Introduction
Liver fibrosis and, ultimately, liver cirrhosis are
the common end stages of all chronic liver diseases (1). The initiation of fibrogenesis is
characterized by a chronic inflammatory condition. The activation
of immunocompetent cells (including Kupffer cells), and not virus-
or toxin-induced hepatocellular damage, is the main cause of an
increase in proinflammatory cytokines, including tumor necrosis
factor (TNF)-α, interleukin (IL)-6 and IL-12 (2). These mediators and the accumulation
of free fatty acids generate highly reactive oxygen species (ROS),
which cause oxidative stress and hepatocyte damage, leading to
hepatocellular injury (3). The
activation of mesenchymal cells, such as hepatic stellate cells
(HSCs, or Ito cells), results in the increased synthesis and
interstitial deposition of extracellular matrix (ECM) components
(4). Following liver injury, HSCs
undergo ‘activation’, which indicates a transition from quiescent
vitamin A-rich cells into proliferative, fibrogenic and contractile
myofibroblasts (α-smooth muscle actin-expressing) that produce ECM
proteins. This pathway has been considered the canonical pathway in
the pathogenesis of liver fibrogenesis (4). Hepatocytes are the major targets in
liver fibrosis (5). Damaged
hepatocytes release ROS as well as fibrogenic mediators, and induce
the recruitment of white blood cells by inflammatory cells.
Apoptosis of damaged hepatocytes also stimulates fibrogenic
processes in the liver (6). Liver
cirrhosis, which is the end-stage of liver fibrosis, is associated
with high mortality (7).
Fibrogenic mechanisms depend on the interplay of
numerous cytokines (8).
Platelet-derived growth factor and transforming growth factor
(TGF)-β, which is designated as a fibrogenic master cytokine with
multiple effects, are profibrogenic growth factors (9). The natural antagonist of TGF-β is
bone morphogenetic protein (BMP)-7 (10). HSCs respond markedly to TGF-β.
During liver damage, hepatocyte apoptosis and growth control for
regeneration are the primary roles of TGF-β (11). Connective tissue growth factor
(CTGF) mediates fiber and matrix interactions, serves a role in
TGF-β signaling and suppresses BMP-7 (12). Generally, CTGF acts as a fibrogenic
master switch in the genesis of fibroblasts that produce ECM during
organ fibrosis (13). An increase
in CTGF expression occurs spontaneously in cultured hepatocytes but
not in fresh hepatocytes; TGF-β treatment enhances CTGF expression
(14). Therefore, CTGF has been
suggested as a biomarker for hepatic fibrosis (15). Additionally, CTGF is constantly
expressed in activated HSCs in liver fibrosis (16).
Carbon tetrachloride (CCl4) has been
widely used as an experimental tool in the study of certain
hepatotoxic effects (17).
CCl4 may form the trichloromethyl radical,
CCl3*, which reacts with oxygen to form the
trichloromethyl peroxyl radical (CCl3OO*), a highly
reactive species that causes oxidative stress.
CCl4-induced hepatotoxicity occurs in two phases. In the
initial phase, CCl4 is metabolized to form
trichloromethyl radicals (CCl3* or CCl3OO*),
which cause membrane lipid peroxidation and finally induce cell
necrosis (18). In the second
phase of CCl4-induced hepatotoxicity, Kupffer cells in
the liver are activated and proinflammatory mediators are produced
(19). Additionally,
CCl4 activates TNF-α, nitric oxide and TGF-α and TGF-β
in hepatocytes; TNF-α promotes apoptosis and TGFs appear to promote
fibrosis (20). Overall,
CCl4-induced hepatic fibrosis is associated with
multiple factors (21); notably,
CCl4-induced liver fibrosis is associated with a higher
expression of CTGF in rats (22).
Signal transducer and activator of transcription 3
(STAT3) is a transcription factor associated with liver injury,
inflammation and regeneration (23,24).
