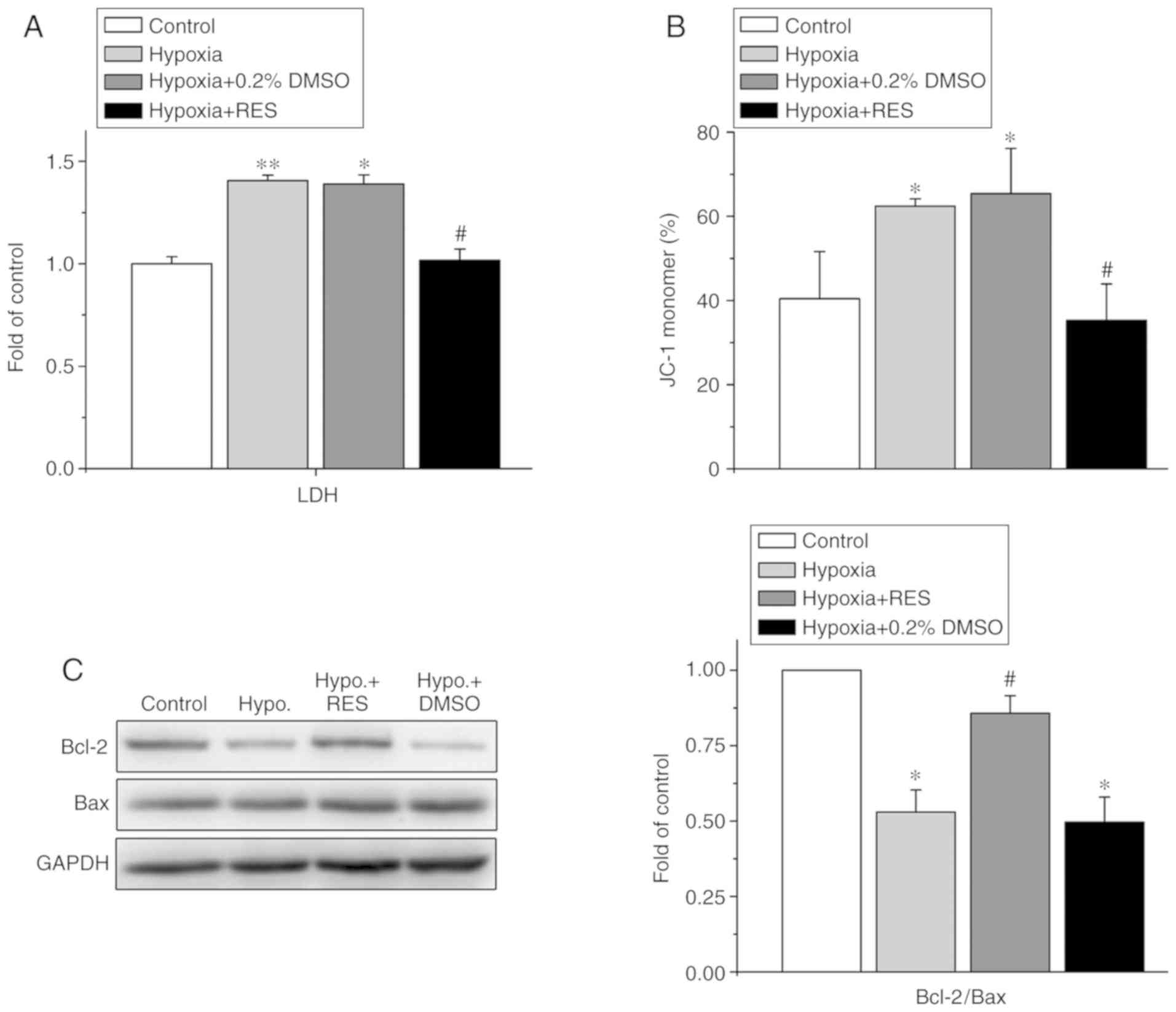
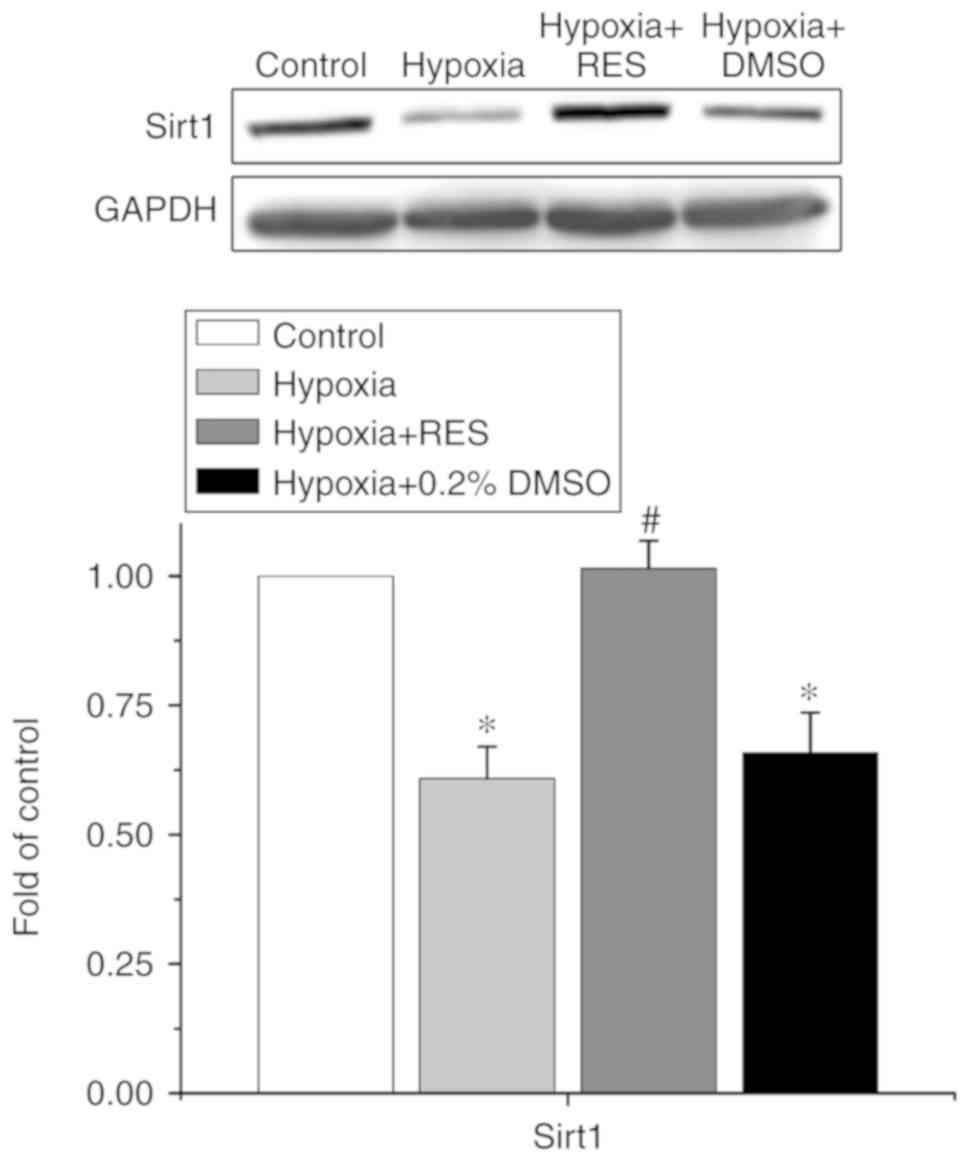
|
1
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paradies G, Paradies V, Ruggiero FM and
Petrosillo G: Mitochondrial bioenergetics and cardiolipin
alterations in myocardial ischemia/reperfusion injury. Implications
for pharmacological cardioprotection. Am J Physiol Heart Circ
Physiol. Aug 10–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cadenas S: ROS and redox signaling in
myocardial ischemia-reperfusion injury and cardioprotection. Free
Radic Biol Med. 117:76–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suen DF, Norris KL and Youle RJ:
Mitochondrial dynamics and apoptosis. Genes Dev. 22:1577–1590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
von Harsdorf R, Li PF and Dietz R:
Signaling pathways in reactive oxygen species-induced cardiomyocyte
apoptosis. Circulation. 99:2934–2941. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonnefont-Rousselot D: Resveratrol and
cardiovascular diseases. Nutrients. 8(pii): E2502016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nicholson SK, Tucker GA and Brameld JM:
Effects of dietary polyphenols on gene expression in human vascular
endothelial cells. Proc Nutr Soc. 67:42–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrovski G, Gurusamy N and Das DK:
Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci.
1215:22–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolinsky VW and Dyck JR: Calorie
restriction and resveratrol in cardiovascular health and disease.
Biochim Biophys Acta. 1812:1477–1489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ge L, Li C, Wang Z, Zhang Y and Chen L:
Suppression of oxidative stress and apoptosis in electrically
stimulated neonatal rat cardiomyocytes by resveratrol and
underlying mechanisms. J Cardiovasc Pharmacol. 70:396–404.
