Introduction
Glaucoma is a common ophthalmic disease, and it is
the second leading cause of blindness worldwide (1). It is estimated that by 2020, the
number of patients with glaucoma will reach approximately 80
million (2). Glaucoma can be
divided into open-angle glaucoma (OAG) and angle-closure glaucoma
(ACG), and in Western countries, approximately 75% of glaucoma
patients are diagnosed with OAG (3), while in Asian countries, ACG is more
prevalent than OAG (4). In 2010,
86.5% of ACG patients were resident in Asia, and the largest number
of patients affected by ACG was in China (47.5% of the total)
(5). In recent years, increasing
evidence has indicated that the oxidative stress-induced senescence
of trabecular meshwork cells (TMCs) may play a key role in the
occurrence and development of glaucoma (6,7);
however, the specific mechanisms still require further
investigation.
MicroRNAs (miRNAs) are short non-coding RNAs
(length, ~22 nucleotides) that can bind to the 3′-UTR of target
mRNAs and consequently suppress the expression of target genes.
miRNAs can regulate a variety of biological activities, including
embryonic development, angiogenesis, cell differentiation,
proliferation and apoptosis (8–10).
With the development of next-generation sequencing and
bioinformatics methods, differentially expressed miRNAs that are
potentially involved in the pathogenesis of diseases, including
cancers (11,12) and glaucoma (13–15),
have been successfully identified. The targets of these miRNAs, as
well as related signaling pathways, have also been predicted.
miR-17-5p is located on human chromosome 13q31, and it belongs to
the miR-17-92 cluster (16).
miR-17-5p is up-regulated in many tumor types and participates in
the pathogenesis of different cancers as an oncogene (17–19).
Besides cancer-related studies, miR-17-5p has been reported to
promote oxidative stress-induced apoptosis of cardiomyocytes in
ischemia/reperfusion-induced cardiac injury animal models (20). It has also been observed that
knockdown of miR-17-5p can restore the expression of the
very-low-density lipoprotein receptor and alleviate the symptoms of
atherosclerosis in ApoE-/- mouse models (21). It has been observed that miR-17-5p
is down-regulated in the trabecular meshwork under oxidative stress
(22); however, the specific
mechanisms have not yet been investigated.
In the present study, we focused on the roles of
miR-17-5p in glaucoma and its related mechanisms. Human TMCs
(HTMCs) were treated with H2O2 to induce
oxidative stress, and the effects of miR-17-5p on the behaviors of
HTMCs under oxidative stress were explored. We hypothesized that
miR-17-5p would regulate the proliferation and apoptosis of HTMCs
by targeting phosphatase and tensin homolog (PTEN). The present
study may provide a theoretical basis and novel therapeutic target
for the treatment of glaucoma.
Materials and methods
HTMC culture
HTMCs were purchased from Sciencell Research
Laboratories (San Diego, CA, USA). According to the product
information, HTMCs were isolated from the juxtacanalicular and
corneoscleral regions of human eyes, and characterized by
immunofluorescence methods using α-smooth muscle actin- and
fibronectin-specific antibodies. Cells were cultured in a
humidified incubator at 37°C with 5% CO2 using
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplied with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin solution (Gibco; Thermo Fisher Scientific,
Inc.). Oxidative stress was induced by treating HTMCs with DMEM
that contained 300 µM H2O2 (Beyotime
Institute of Biotechnology, Shanghai, China) for 3–7 h. Then, the
cells were harvested for future analysis.
Cell transfection
The miR-17-5p inhibitor and miR-17-5p mimic
oligonucleotides were synthesized by GenePharma Co., Ltd.,
(Shanghai, China). HTMCs were cultured until confluence,
trypsinized and seeded onto new six-well plates at 100,000
cells/well. Cells were then transfected with either miR-17-5p
inhibitor or miR-17-5p mimic using Lipofectamine® 3000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. After transfection, cells were
cultured for 48 h and harvested for future analysis.
Cell proliferation analysis
An MTT assay was performed to determine the
viability of HTMCs following different treatments. An MTT
proliferation assay kit (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was used, according to the manufacturer's protocol.
Cellular apoptosis analysis
For cellular apoptosis analysis, HTMCs subjected to
different treatments were stained using an Annexin V-FITC apoptosis
detection kit (Sigma-Aldrich; Merck KGaA). The apoptosis rate of
the cells in different groups was detected and analyzed using a BD
FACSVerse flow cytometer (BD Biosciences, San Jose, CA, USA),
according to the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from HTMCs using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.), and reverse
transcribed into cDNA using the PrimeScript™ RT Master Mix (Perfect
Real Time) kit (Takara Biotechnology Co., Ltd., Dalian, China),
then qPCR was performed using the SYBR ExScript RT-PCR kit (Takara
Biotechnology Co., Ltd.) on an ABI 7300 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The thermocycling profiles are shown
in Table I. The relative
expression of PTEN in each sample was normalized to the level of
GAPDH using the 2−ΔΔCq method. The expression of
miR-17-5p was examined using the Hairpin-it™ miRNAs qPCR
Quantitation Kit (GenePharma Co., Ltd.) according to the
manufacturer's protocol, and U6 (RNU6B; GenePharma Co., Ltd.) was
used for normalization. Primers were synthesized by GenScript
(Nanjing, China), and the sequences of the primers are shown in
Table II.
 | Table I.Thermocycling profiles for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Thermocycling profiles for reverse
transcription-quantitative polymerase chain reaction.
| Cycle no. | Temperature | Time |
|---|
| 1 | 95°C | 30 Sec |
| 40 | 95°C | 5
Sec |
|
| 60°C | 4
Sec |
 | Table II.Sequences of the primers used in the
present study. |
Table II.