IL-6 has been reported to activate STAT3 in HSCs, and promote HSC
survival, proliferation and activation, thereby contributing to
liver fibrogenesis (25). In
addition, therapeutic agents ameliorate liver fibrosis through
STAT3 inhibition in HSCs and STAT3 is considered a promising
fibrotic biomarker and/or therapeutic target in liver fibrosis
(26,27). In the rat liver, an increase in
STAT3 phosphorylation caused by CCl4 has been
demonstrated (28). However, the
direct effect of CCl4 on STAT3 expression in the liver
remains to be elucidated.
The present study focused on changes in the
expression levels of CTGF, an established biomarker of hepatic
fibrosis (15), and revealed that
the changes were associated with STAT3 activation in the livers of
rats who received chronic treatment with CCl4.
Additionally, α mouse liver 12 (AML-12) cells, which are
immortalized hepatocytes, were used to determine the role of
oxidative stress in the increase in CTGF expression through
CCl4-induced STAT3 activation.
Materials and methods
Animals
A total of 18 male Sprague-Dawley rats (weight,
250–280 g; age, 8 weeks) were obtained from the National Laboratory
Animal Center (Taipei, Taiwan). Rats were fed in a
climate-controlled room (23±1°C; 55±5% humidity) under a 12-h
light/dark cycle and were supplied ad libitum with clean water and
food. The experimental protocols were approved by the Institutional
Animal Ethics Committee (2017-047) of China Medical University. All
experimental procedures were performed in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals as well as the guidelines of the Animal Welfare Act (eighth
edition; grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf)
to avoid all stressful conditions.
Drug-induced liver injury
The rats were randomly divided into the following
three groups (n=6/group): i) Normal control ii) vehicle (0.9%
saline) plus CCl4 (Thermo Fisher Scientific, Inc.)
treatment; and iii) 200 mg/kg silymarin (Sigma-Aldrich; Merck KGaA)
plus CCl4 treatment. Silymarin is a flavonolignan, which
is obtained from the plant milk thistle (29). Liver injury was induced in rats by
an intraperitoneal injection of 20% CCl4 (diluted in
olive oil) at 2 ml/kg twice a week (Monday and Thursday) for 8
weeks (30,31). Additionally, the
CCl4-treated rats received a daily oral administration
of silymarin at the indicated dose, or were administered the same
volume of vehicle used to dissolve silymarin daily. The
morphological and behavioral changes of the rats were monitored
daily. No sudden death of rats was observed during the present
study. A total of 4 h after the last dosing, rats were anesthetized
with 2% isoflurane and were confirmed to be unresponsive to all
stimuli. Blood samples were collected from the descending aorta.
Subsequently, the animals were sacrificed by inhalation of
CO2 at the rate of 3 l/min with displacement of 30% of
the cage volume per minute. Once the rats were confirmed to be
non-responsive to external stimuli and respiration had ceased, the
livers were rapidly excised and weighed. All samples were stored at
−80°C until extraction.
Identification of liver injury
Blood samples were collected in heparin-containing
tubes and were centrifuged at 2,000 × g for 10 min at 4°C to obtain
the plasma. Subsequently, plasma alanine transaminase (ALT) and
aspartate aminotransferase (AST) levels were measured using an
autoanalyzer and commercially available ALT (cat. no. 700260;
Cayman Chemical Company) and AST (cat. no. MAK055; Sigma-Aldrich;
Merck KGaA) kits. The plasma albumin concentration was determined
using an ELISA kit (cat. no. ab108789; Abcam). The hydroxyproline
levels were also determined using an ELISA kit (cat. no. MAK008;
Sigma-Aldrich; Merck KGaA). All the aforementioned kits were used
according to manufacturer's protocol. Liver homogenates (10%, w/v)
were prepared by homogenizing the liver tissues isolated from each
rat in 150 mM Tris-HCl-buffered saline (Ph 7.2; Sigma-Aldrich;
Merck KGaA). The homogenates were used for western blotting and
other assays.