2017.PubMed/NCBI
|
|
12
|
Meng Z, Jing H, Gan L, Li H and Luo B:
Resveratrol attenuated estrogen-deficient-induced cardiac
dysfunction: Role of AMPK, SIRT1, and mitochondrial function. Am J
Transl Res. 8:2641–2649. 2016.PubMed/NCBI
|
|
13
|
Wu Z, Huang A, Yan J, Liu B, Liu Q, Zhang
J, Zhang X, Ou C and Chen M: Resveratrol ameliorates cardiac
dysfunction by inhibiting apoptosis via the PI3K/Akt/FoxO3a pathway
in a rat model of diabetic cardiomyopathy. J Cardiovasc Pharmacol.
70:184–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Della-Morte D, Dave KR, DeFazio RA, Bao
YC, Raval AP and Perez-Pinzon MA: Resveratrol pretreatment protects
rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling
protein 2 pathway. Neuroscience. 159:993–1002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gurusamy N, Lekli I, Mukherjee S, Ray D,
Ahsan MK, Gherghiceanu M, Popescu LM and Das DK: Cardioprotection
by resveratrol: A novel mechanism via autophagy involving the
mTORC2 pathway. Cardiovasc Res. 86:103–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma XQ, Fu RF, Feng GQ, Wang ZJ, Ma SG and
Weng SA: Hypoxia-reoxygenation-induced apoptosis in cultured
neonatal rat cardiomyocyets and the protective effect of
prostaglandin E. Clin Exp Pharmacol Physiol. 32:1124–1130. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Addabbo F, Montagnani M and Goligorsky MS:
Mitochondria and reactive oxygen species. Hypertension. 53:885–892.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu XR, Li T, Cao L, Yu YY, Chen LL, Fan
XH, Yang BB and Tan XQ: Dexmedetomidine attenuates H2O2-induced
neonatal rat cardiomyocytes apoptosis through mitochondria- and
ER-medicated oxidative stress pathways. Mol Med Rep. 17:7258–7264.
2018.PubMed/NCBI
|
|
19
|
Tatlidede E, Sehirli O, Velioğlu-Oğünc A,
Cetinel S, Yeğen BC, Yarat A, Süleymanoğlu S and Sener G:
Resveratrol treatment protects against doxorubicin-induced
cardiotoxicity by alleviating oxidative damage. Free Radic Res.
43:195–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bargiela D, Burr SP and Chinnery PF:
Mitochondria and hypoxia: Metabolic crosstalk in cell-fate
decisions. Trends Endocrinol Metab. 29:249–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li F, You Y and Zhu H: 15-HETE protects
pulmonary artery smooth muscle cells against apoptosis via SIRT1
regulation during hypoxia. Biomed Pharmacother. 108:325–330. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu XR, Cao L, Li T, Chen LL, Yu YY, Huang
WJ, Liu L and Tan XQ: Propofol attenuates
H2O2-induced oxidative stress and apoptosis
via the mitochondria- and ER-medicated pathways in neonatal rat
cardiomyocytes. Apoptosis. 22:639–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burgoyne JR, Mongue-Din H, Eaton P and
Shah AM: Redox signaling in cardiac physiology and pathology. Circ
Res. 111:1091–1106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rajendran P, Nandakumar N, Rengarajan T,
Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J and
Nishigaki I: Antioxidants and human diseases. Clin Chim Acta.
436:332–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Gao M, Chen J, Yang Z, Sun J, Wang
Z, Huang X, Yuan T, Shen X and Xian S: Resveratrol ameliorates
pressure overload-induced cardiac dysfunction and attenuates
autophagy in rats. J Cardiovasc Pharmacol. 66:376–382. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong Q, Wu Z, Li X, Yan J, Zhao L, Yang C,
Lu J, Deng J and Chen M: Resveratrol ameliorates cardiac
dysfunction induced by pressure overload in rats via structural
protection and modulation of Ca(2+) cycling proteins. J Transl Med.
12:3232014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou L and Chang DC: Dynamics and
structure of the Bax-Bak complex responsible for releasing
mitochondrial proteins during apoptosis. J Cell Sci. 121:2186–2196.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo R, Liu W, Liu B, Zhang B, Li W and Xu
Y: SIRT1 suppresses cardiomyocyte apoptosis in diabetic
cardiomyopathy: An insight into endoplasmic reticulum stress
response mechanism. Int J Cardiol. 191:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen CJ, Yu W, Fu YC, Wang X, Li JL and
Wang W: Resveratrol protects cardiomyocytes from hypoxia-induced
apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res
Commun. 378:389–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sin TK, Tam BT, Yung BY, Yip SP, Chan LW,
Wong CS, Ying M, Rudd JA and Siu PM: Resveratrol protects against
doxorubicin-induced cardiotoxicity in aged hearts through the
SIRT1-USP7 axis. J Physiol. 593:1887–1899. 2015. View Article : Google Scholar : PubMed/NCBI
|