Sequences of the primers used in the
present study.
| Gene name | Sequences of the
primer |
|---|
| miR-17-5p | F:
5′-TCTAGATCCCGAGGACTG-3′ |
|
| R:
5′-ATCGTGACCTGAACC-3′ |
| U6 | F:
5′-CTCGCTTTGGCAGCACA-3′ |
|
| R:
5′-AACGCTTCACGAATTTGCGT-3′ |
| PTEN | F:
5′-CGACGGGAAGACAAGTTCAT-3′ |
|
| R:
5′-AGGTTTCCTCTGGTCCTGGT-3′ |
| GAPDH | F:
5′-GACAGTCAGCCGCATCTTCT-3′ |
|
| R:
5′-TTAAAAGCAGCCCTGGTGAC-3′ |
Western blot analysis
Cells were lysed using RIPA buffer (Beyotime
Institute of Biotechnology), and the concentration of total protein
was measured using a BCA Kit (Beyotime Institute of Biotechnology).
Next, SDS-PAGE was performed to separate the proteins, and the
proteins were then transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% non-fat milk and incubated with primary antibodies
(anti-Bax, anti-PTEN, anti-Bcl-2, anti-Bcl-xL and anti-GAPDH, all
purchased from Abcam, Cambridge, MA, USA) overnight at 4°C. The
following day, the membranes were incubated with HRP-conjugated
secondary antibodies (purchased from Abcam, Cambridge, MA, USA),
then washed and incubated with the enhanced chemiluminescence
reagent (Beyotime Institute of Biotechnology). Finally, the signals
were detected using the ChemiDoc™XRS+ imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Immunocytochemistry
HTMCs were fixed in 4% paraformaldehyde and
permeabilized with 0.1% Triton X-100, then incubated with the
anti-PTEN primary antibodies for 30 min. Cells were incubated with
Alexa Fluor 488-conjugated secondary antibodies, then visualized
using an Olympus fluorescent microscope (Olympus Corporation,
Tokyo, Japan).
Computational methods
The target genes of miR-17-5p were predicted using
online bioinformatics tools, TargetScan (http://www.targetscan.org) and miRanda (http://www.microrna.org).
Dual luciferase reporter assay
Fragments of either the wild-type PTEN 3′-UTR
(PTEN-3′UTR) or mutant PTEN 3′-UTR (PTEN-MUT) region that contains
the miR-17-5p binding site were synthesized and cloned into the
pGL6-TA-reporter plasmid (Beyotime Institute of Biotechnology). The
plasmids were transfected into 293 cells using
Lipofectamine® 3000 for 48 h, and the activity of the
luciferase in each group was examined using the dual-luciferase
reporter system (Beyotime Institute of Biotechnology), according to
the manufacturer's protocol.
Statistics
All statistical analyses were conducted using SPSS
v.22.0 software (IBM Corp., Armonk, NY, USA). Data are presented as
the mean ± standard deviation. Two independent sample t-tests were
performed for comparisons between two groups. One-way analysis of
variance followed by Dunnett's post-hoc test was performed for
comparisons among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of oxidative stress on the
proliferation and apoptosis of HTMCs in vitro
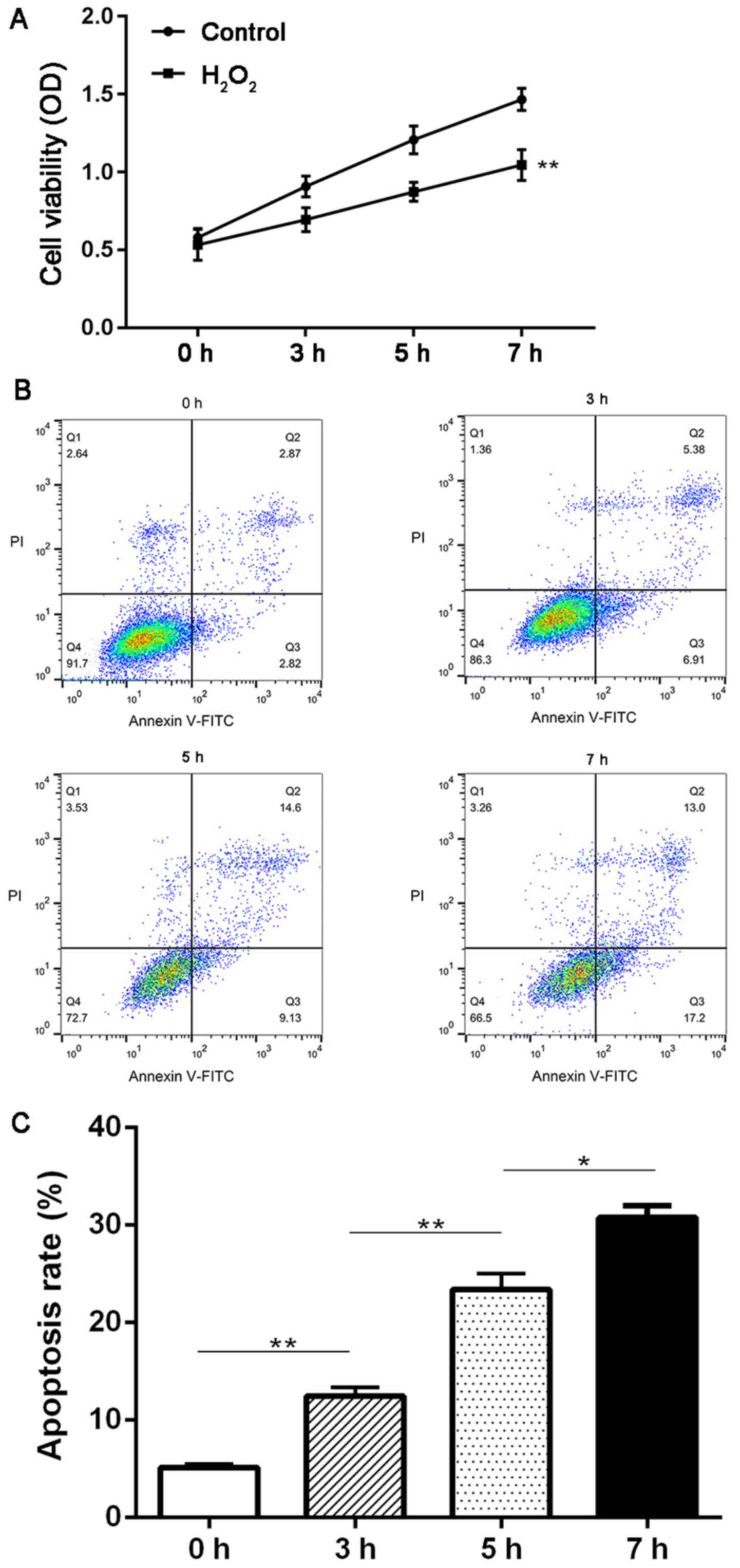
First, we investigated the effect of
H2O2 treatment on the proliferation and
apoptosis of HTMCs using MTT and flow cytometry methods. It was
observed that the cell viability was significantly decreased in
cells treated with H2O2 compared with the
control group at 3, 5 and 7 h (P<0.01; Fig. 1A). Moreover,
H2O2 treatment also induced a continuous
increase in the apoptosis rate of HTMCs from 0 to 7 h (P<0.05;
Fig. 1B and C).