Cell culture
A mouse hepatocyte-derived cell line (AML-12) was
obtained from the Culture Collection and Research Center of the
Food Industry Institute (BCRC No. 60326) and was cultured in a 1:1
mixture of Dulbecco's Modified Eagle Medium (GE Healthcare Life
Sciences) and Ham's F12 medium (Thermo Fisher Scientific, Inc.)
supplemented with 0.005 mg/ml insulin, 0.005 mg/ml transferrin, 5
ng/ml selenium, 40 ng/ml dexamethasone, 10% fetal bovine serum (GE
Healthcare Life Sciences), 100 IU/ml penicillin and 100 U/ml
streptomycin (GE Healthcare Life Sciences). All the cells were
cultured in a humidified incubator at 37°C in an atmosphere
containing 5% CO2. AML-12 cells were pretreated with
stattic, tiron (4,5-dihydroxybenzene-1,3-disulfonic acid disodium
salt; Sigma-Aldrich; Merck KGaA) or silymarin, for 30 min, prior to
incubation with CCL4 (dissolved in DMSO; Sigma-Aldrich;
Merck KGaA) for 24 h at 37°C. Control cells were treated with 0.1%
DMSO only.
Small interfering RNA (siRNA)
transfection
According to a previously reported protocol
(32), duplexed RNA
oligonucleotides for rat STAT3 were prepared using siGENOME
SMARTpool (Thermo Fisher Scientific, Inc.). The AML-12 cells were
transfected with 40 pmol STAT3-specific siRNAs (siSTAT3) or
scrambled siRNA (Sc) using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The transfected cells were incubated at
37°C for 48 h prior to use. Successful transfection was confirmed
through western blotting.
Western blotting
Total protein was extracted from rat liver tissues
and AML12 cells using RIPA lysis buffer (25 mM Tris, 150 mM NaCl,
0.5% sodium deoxycholate, 0.1% SDS, 1% Triton-X-100, pH 7.6)
supplemented with phosphatase (cat. no. 78420; Thermo Fisher
Scientific, Inc.) and protease inhibitors (cat. no. 539131;
Sigma-Aldrich; Merck KGaA). The concentration of sample was
quantified using a bicinchoninic acid protein assay kit (Thermo
Scientific, Inc.). Equal amounts of protein samples (20 µg) were
resolved by SDS-PAGE gel electrophoresis using 10% gels. Following
electrophoresis, the proteins were transferred to expanded
polyvinylidene difluoride membranes (EMD Millipore) and blocked
using 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in
Tris-buffered saline and 0.1% Tween 20 (TBST) for 2 h at room
temperature. The membranes were then incubated overnight at 4°C
with primary antibodies specific to β-actin (1:5,000;
Sigma-Aldrich; Merck KGaA; cat. no. A5441), phosphorylated
(p)-STAT3 (1:1,000; cat. no. ab76315), STAT3 (1:1,000; cat. no.
ab68153) and CTGF (1:1,000; cat. no. ab6992). The aforementioned
primary antibodies were purchased from Abcam. Following incubation,
the membranes were washed with TBST and incubated for 1 h at room
temperature with horseradish peroxidase conjugated secondary
antibodies (goat anti-rabbit IgG and goat anti-mouse IgG; cat. nos.
AP132P and AP124P; 1:5,000; Sigma-Aldrich; Merck KGaA). The blots
were developed using a chemiluminescence kit (Thermo Scientific,
Inc.) and immunoblot densities were semi-quantified using a
CHEMX400 laser densitometer (Avegene Life Science). The optical
densities of the bands corresponding to p-STAT3 (88 kDa), STAT3 (92
kDa), CTGF (36 kDa) and β-actin (43 kDa) were semi-quantified using
ImageJ (version 1.46; National Institutes of Health).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was isolated from the prepared liver
homogenates and AML-12 cells using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.), followed by chloroform extraction.
The extracted mRNA (2 µg/sample) was reverse-transcribed into cDNA
using the Transcriptor First Strand cDNA Synthesis kit (Roche
Diagnostics) according to the manufacturer's instructions. The mRNA
expression levels of CTGF and STAT3 were measured through RT-qPCR
using Taqman probes (Roche Diagnostics) and specific primers on a
LightCycler 480 system (Roche Diagnostics). The qPCR cycling
conditions were as follows: Initial denaturation at 95°C for 10
min; 30 cycles at 96°C for 10 sec, 60°C for 30 sec; and final
extension at 72°C for 10 min. Signal intensities were normalized to
GAPDH. The primers used were: CTGF forward,
5′-CCAACTATGATGCGAGCCAACT-3′ and reverse,
5′-TTAGCCCGGTAGGTCTTCACACT-3′; STAT3 forward,
5′-CTGGCACCTTGGATTGAGAG-3′ and reverse, 5′-CAACGTGGCATGTGACTCTT-3′;
and GAPDH forward, 5′-GACATGCCGCCTGGAGAAAC-3′ and reverse,
5′-AGCCCAGGATGCCCTTTAGT-3′. Gene expression was quantified using
the 2−ΔΔCq method (33)
and normalized to β-actin.
