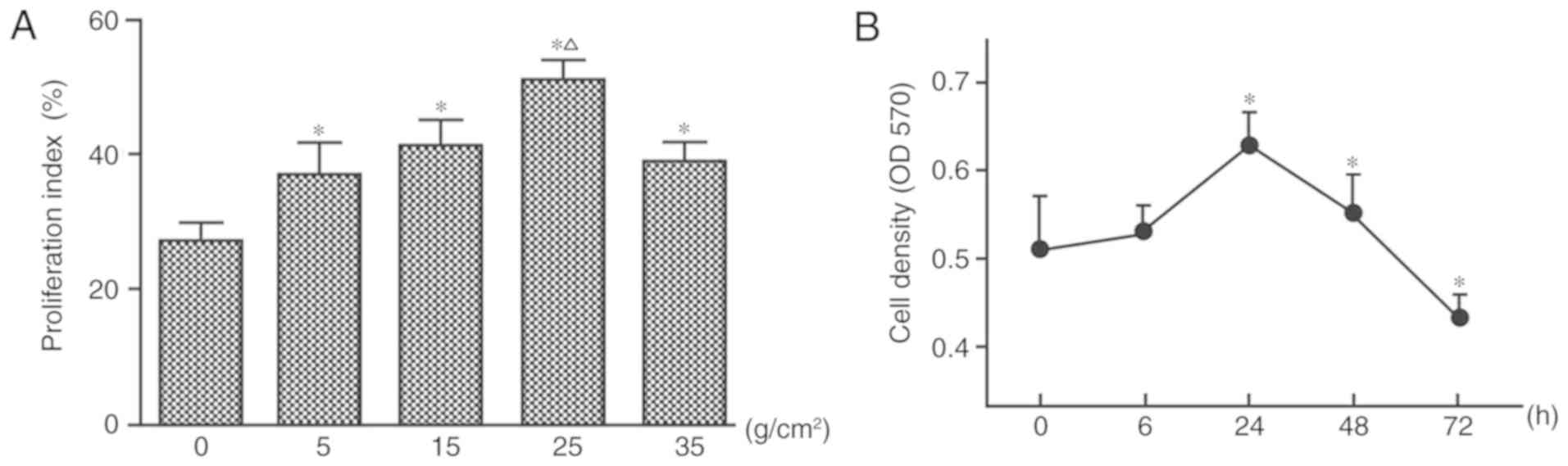
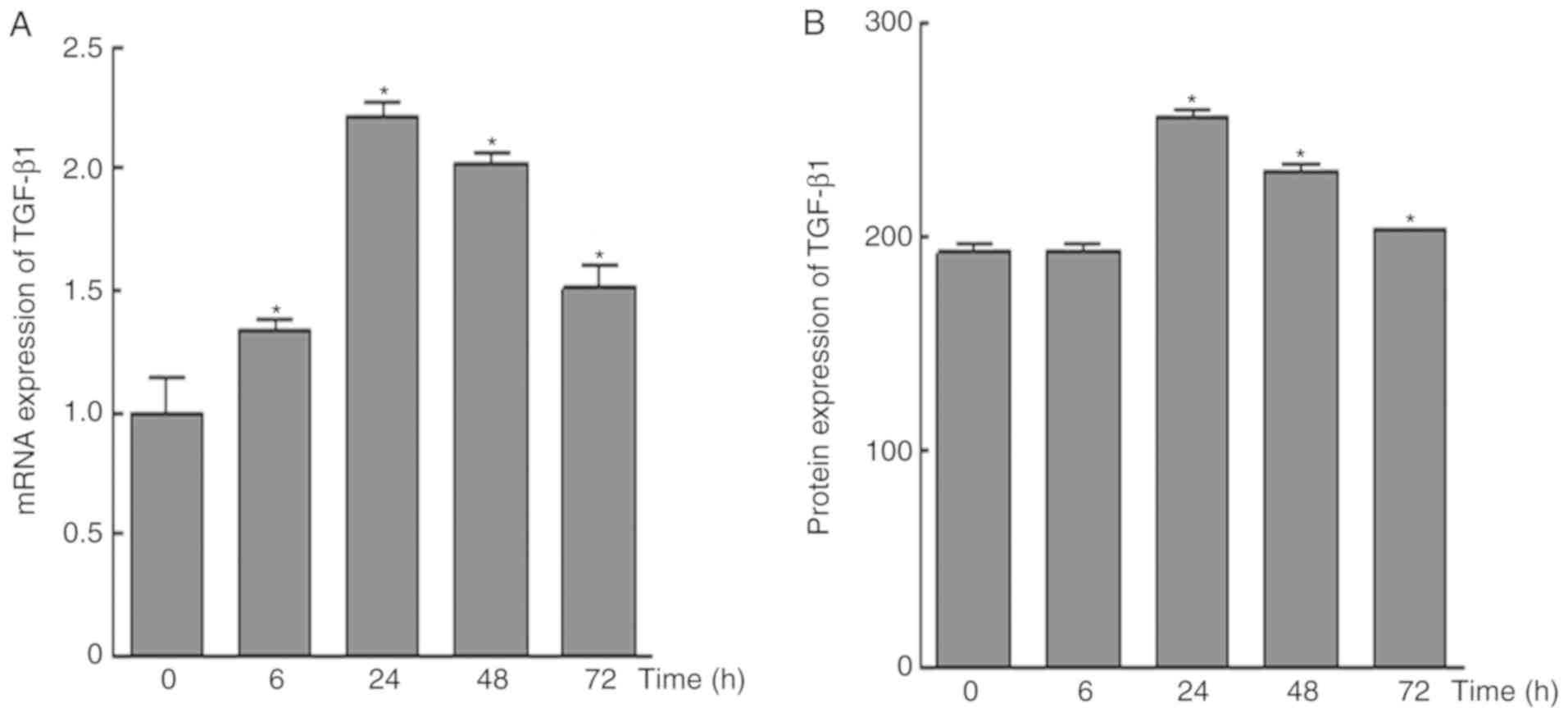
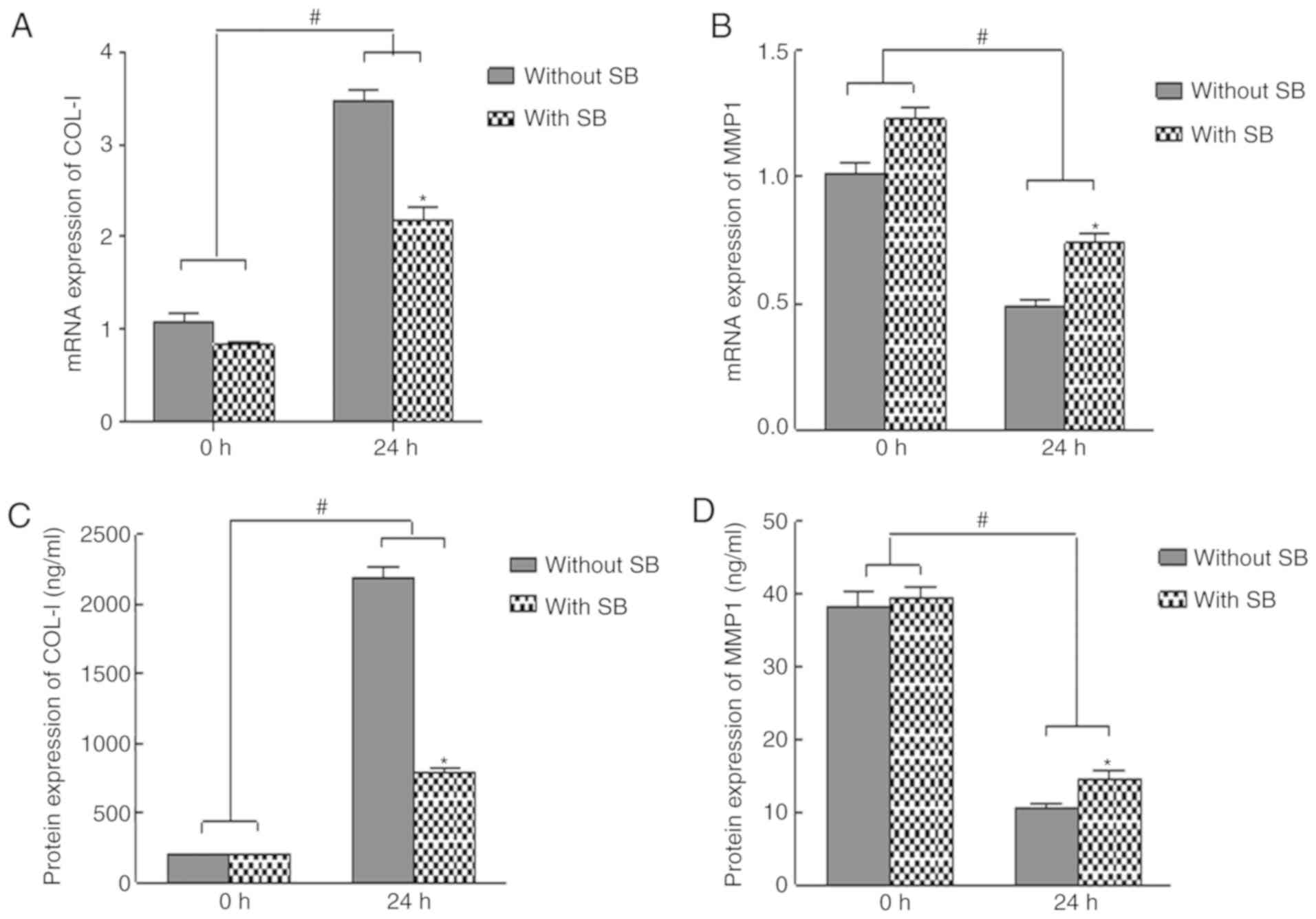
|
1
|
Dutra EH, Ahmida A, Lima A, Schneider S,
Nanda R and Yadav S: The effects of alveolar decortications on
orthodontic tooth movement and bone remodelling in rats. Eur J
Orthod. 40:423–429. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pu H and Hua Y: Hydrogen sulfide regulates
bone remodeling and promotes orthodontic tooth movement. Mol Med
Rep. 16:9415–9422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang N, Guo W, Chen M, Zheng Y, Zhou J,
Kim SG, Embree MC, Songhee Song K, Marao HF and Mao JJ: Periodontal
ligament and alveolar bone in health and adaptation: Tooth
movement. Front Oral Biol. 18:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai J, Bi L, Lin J and Qi F: Evaluation of
interleukin-10 producing CD19+ B cells in human gingival
tissue. Arch Oral Biol. 84:112–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Viecilli RF, Katona TR, Chen J, Hartsfield
JK Jr and Roberts WE: Three-dimensional mechanical environment of
orthodontic tooth movement and root resorption. Am J Orthod