miR-17-5p was down-regulated in HTMCs
under oxidative stress in vitro
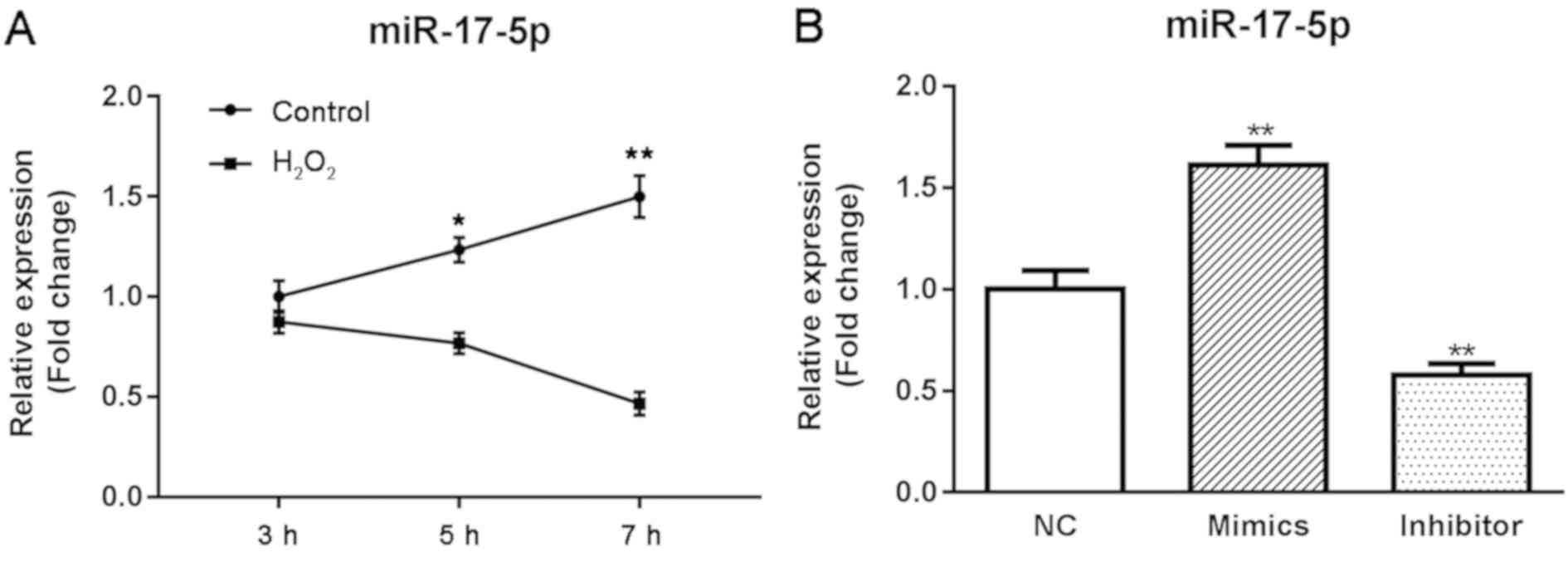
In order to explore the roles of miR-17-5p in HTMCs
under oxidative stress, we compared the expression of miR-17-5p in
control and H2O2 groups at different time
points using RT-qPCR methods. As shown in Fig. 2A, H2O2
induced a significant decrease in the expression of miR-17-5p at 5
and 7 h (P<0.05; Fig. 2A).
