal
ligament fibroblasts secrete relatively higher levels of tumor
necrosis factor-α in the compression side compared with the tension
side following application of mechanical stimuli, thereby
facilitating bone resorption during orthodontic tooth movement
(27,28). Improved simulation of orthodontic
treatment in vitro is an important branch of cell
biomechanics research. The concept of optimal orthodontic force in
orthodontic treatment was first described in 1932 by Schwarz
(29). The optimal orthodontic
force was calculated by analyzing the capillary pressure at the
periodontal ligament on the root surface. Notably, periodontal
tissue may undergo ischemia and necrosis under high orthodontic
force. In the majority of mammals and humans, the force value is
20–26 g/cm2; therefore, in the present study, force
magnitudes of 5, 15, 25 and 35 g/cm2 were applied. It
was revealed that when the force magnitude was 5 and 15
g/cm2, HGF proliferation was enhanced, and cell
proliferation was the highest at 25 g/cm2. In addition,
when the force magnitude reached 35 g/cm2, HGF
proliferation was inhibited. These results were consistent with
those of a previous study (30).
Thus, 25 g/cm2 was selected for the subsequent
experiments.
Mechanical forces of various magnitudes applied to
HGFs affect cell proliferation, function and ECM metabolism through
the autocrine or paracrine secretion of various cytokines (31). In the present study, HGF
proliferation and COL-1 expression was increased after 6 h and
peaked at 24 h under 25 g/cm2. COL-1 expression began to
decrease after 48 h, and cell proliferation activity began to
decrease after 72 h. In addition, MMP-1 expression was decreased
after 6 h of force application under 25 g/cm2, and
reached a nadir at 24 h. Its expression subsequently increased
after 48 h. Previous studies have demonstrated that orthodontic
force as an extrinsic mechanical stimulus not only moves the teeth,
but also rebuilds equilibrium through evoking a biological cellular
response within periodontal tissues (32). The alterations in HGF proliferation
by different mechanical forces may have reflected the establishment
of a new equilibrium.
TGF-β is expressed in several cell types, including
HGFs, and is involved in the proliferation and differentiation of
these cells (33,34). The functions of TGF-β are autocrine
or paracrine, since they are involved in the regulation of HGFs
during oral inflammation as well as wound healing processes at the
site of injury (35). Previous
studies have evaluated the effects of TGF-β on cell proliferation
and collagen metabolism in primary human periodontal ligament cells
in vitro. It was revealed that TGF-β significantly increases
cell proliferation, as well as COL-1 collagen expression (36). Furthermore, Kimoto et al
(37) focused on the effects of
intermittent mechanical strain on the cytokine synthesis of HGFs
in vitro in a 2D cell culture model, revealing that
mechanical stretching induced TGF-β secretion. These findings
suggest that HGFs synthesize and secrete TGF-β as autocrine or
paracrine factors that affect bone remodeling and root resorption.
The levels of these factors are altered in response to mechanical
stress. Consistent with previous studies, the results of the
present study determined that TGF-β levels were increased 6 h after
force application under 25 g/cm2, whereas TGF-β levels
began to decrease after 48 h. The selective TGF-β inhibitor
SB431642 prevented the effects of mechanical force on HGF
proliferation, upregulation of COL-1 expression and downregulation
of MMP-1, indicating that TGF-β served an important role in
gingival tissue remodeling mediated by mechanical force.
Nevertheless, the effects of TGF-β on HGFs should be further
demonstrated using a more rigorous approach that includes genetic
knockouts, small interfering RNAs and overexpression vectors. This
will be performed in future experiments.
In conclusion, HGFs were cultured on 3D PLGA
scaffolds and the results strongly suggested that inhibition or
reduction of local TGF-β expression decreased HGF proliferation and
COL-1 expression, and increased MMP-1 expression. These effects may
lead to an increased degradation of collagen fibrils in the ECM and
decreased gingival hyperplasia following orthodontic treatments.
The modulation of TGF-β pathway may contribute to the development
of novel therapeutic strategies to promote gingival tissue
remodeling.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81070860)
and the Natural Science Foundation of Guangxi Province (grant no.
2013GXNSFAA019183).
Availability of data and materials
The datasets used and/or analyzed during the current
study are included in this published article.