Statistical analysis
The results are presented as the mean ± standard
deviation from the sample number of each group. Each experiment was
performed at least three times. For multiple group comparison, data
were statistically analyzed using one-way analysis of variance
followed by Tukey's multiple comparison test. The non-parametric
Mann-Whitney U test was performed for two-group comparisons. The
results were analyzed using SPSS software (version 17; SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Silymarin alleviates hepatic fibrosis
induced by CCl4 in rats
Consistent with a previous report (34), the rats exposed to CCl4
for 8 weeks presented marked changes in the levels of hepatic
fibrosis biomarkers, including plasma AST, ALT, hydroxyproline and
albumin levels, compared with vehicle-treated normal rats (Fig. 1). Consistent with a previous study
(35), oral administration of
silymarin (200 mg/kg) significantly attenuated high plasma AST and
ALT levels in the CCl4-treated rats. However, silymarin
alone did not modify AST or ALT levels in the vehicle-treated
normal rats. Similar results were observed with other biomarkers,
namely hydroxyproline and albumin (Fig. 1). Hydroxyproline is a major
component of collagen and changes in hydroxyproline levels are
widely used to quantify collagen content. Additionally, albumin is
synthesized by the liver and lower-than-normal levels of plasma
albumin indicate liver injury (36). The plasma albumin levels were
decreased in CCl4-treated rats and this decrease was
markedly reversed by silymarin (Fig.
1). Thus, hepatic fibrosis was identified in the rats
chronically exposed to CCl4 for 8 weeks.
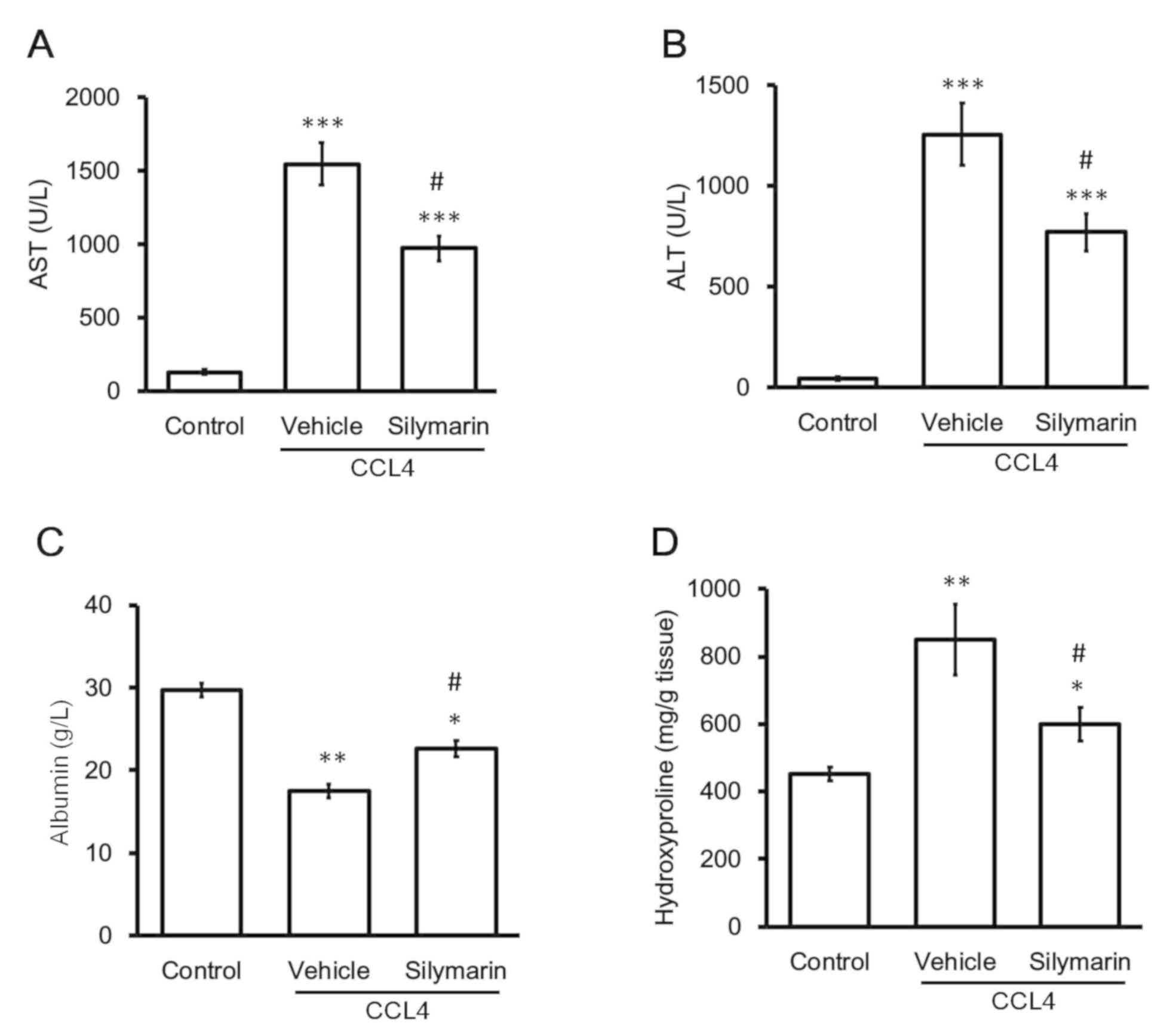 | Figure 1.Changes in plasma levels of
biomarkers in CCl4-treated rats. Hepatic injury was
induced in rats by intraperitoneally injecting 20% CCl4
at a dose of 2 ml/kg twice a week for 8 weeks. Plasma biomarkers
for hepatic fibrosis, including (A) AST, (B) ALT, (C) albumin and
(D) hydroxyproline, were used to compare the three groups of rats:
Normal control (control), vehicle plus CCl4 treatment
and 200 mg/kg silymarin plus CCl4. *P<0.05,
**P<0.01, ***P<0.001 vs. the normal control group;
#P<0.05 vs. the vehicle plus CCl4 group.
ALT, alanine transaminase; AST, aspartate aminotransferase;
CCl4, carbon tetrachloride. |
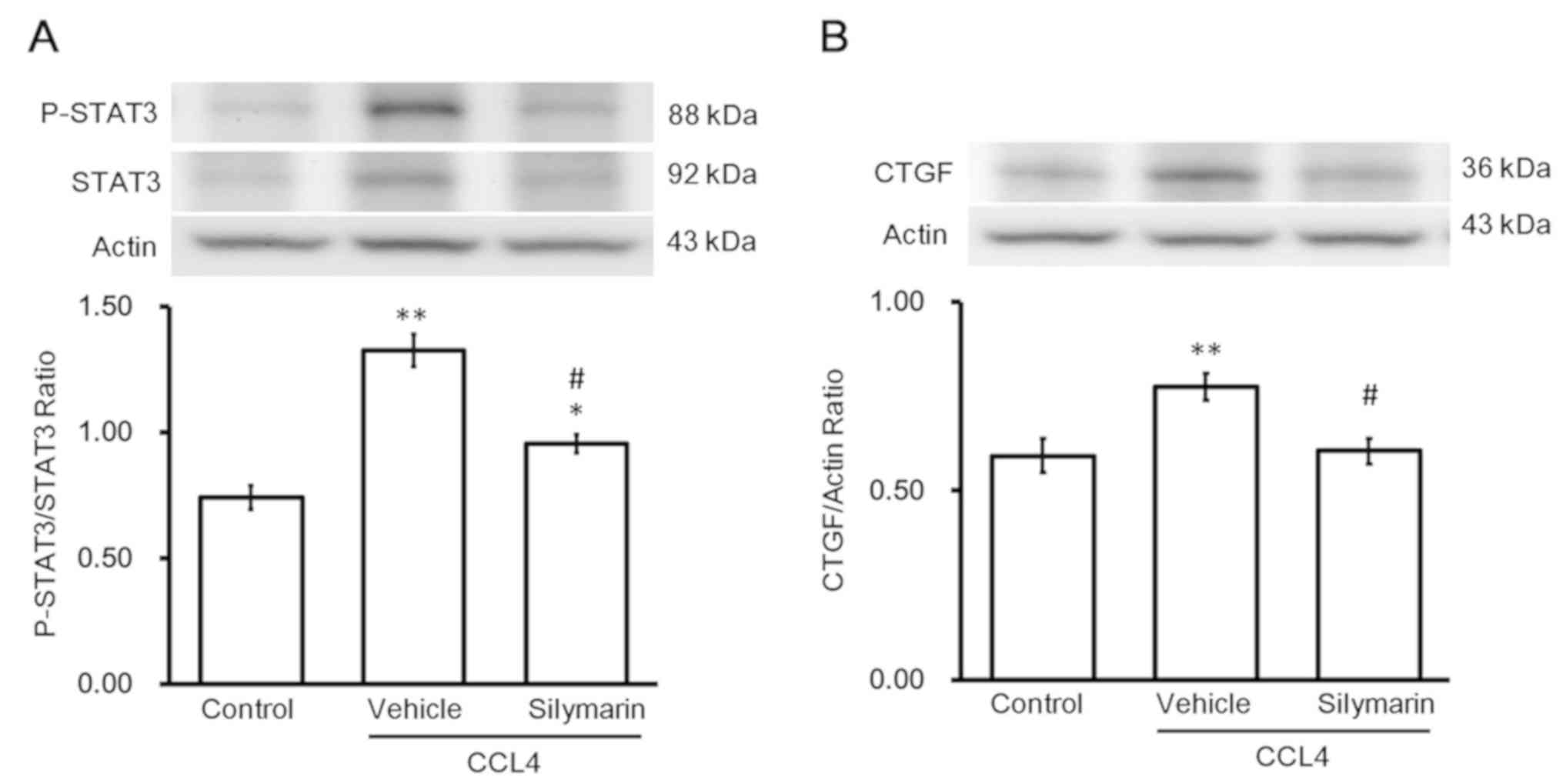
Changes in fibrotic gene expression
during hepatic fibrosis