miR-17-5p can regulate the
proliferation and apoptosis of HTMCs in vitro
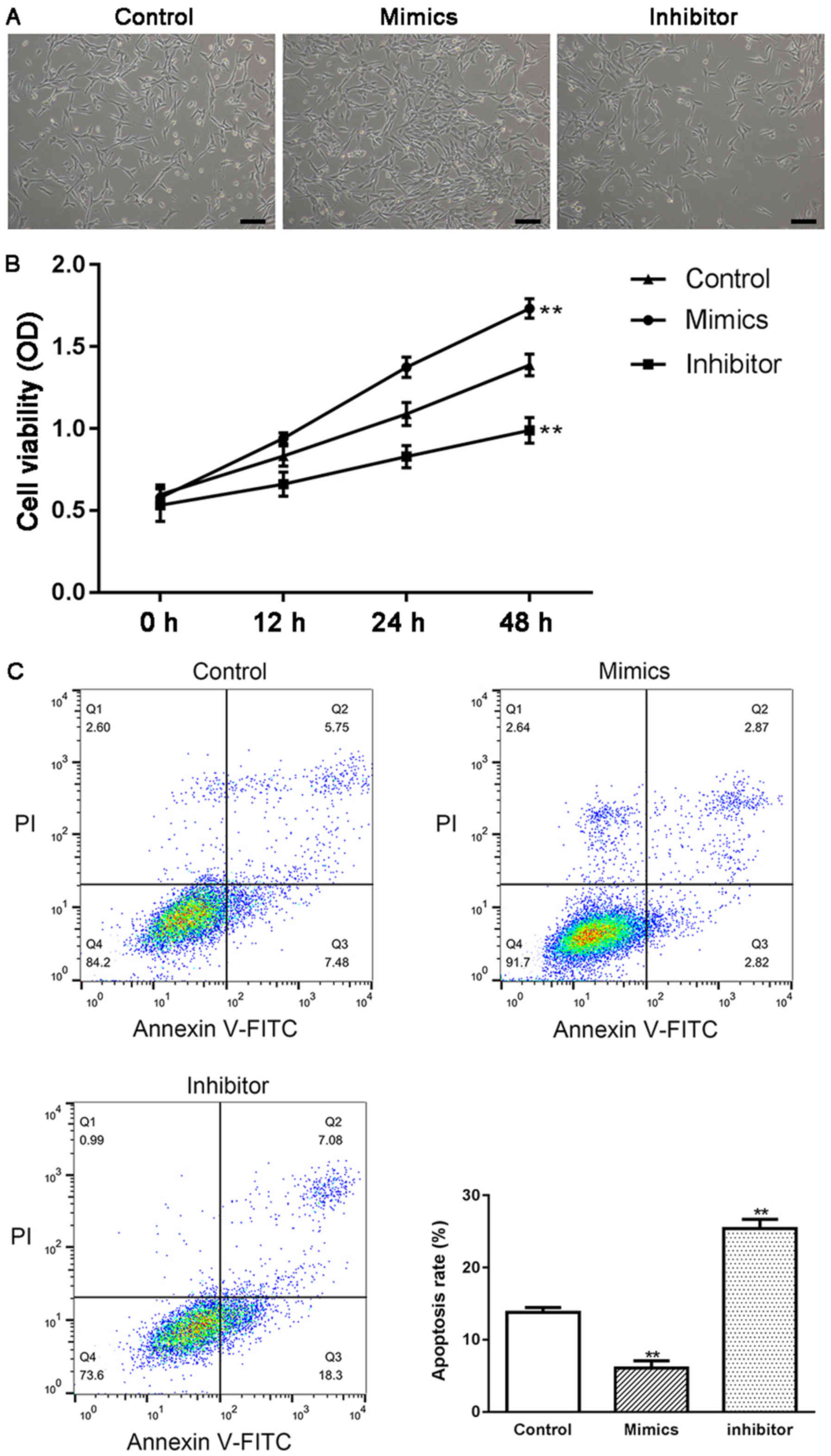
HTMCs were transfected with either miR-17-5p mimics
or miR-17-5p inhibitor, and the effect of miR-17-5p on the
proliferation and apoptosis of HTMCs was examined using MTT and
flow cytometry methods. As shown in Fig. 2B, the expression of miR-17-5p was
significantly increased in miR-17-5p mimic-transfected HTMCs and
markedly decreased in miR-17-5p inhibitor-transfected HTMCs,
suggesting that transfection had been successfully performed.
Moreover, transient overexpression of miR-17-5p induced a
significant increase in the proliferation (P<0.01; Fig. 3A and B) and a significant decrease
in the apoptosis of HTMCs (P<0.01; Fig. 3C). Knockdown of miR-17-5p exhibited
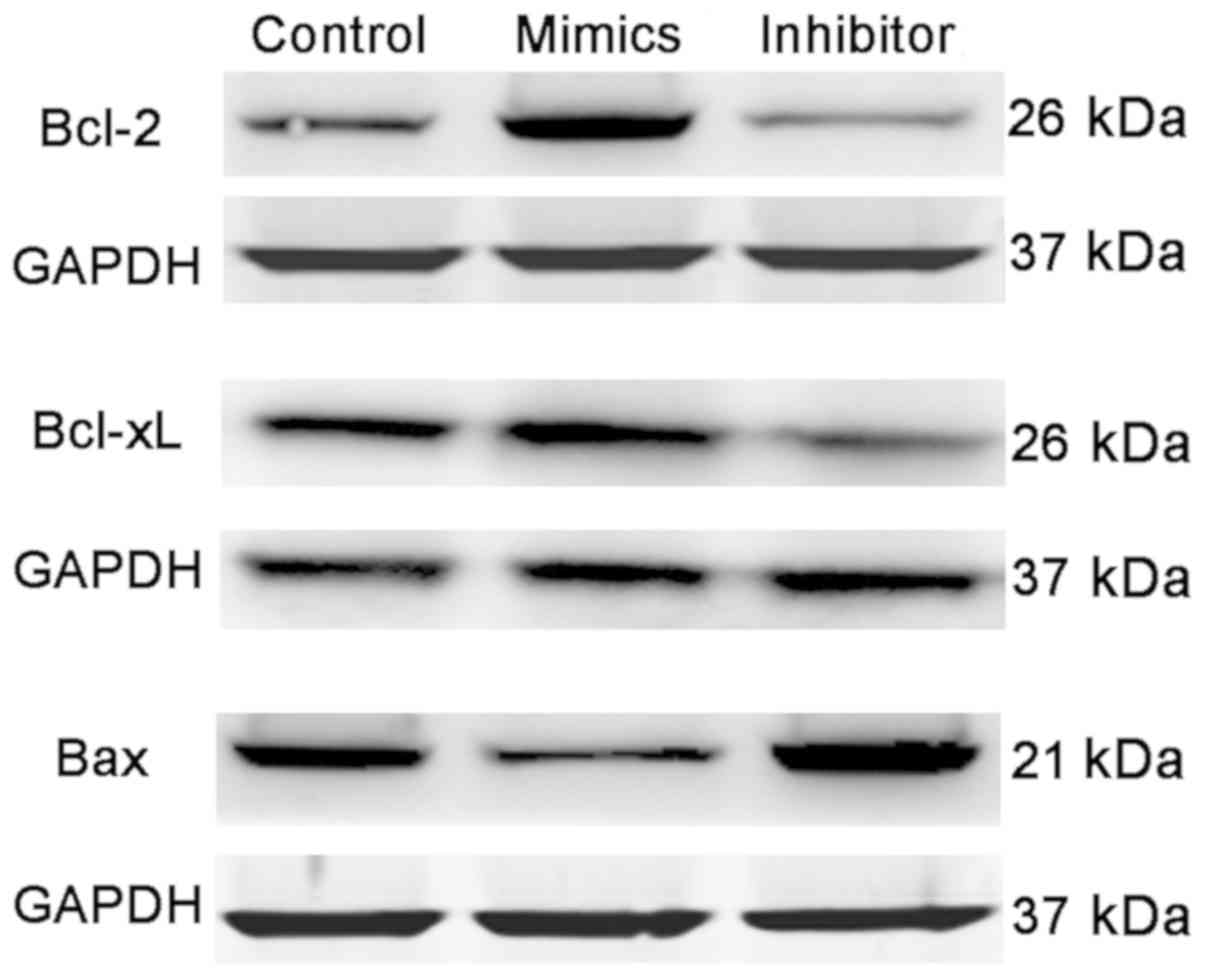
the opposite results. In addition, transfection of miR-17-5p mimics
also induced a significant increase in the expression of Bcl-2 and
Bcl-xL, and a marked decrease in the expression of Bax, while
knockdown of miR-17-5p exhibited the opposite results (Fig. 4).