Authors' contributions
LN conceived and coordinated the study, designed,
performed and analyzed the experiments, and wrote the paper. YZ,
NL, SL, YW, ZC, LW, SZ and SM carried out data collection, data
analysis and manuscript revisions. All authors reviewed the results
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Hospital Institutional
Review Board (approval no. 20150304-22) of Guangxi Medical
University (Nanning, China). All donors and their guardians signed
an informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ECM
|
extracellular matrix
|
|
HGFs
|
human gingival fibroblasts
|
|
MMP-1
|
matrix metallopeptidase 1
|
|
OD
|
optical density
|
|
PGA
|
polyglycolic acid
|
|
PLA
|
polylactic acid
|
|
PLGA
|
poly(lactide-co-glycolide)
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Dutra EH, Ahmida A, Lima A, Schneider S,
Nanda R and Yadav S: The effects of alveolar decortications on
orthodontic tooth movement and bone remodelling in rats. Eur J
Orthod. 40:423–429. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pu H and Hua Y: Hydrogen sulfide regulates
bone remodeling and promotes orthodontic tooth movement. Mol Med
Rep. 16:9415–9422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang N, Guo W, Chen M, Zheng Y, Zhou J,
Kim SG, Embree MC, Songhee Song K, Marao HF and Mao JJ: Periodontal
ligament and alveolar bone in health and adaptation: Tooth
movement. Front Oral Biol. 18:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai J, Bi L, Lin J and Qi F: Evaluation of
interleukin-10 producing CD19+ B cells in human gingival
tissue. Arch Oral Biol. 84:112–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Viecilli RF, Katona TR, Chen J, Hartsfield
JK Jr and Roberts WE: Three-dimensional mechanical environment of
orthodontic tooth movement and root resorption. Am J Orthod
Dentofacial Orthop. 133:791.e11–e26. 2008. View Article : Google Scholar
|
|
6
|
Kong S, Aoki A, Iwasaki K, Mizutani K,
Katagiri S, Suda T, Ichinose S, Ogita M, Pavlic V and Izumi Y:
Biological effects of Er:YAG laser irradiation on the proliferation
of primary human gingival fibroblasts. J Biophotonics. Nov
2–2017.(Epub ahead of print). doi: 10.1002/jbio.201700157.
|
|
7
|
Belibasakis GN and Guggenheim B: Induction
of prostaglandin E(2) and interleukin-6 in gingival fibroblasts by
oral biofilms. FEMS Immunol Med Microbiol. 63:381–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Belibasakis GN, Meier A, Guggenheim B and
Bostanci N: The RANKL-OPG system is differentially regulated by
supragingival and subgingival biofilm supernatants. Cytokine.
55:98–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bostanci N, Meier A, Guggenheim B and
Belibasakis GN: Regulation of NLRP3 and AIM2 inflammasome gene
expression levels in gingival fibroblasts by oral biofilms. Cell
Immunol. 270:88–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartold PM and McCulloch CA: Information
generation and processing systems that regulate periodontal
structure and function. Periodontol 2000. 63:7–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawahara T, Yamashita M, Ikegami K,
Nakamura T, Yanagita M, Yamada S, Kitamura M and Murakami S:
TGF-Beta negatively regulates the BMP2-dependent early commitment
of periodontal ligament cells into hard tissue forming cells. PLoS
One. 10:e01255902015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aukkarasongsup P, Haruyama N, Matsumoto T,
Shiga M and Moriyama K: Periostin inhibits hypoxia-induced
apoptosis in human periodontal ligament cells via TGF-beta
signaling. Biochem Biophys Res Commun. 441:126–132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu H, He Y, Feng JQ, Shu R, Liu Z, Li J,
Wang Y, Xu Y, Zeng H, Xu X, et al: Wnt3α and transforming growth
factor-β induce myofibroblast differentiation from periodontal
ligament cells via different pathways. Exp Cell Res. 353:55–62.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo F, Carter DE and Leask A: Mechanical
tension increases CCN2/CTGF expression and proliferation in
gingival fibroblasts via a TGFβ-dependent mechanism. PLoS One.