Dentofacial Orthop. 133:791.e11–e26. 2008. View Article : Google Scholar
|
|
6
|
Kong S, Aoki A, Iwasaki K, Mizutani K,
Katagiri S, Suda T, Ichinose S, Ogita M, Pavlic V and Izumi Y:
Biological effects of Er:YAG laser irradiation on the proliferation
of primary human gingival fibroblasts. J Biophotonics. Nov
2–2017.(Epub ahead of print). doi: 10.1002/jbio.201700157.
|
|
7
|
Belibasakis GN and Guggenheim B: Induction
of prostaglandin E(2) and interleukin-6 in gingival fibroblasts by
oral biofilms. FEMS Immunol Med Microbiol. 63:381–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Belibasakis GN, Meier A, Guggenheim B and
Bostanci N: The RANKL-OPG system is differentially regulated by
supragingival and subgingival biofilm supernatants. Cytokine.
55:98–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bostanci N, Meier A, Guggenheim B and
Belibasakis GN: Regulation of NLRP3 and AIM2 inflammasome gene
expression levels in gingival fibroblasts by oral biofilms. Cell
Immunol. 270:88–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartold PM and McCulloch CA: Information
generation and processing systems that regulate periodontal
structure and function. Periodontol 2000. 63:7–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawahara T, Yamashita M, Ikegami K,
Nakamura T, Yanagita M, Yamada S, Kitamura M and Murakami S:
TGF-Beta negatively regulates the BMP2-dependent early commitment
of periodontal ligament cells into hard tissue forming cells. PLoS
One. 10:e01255902015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aukkarasongsup P, Haruyama N, Matsumoto T,
Shiga M and Moriyama K: Periostin inhibits hypoxia-induced
apoptosis in human periodontal ligament cells via TGF-beta
signaling. Biochem Biophys Res Commun. 441:126–132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu H, He Y, Feng JQ, Shu R, Liu Z, Li J,
Wang Y, Xu Y, Zeng H, Xu X, et al: Wnt3α and transforming growth
factor-β induce myofibroblast differentiation from periodontal
ligament cells via different pathways. Exp Cell Res. 353:55–62.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo F, Carter DE and Leask A: Mechanical
tension increases CCN2/CTGF expression and proliferation in
gingival fibroblasts via a TGFβ-dependent mechanism. PLoS One.