PTEN is a direct target of miR-17-5p
in HTMCs
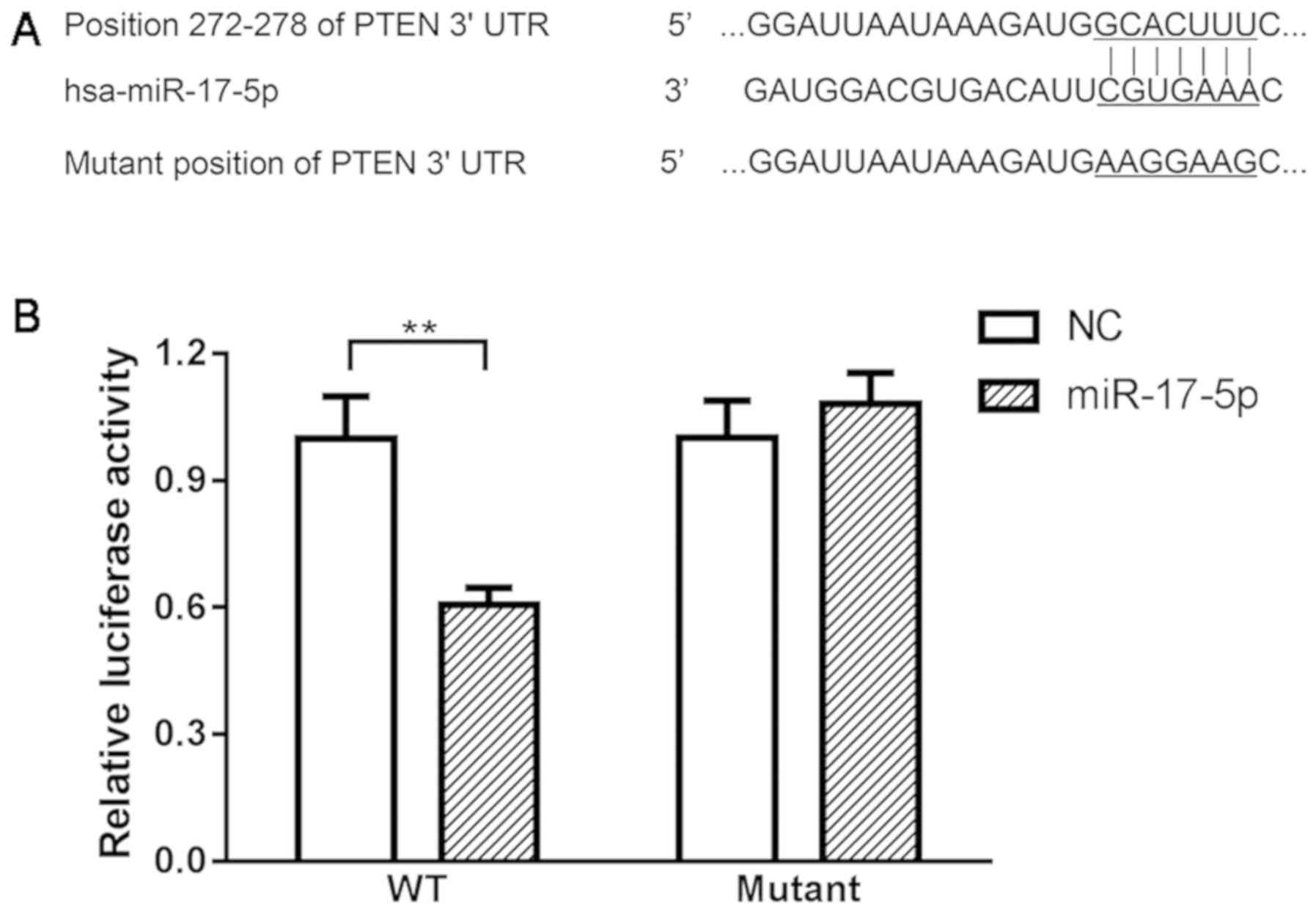
Using online bioinformatics tools, PTEN was
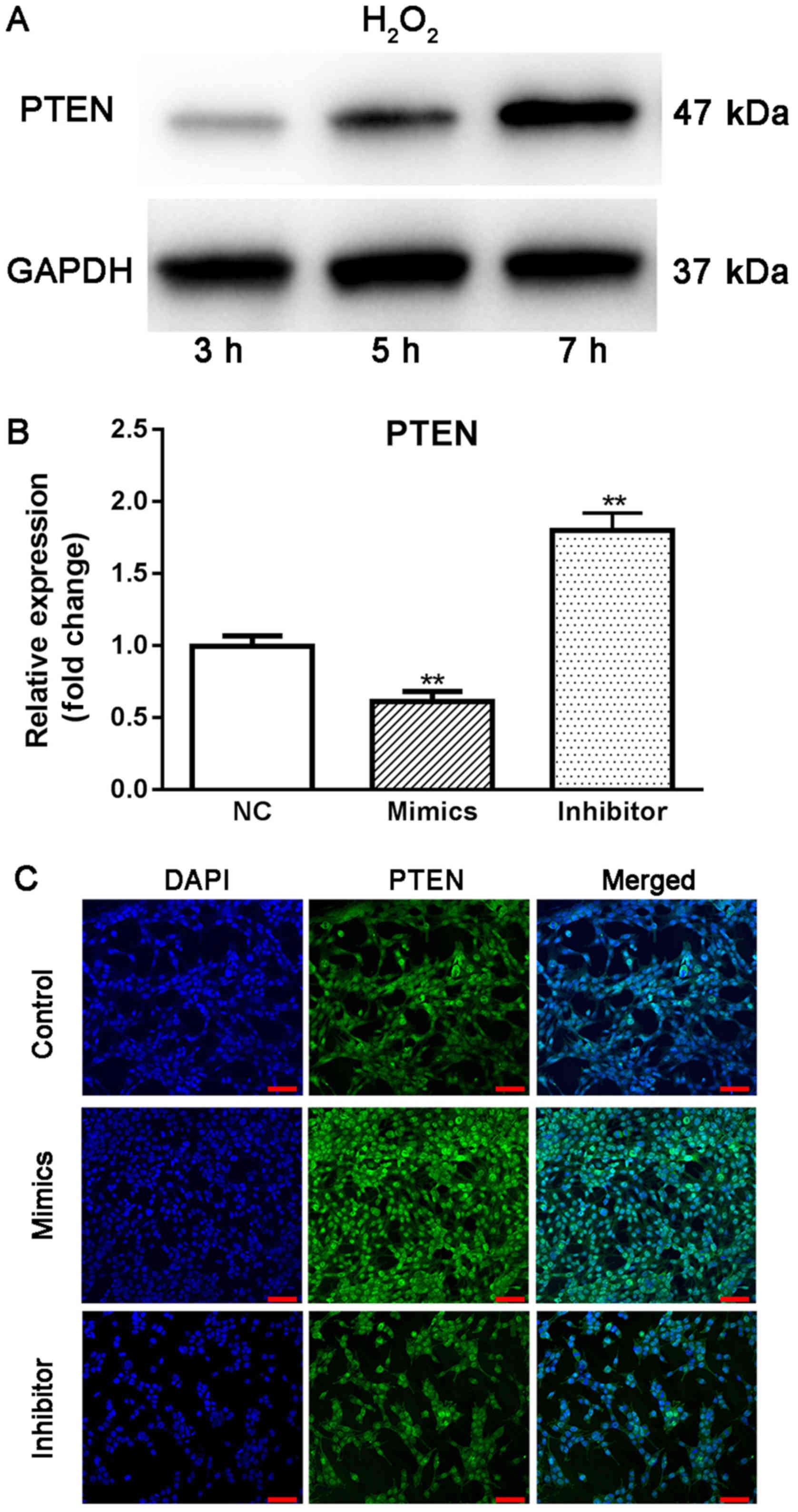
predicted to be a direct target of miR-17-5p. Using western blot
analysis, it was observed that the expression of PTEN continuously
increased from 3 to 7 h under oxidative stress (Fig. 5A), suggesting that increased PTEN
may be associated with the pathogenesis of glaucoma. Moreover,
transient overexpression of miR-17-5p in HTMCs induced a
significant decrease in the expression of PTEN, while knockdown of
miR-17-5p promoted the expression of PTEN in HTMCs (Fig. 5B and C). Finally, the results from
the dual luciferase reporter assay indicated that co-transfection
of miR-17-5p mimics and wild-type PTEN-3′UTR plasmids significantly
decreased the activity of the luciferases. Co-transfection of
miR-17-5p mimic and PTEN-MUT exhibited no effect on the activity of
the luciferases (Fig. 6),
suggesting that PTEN is a direct target of miR-17-5p.
Discussion
Glaucoma is a common ophthalmic disorder that can
cause damage to the optic nerve and loss of vision. Increasing
evidence has indicated that oxidative stress-induced senescence of
HTMCs is involved in the pathogenesis of glaucoma (7). H2O2 is an
important oxidant in the human eye. It has been demonstrated that
oxidative levels significantly increase in the aqueous humor of
patients with glaucoma, which means that TMCs will also be exposed
to high concentrations of H2O2. Thus, in
previous studies, a high concentration of
H2O2 has been used to induce oxidative stress
in HTMCs, and this has now become a widely applied in vitro
model in glaucoma-related studies (23,24).
In recent years, the effects of miRNAs on TMCs have
been discussed in many studies (25,26).
Li et al (22), performed
miRNA array analysis to examine alterations in the expression
levels of microRNAs in oxidative stress-induced cellular senescence
in HTMCs. It was observed that the expression of miR-17-5p was
significantly decreased by treatment with a high concentration of
H2O2. However, the specific mechanisms
underlying this remain unclear. In the present study, we identified
that compared with untreated cells, H2O2
treatment induced a significant decrease in the proliferation and a
marked increase in the apoptosis of HTMCs at each time point
examined (P<0.05). Moreover, H2O2
treatment also induced a significant decrease in the expression of
miR-17-5p, which was consistent with the finding from Li et
al. These results indicate that miR-17-5p is down-regulated in
HTMCs under oxidative stress, and miR-17-5p may participate in the
mechanism of H2O2-induced increase in
apoptosis and decrease in the proliferation of HTMCs.