Western blotting revealed changes in protein levels
of p-STAT3 and STAT3. Administration of the effective dose of
silymarin to alleviate hepatic fibrosis reversed the increase in
p-STAT3/STAT3 (Fig. 2A). Changes
in protein levels of CTGF were similar to the changes in
p-STAT3/STAT3 levels (Fig. 2B).
Notably, activation of STAT3 (p-STAT3/STAT3) was observed in the
livers with fibrosis. Silymarin inhibited STAT3 activation and
thus, CTGF expression, which alleviated hepatic fibrosis.
Effects of CCl4 on
hepatocytes in vitro
The direct effect of CCl4 on hepatocytes
was investigated using the cultured AML-12 cell line. Incubation of
AML-12 cells with CCl4 resulted in increased expression
of CTGF mRNA and protein. The protein (Fig. 3A) and mRNA (Fig. 3B) expression levels of CTGF were
increased after CCl4 treatment in a dose-dependent
manner. Additionally, administration of the same dose of
CCl4 enhanced the mRNA expression of STAT3 in the AML-12
cells (Fig. 3C). Observation of
the liver tissues revealed that CCl4 was directly toxic
to hepatocytes; however, CCl4 at 20 mM did not induce
damage in the AML-12 cells, which was verified by the results of
MTT assay and lactate dehydrogenase measurements (data not
shown).