6:e197562011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeon YM, Kook SH, Son YO, Kim EM, Park SS,
Kim JG and Lee JC: Role of MAPK in mechanical force-induced
up-regulation of type I collagen and osteopontin in human gingival
fibroblasts. Mol Cell Biochem. 320:45–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ravi M, Paramesh V, Kaviya SR, Anuradha E
and Solomon FD: 3D cell culture systems: Advantages and
applications. J Cell Physiol. 230:16–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sachar A, Strom TA, San Miguel S, Serrano
MJ, Svoboda KK and Liu X: Cell-matrix and cell-cell interactions of
human gingival fibroblasts on three-dimensional nanofibrous gelatin
scaffolds. J Tissue Eng Regen Med. 8:862–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei G, Jin Q, Giannobile WV and Ma PX:
Nano-fibrous scaffold for controlled delivery of recombinant human
PDGF-BB. J Control Release. 112:103–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harrington H, Rose FR, Aylott JW and
Ghaemmaghami AM: Self-reporting scaffolds for 3-dimensional cell
culture. J Vis Exp. e506082013.PubMed/NCBI
|
|
20
|
Thadavirul N, Pavasant P and Supaphol P:
Improvement of dual-leached polycaprolactone porous scaffolds by
incorporating with hydroxyapatite for bone tissue regeneration. J
Biomater Sci Polym Ed. 25:1986–2008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang KL, Lee SW, Ahn YS, Kim SH and Kang
YG: Bioinformatic analysis of responsive genes in two-dimension and
three-dimension cultured human periodontal ligament cells subjected
to compressive stress. J Periodontal Res. 48:87–97. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramos T, Ahmed M, Wieringa P and Moroni L:
Schwann cells promote endothelial cell migration. Cell Adh Migr.
9:441–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang L, Yang Y, Wang S, Li Y and Zhao Z:
In vitro mechanical loading models for periodontal ligament cells:
From two-dimensional to three-dimensional models. Arch Oral Biol.
60:416–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ugolini GS, Pavesi A, Rasponi M, Fiore GB,
Kamm R and Soncini M: Human cardiac fibroblasts adaptive responses
to controlled combined mechanical strain and oxygen changes in
vitro. Elife. 6(pii): e228472017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riddle RC, Leslie JM, Gross TS and Clemens
TL: Hypoxia-inducible factor-1alpha protein negatively regulates
load-induced bone formation. J Biol Chem. 286:44449–44456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kook SH, Jang YS and Lee JC: Human
periodontal ligament fibroblasts stimulate osteoclastogenesis in
response to compression force through TNF-α-mediated activation of
CD4+ T cells. J Cell Biochem. 112:2891–2901. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kook SH, Son YO, Hwang JM, Kim EM, Lee CB,
Jeon YM, Kim JG and Lee JC: Mechanical force inhibits
osteoclastogenic potential of human periodontal ligament
fibroblasts through OPG production and ERK-mediated signaling. J
Cell Biochem. 106:1010–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwarz A: Tissue changes incident to
orthodontic tooth movement. Int J Orthodontics. 18:331–352.
1932.
|
|
30
|
Li M, Yi J, Yang Y, Zheng W, Li Y and Zhao
Z: Investigation of optimal orthodontic force at the cellular level
through three-dimensionally cultured periodontal ligament cells.
Eur J Orthod. 38:366–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang JH and Thampatty BP: An introductory
review of cell mechanobiology. Biomech Model Mechanobiol. 5:1–16.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krishnan V and Davidovitch Z: Cellular,
molecular, and tissue-level reactions to orthodontic force. Am J
Orthod Dentofacial Orthop. 129:469.e1–e32. 2006. View Article : Google Scholar
|
|
33
|
Cotrim P, Martelli-Junior H, Graner E,
Sauk JJ and Coletta RD: Cyclosporin A induces proliferation in
human gingival fibroblasts via induction of transforming growth
factor-beta1. J Periodontol. 74:1625–1633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Komatsu Y, Ibi M, Chosa N, Kyakumoto S,
Kamo M, Shibata T, Sugiyama Y and Ishisaki A: Zoledronic acid
suppresses transforming growth factor-β-induced fibrogenesis by
human gingival fibroblasts. Int J Mol Med. 38:139–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Komatsu Y, Ibi M, Chosa N, Kyakumoto S,
Kamo M, Shibata T, Sugiyama Y and Ishisaki A: Zoledronic acid
suppresses transforming growth factor-β-induced fibrogenesis by
human gingival fibroblasts. Int J Mol Med. 38:139–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Watanabe T, Yasue A and Tanaka E:
Hypoxia-inducible factor-1α is required for transforming growth
factor-β1-induced type I collagen, periostin and α-smooth muscle
actin expression in human periodontal ligament cells. Arch Oral
Biol. 59:595–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kimoto S, Matsuzawa M, Matsubara S,
Komatsu T, Uchimura N, Kawase T and Saito S: Cytokine secretion of
periodontal ligament fibroblasts derived from human deciduous
teeth: Effect of mechanical stress on the secretion of transforming
growth factor-beta 1 and macrophage colony stimulating factor. J
Periodontal Res. 34:235–243. 1999. View Article : Google Scholar : PubMed/NCBI
|