Next, we transfected HTMCs with either miR-17-5p
mimics or miR-17-5p inhibitor and evaluated the effect of miR-17-5p
on the proliferation and apoptosis of HTMCs. We observed that
transient overexpression of miR-17-5p induced a significant
increase in the proliferation and a significant decrease in the
apoptosis of HTMCs (P<0.05), while knockdown of miR-17-5p
exhibited the opposite results. Moreover, Bcl-2 and Bcl-xL are
known to be anti-apoptotic proteins, and Bax is a pro-apoptotic
protein (27,28). Our data indicated that transfection
of miR-17-5p mimics promoted the expression of Bcl-2 and Bcl-xL,
and suppressed the expression of Bax, while knockdown of miR-17-5p
exhibited the opposite effects. Taken together, these results
indicate that miR-17-5p can regulate the proliferation and
apoptosis of HTMCs in vitro.
PTEN is known to be a tumor suppressor in the field
of cancer studies (29). Knockdown
of PTEN can lead to an increase in the proliferation and a decrease
in the apoptosis rate of many cancer cell types, leading to the
incidence and development of various diseases (30–32).
However, in the case of glaucoma, PTEN may play a different role.
It has been observed that increased expression of PTEN can lead to
the degeneration of optic neurons, and knockdown of PTEN can
significantly alleviate optic nerve damage in glaucoma. In a very
recent study, Tellios et al (33) demonstrated that phosphorylation of
PTEN is significantly increased in TMCs upon treatment with TGF-β
(increased levels of TGF-β in the aqueous humor is one of the main
causes of fibrosis in OAG), and an increase in the expression of
PTEN may contribute to the fibrosis of the HTMCs in glaucoma. In
the present study, we observed that the expression of PTEN was
markedly increased in HTMCs following exposure to
H2O2 (P<0.01), suggesting that increased
PTEN may be involved in the pathogenesis of glaucoma. Moreover,
transfection of miR-17-5p mimic or inhibitor induced a significant
decrease or increase in the expression of PTEN, respectively. The
results from the dual luciferase reporter assay confirmed that PTEN
is a direct target of miR-17-5p. In summary, these data indicated
that miR-17-5p can regulate the proliferation and apoptosis of
HTMCs by targeting PTEN.
The present study has certain limitations. We only
performed cellular experiments, and the expression levels of
miR-17-5p in tissue samples from patients and normal controls
should be evaluated to confirm our findings from the clinical
perspective. However, due to ethical issues, it is difficult to
obtain the trabecular meshwork of healthy volunteers; thus, in
vivo studies using animal models could be a better option.
In conclusion, our data demonstrated that miR-17-5p
is down-regulated and PTEN is up-regulated in HTMCs under oxidative
conditions. We also identified that miR-17-5p can regulate the
proliferation and apoptosis of HTMCs through targeting PTEN. Our
data indicated that miR-17-5p has the potential to become a novel
therapeutic target for the treatment of glaucoma.
Acknowledgements
Not applicable.
Funding
The persent study was sponsored by the funds from
the project of the Department of Health, Heilongjiang Province
(project no. 2011-005).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ designed the study and wrote the majority of the
manuscript. XW performed most of the experiments and wrote part of
the manuscript. ZL and JB performed some of the experiments. WS
performed some of the statistical analysis.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao R, Yin D, Wang E and Si B: The effect
of MTHFR ala222val polymorphism on open-angle glaucoma: a
meta-analysis. Ophthalmic Genet. 36:27–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tham YC, Li X, Wong TY, Quigley HA, Aung T
and Cheng CY: Global prevalence of glaucoma and projections of
glaucoma burden through 2040: A systematic review and
meta-analysis. Ophthalmology. 121:2081–2090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bailey JN, Loomis SJ, Kang JH, Allingham
RR, Gharahkhani P, Khor CC, Burdon KP, Aschard H, Chasman DI, Igo
RP Jr, et al: Genome-wide association analysis identifies TXNRD2,
ATXN2 and FOXC1 as susceptibility loci for primary open-angle
glaucoma. Nat Genet. 48:189–194. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang YB, Wang NL, Rong SS and Thomas R:
Initial treatment for primary angle-closure glaucoma in China. J
Glaucoma. 24:469–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nongpiur ME, Khor CC, Jia H, Cornes BK,
Chen LJ, Qiao C, Nair KS, Cheng CY, Xu L, George R, et al: ABCC5, a
gene that influences the anterior chamber depth, is associated with
primary angle closure glaucoma. PLoS Genet. 10:e10040892014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen W, Han Y, Huang B, Qi Y, Xu L, Guo R,
Wang X and Wang J: MicroRNA-483-3p inhibits extracellular matrix
production by targeting Smad4 in human trabecular meshwork cells.
Invest Ophthalmol Vis Sci. 56:8419–8427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Zhang Y, Guo R, Shen W, Qi Y, Wang
Q, Guo Z, Qi C, Yin H and Wang J: HES1 promotes extracellular
matrix protein expression and inhibits proliferation and migration
in human trabecular meshwork cells under oxidative stress.
Oncotarget. 8:21818–21833. 2017.PubMed/NCBI
|
|
8
|
Feng R, Sang Q, Zhu Y, Fu W, Liu M, Xu Y,
Shi H, Xu Y, Qu R, Chai R, et al: MiRNA-320 in the human follicular
fluid is associated with embryo quality in vivo and affects mouse
embryonic development in vitro. Sci Rep. 5:86892015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Cai B, Shen L, Dong Y, Lu Q, Sun S,
Liu S, Ma S, Ma PX and Chen J: MiRNA-29b suppresses tumor growth
through simultaneously inhibiting angiogenesis and tumorigenesis by
targeting Akt3. Cancer Lett. 397:111–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qun L, Wenda X, Weihong S, Jianyang M, Wei
C, Fangzhou L, Zhenyao X and Pingjin G: miRNA-27b modulates
endothelial cell angiogenesis by directly targeting Naa15 in
atherogenesis. Atherosclerosis. 254:184–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falzone L, Candido S, Salemi R, Basile MS,
Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M and
Libra M: Computational identification of microRNAs associated to
both epithelial to mesenchymal transition and NGAL/MMP-9 pathways
in bladder cancer. Oncotarget. 7:72758–72766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bagnoli M, De Cecco L, Granata A,
Nicoletti R, Marchesi E, Alberti P, Valeri B, Libra M, Barbareschi
M, Raspagliesi F, et al: Identification of a chrXq27.3 microRNA
cluster associated with early relapse in advanced stage ovarian
cancer patients. Oncotarget. 2:1265–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo R, Shen W, Su C, Jiang S and Wang J:
Relationship between the pathogenesis of glaucoma and miRNA.