6:e197562011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeon YM, Kook SH, Son YO, Kim EM, Park SS,
Kim JG and Lee JC: Role of MAPK in mechanical force-induced
up-regulation of type I collagen and osteopontin in human gingival
fibroblasts. Mol Cell Biochem. 320:45–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ravi M, Paramesh V, Kaviya SR, Anuradha E
and Solomon FD: 3D cell culture systems: Advantages and
applications. J Cell Physiol. 230:16–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sachar A, Strom TA, San Miguel S, Serrano
MJ, Svoboda KK and Liu X: Cell-matrix and cell-cell interactions of
human gingival fibroblasts on three-dimensional nanofibrous gelatin
scaffolds. J Tissue Eng Regen Med. 8:862–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei G, Jin Q, Giannobile WV and Ma PX:
Nano-fibrous scaffold for controlled delivery of recombinant human
PDGF-BB. J Control Release. 112:103–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harrington H, Rose FR, Aylott JW and
Ghaemmaghami AM: Self-reporting scaffolds for 3-dimensional cell
culture. J Vis Exp. e506082013.PubMed/NCBI
|
|
20
|
Thadavirul N, Pavasant P and Supaphol P:
Improvement of dual-leached polycaprolactone porous scaffolds by
incorporating with hydroxyapatite for bone tissue regeneration. J
Biomater Sci Polym Ed. 25:1986–2008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang KL, Lee SW, Ahn YS, Kim SH and Kang
YG: Bioinformatic analysis of responsive genes in two-dimension and
three-dimension cultured human periodontal ligament cells subjected
to compressive stress. J Periodontal Res. 48:87–97. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramos T, Ahmed M, Wieringa P and Moroni L:
Schwann cells promote endothelial cell migration. Cell Adh Migr.
9:441–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang L, Yang Y, Wang S, Li Y and Zhao Z:
In vitro mechanical loading models for periodontal ligament cells:
From two-dimensional to three-dimensional models. Arch Oral Biol.
60:416–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ugolini GS, Pavesi A, Rasponi M, Fiore GB,
Kamm R and Soncini M: Human cardiac fibroblasts adaptive responses
to controlled combined mechanical strain and oxygen changes in
vitro. Elife. 6(pii): e228472017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riddle RC, Leslie JM, Gross TS and Clemens
TL: Hypoxia-inducible factor-1alpha protein negatively regulates
load-induced bone formation. J Biol Chem. 286:44449–44456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kook SH, Jang YS and Lee JC: Human
periodontal ligament fibroblasts stimulate osteoclastogenesis in
response to compression force through TNF-α-mediated activation of
CD4+ T cells. J Cell Biochem. 112:2891–2901. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kook SH, Son YO, Hwang JM, Kim EM, Lee CB,
Jeon YM, Kim JG and Lee JC: Mechanical force inhibits
osteoclastogenic potential of human periodontal ligament
fibroblasts through OPG production and ERK-mediated signaling. J
Cell Biochem. 106:1010–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwarz A: Tissue changes incident to
orthodontic tooth movement. Int J Orthodontics. 18:331–352.
1932.
|
|
30
|
Li M, Yi J, Yang Y, Zheng W, Li Y and Zhao
Z: Investigation of optimal orthodontic force at the cellular level
through three-dimensionally cultured periodontal ligament cells.
Eur J Orthod. 38:366–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang JH and Thampatty BP: An introductory
review of cell mechanobiology. Biomech Model Mechanobiol. 5:1–16.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krishnan V and Davidovitch Z: Cellular,
molecular, and tissue-level reactions to orthodontic force. Am J
Orthod Dentofacial Orthop. 129:469.e1–e32. 2006. View Article : Google Scholar
|
|
33
|
Cotrim P, Martelli-Junior H, Graner E,
Sauk JJ and Coletta RD: Cyclosporin A induces proliferation in
human gingival fibroblasts via induction of transforming growth
factor-beta1. J Periodontol. 74:1625–1633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Komatsu Y, Ibi M, Chosa N, Kyakumoto S,
Kamo M, Shibata T, Sugiyama Y and Ishisaki A: Zoledronic acid
suppresses transforming growth factor-β-induced fibrogenesis by
human gingival fibroblasts. Int J Mol Med. 38:139–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Komatsu Y, Ibi M, Chosa N, Kyakumoto S,
Kamo M, Shibata T, Sugiyama Y and Ishisaki A: Zoledronic acid
suppresses transforming growth factor-β-induced fibrogenesis by
human gingival fibroblasts. Int J Mol Med. 38:139–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Watanabe T, Yasue A and Tanaka E:
Hypoxia-inducible factor-1α is required for transforming growth
factor-β1-induced type I collagen, periostin and α-smooth muscle
actin expression in human periodontal ligament cells. Arch Oral
Biol. 59:595–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kimoto S, Matsuzawa M, Matsubara S,
Komatsu T, Uchimura N, Kawase T and Saito S: Cytokine secretion of
periodontal ligament fibroblasts derived from human deciduous
teeth: Effect of mechanical stress on the secretion of transforming
growth factor-beta 1 and macrophage colony stimulating factor. J
Periodontal Res. 34:235–243. 1999. View Article : Google Scholar : PubMed/NCBI
|