Ophthalmic Res. 57:194–199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molasy M, Walczak A, Szaflik J, Szaflik JP
and Majsterek I: MicroRNAs in glaucoma and neurodegenerative
diseases. J Hum Genet. 62:105–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paylakhi SH, Yazdani S, April C, Fan JB,
Moazzeni H, Ronaghi M and Elahi E: Non-housekeeping genes expressed
in human trabecular meshwork cell cultures. Mol Vis. 18:241–254.
2012.PubMed/NCBI
|
|
16
|
Tsuchida A, Ohno S, Wu W, Borjigin N,
Fujita K, Aoki T, Ueda S, Takanashi M and Kuroda M: miR-92 is a key
oncogenic component of the miR-17-92 cluster in colon cancer.
Cancer Sci. 102:2264–2271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Gu J, Li Y, Peng C, Shi M, Wang X,
Wei G, Ge O, Wang D, Zhang B, et al: MiR-17-5p enhances pancreatic
cancer proliferation by altering cell cycle profiles via disruption
of RBL2/E2F4-repressing complexes. Cancer Lett. 412:59–68. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao XH, Xiang Y, Yu CX, Li JP, Li H, Nie
Q, Hu P, Zhou J and Zhang TC: STAT3 is required for
MiR-17-5p-mediated sensitization to chemotherapy-induced apoptosis
in breast cancer cells. Oncotarget. 8:15763–15774. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin T, Wu X, Yang H, Liu M, He Y, He X,
Shi X, Wang F, Du S, Ma Y, et al: Association of the miR-17-5p
variants with susceptibility to cervical cancer in a Chinese
population. Oncotarget. 7:76647–76655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi J, Bei Y, Kong X, Liu X, Lei Z, Xu T,
Wang H, Xuan Q, Chen P, Xu J, et al: miR-17-3p contributes to
exercise-induced cardiac growth and protects against myocardial
ischemia-reperfusion injury. Theranostics. 7:664–676. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan L, Meng L, Shi X and Yu B: Knockdown
of microRNA-17-5p ameliorates atherosclerotic lesions in ApoE-/-
mice and restores the expression of very low density lipoprotein
receptor. Biotechnol Lett. 39:967–976. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li G, Luna C, Qiu J, Epstein DL and
Gonzalez P: Alterations in microRNA expression in stress-induced
cellular senescence. Mech Ageing Dev. 130:731–741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu AL, Fuchshofer R, Kampik A and
Welge-Lüssen U: Effects of oxidative stress in trabecular meshwork
cells are reduced by prostaglandin analogues. Invest Ophthalmol Vis
Sci. 49:4872–4880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen M, Liu B, Gao Q, Zhuo Y and Ge J:
Mitochondria-targeted peptide MTP-131 alleviates mitochondrial
dysfunction and oxidative damage in human trabecular meshwork
cells. Invest Ophthalmol Vis Sci. 52:7027–7037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Li F and Wang S: MicroRNA-93 is
overexpressed and induces apoptosis in glaucoma trabecular meshwork
cells. Mol Med Rep. 14:5746–5750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luna C, Li G, Qiu J, Epstein DL and
Gonzalez P: MicroRNA-24 regulates the processing of latent TGFβ1
during cyclic mechanical stress in human trabecular meshwork cells
through direct targeting of FURIN. J Cell Physiol. 226:1407–1414.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reshi L, Wang HV, Hui CF, Su YC and Hong
JR: Anti-apoptotic genes Bcl-2 and Bcl-xL overexpression can block
iridovirus serine/threonine kinase-induced
Bax/mitochondria-mediated cell death in GF-1 cells. Fish Shellfish
Immunol. 61:120–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J and Yao S: JNK-Bcl-2/Bcl-xL-Bax/Bak
pathway mediates the crosstalk between matrine-induced autophagy
and apoptosis via interplay with Beclin 1. Int J Mol Sci.
16:25744–25758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu H, Pan Y, Han X, Liu J and Li R:
MicroRNA-216a promotes the metastasis and epithelial-mesenchymal
transition of ovarian cancer by suppressing the PTEN/AKT pathway.
Onco Targets Ther. 10:2701–2709. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ronen S, Abbott DW, Kravtsov O, Abdelkader
A, Xu Y, Banerjee A and Iczkowski KA: PTEN loss and p27 loss differ
among morphologic patterns of prostate cancer, including
cribriform. Hum Pathol. 65:85–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng Y, Zou W, Hu C, Li G, Zhou S, He Y,
Ma F, Deng C and Sun L: Modulation of CASC2/miR-21/PTEN pathway
sensitizes cervical cancer to cisplatin. Arch Biochem Biophys.
623-624:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Shen Y, Wang M and Yang J, Lv M, Li
P, Chen Z and Yang J: Loss of PTEN expression in breast cancer:
Association with clinicopathological characteristics and prognosis.
Oncotarget. 8:32043–32054. 2017.PubMed/NCBI
|
|
33
|
Tellios N, Belrose JC, Tokarewicz AC,
Hutnik C, Liu H, Leask A, Motolko M, Iijima M and Parapuram SK:
TGF-β induces phosphorylation of phosphatase and tensin homolog:
Implications for fibrosis of the trabecular meshwork tissue in
glaucoma. Sci Rep. 7:8122017. View Article : Google Scholar : PubMed/NCBI
|