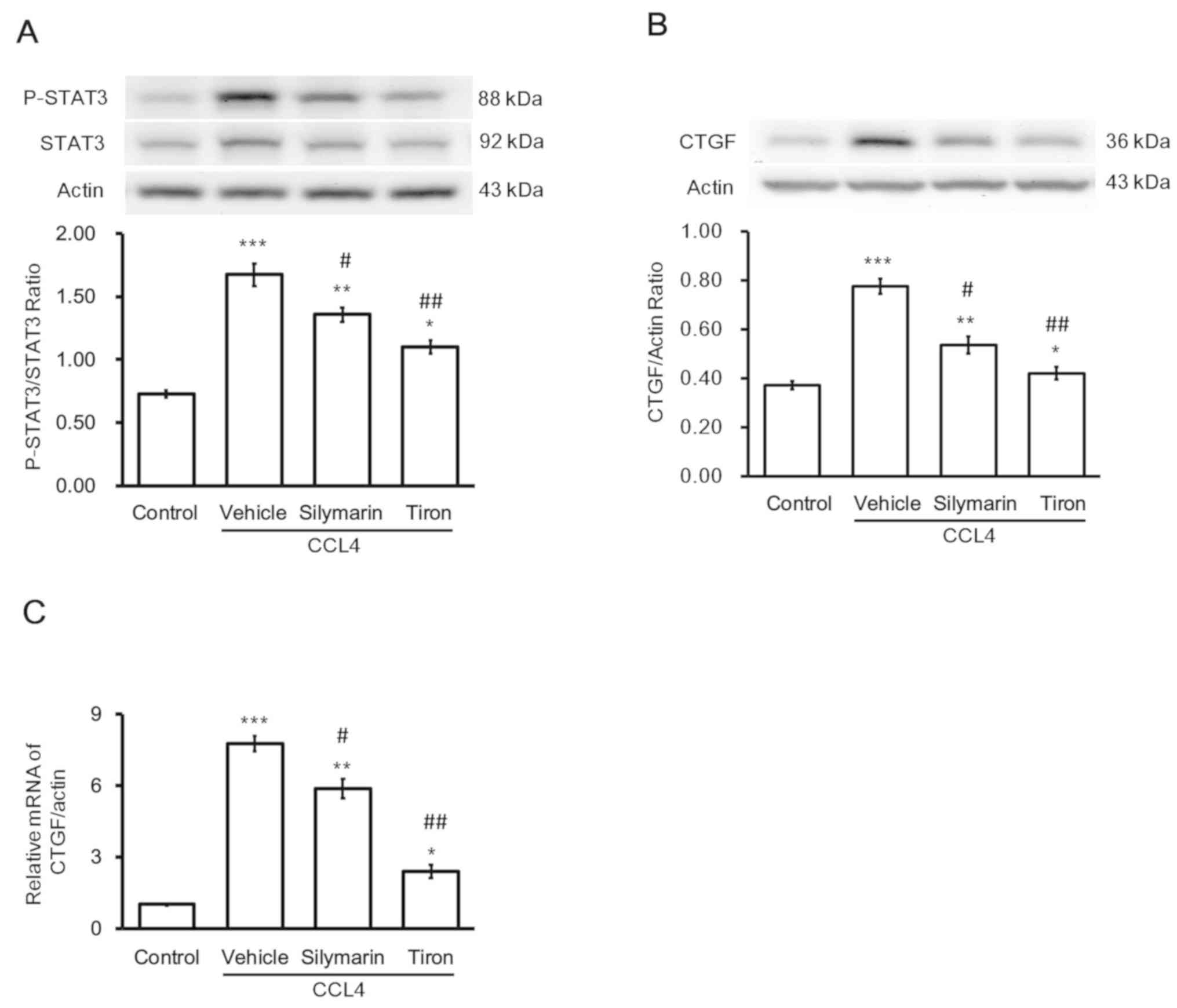
Effects of antioxidants on the
toxicity of CCl4 in hepatocytes
The direct effects of CCl4 on the liver
were reproduced in hepatocytes, namely the increase in STAT3
activation (Fig. 4A) and CTGF
expression (Fig. 4B). Silymarin
inhibited the effects of CCl4 on hepatocytes (Fig. 4). Additionally, another
antioxidant, tiron (37), produced
an effect similar to that of silymarin.
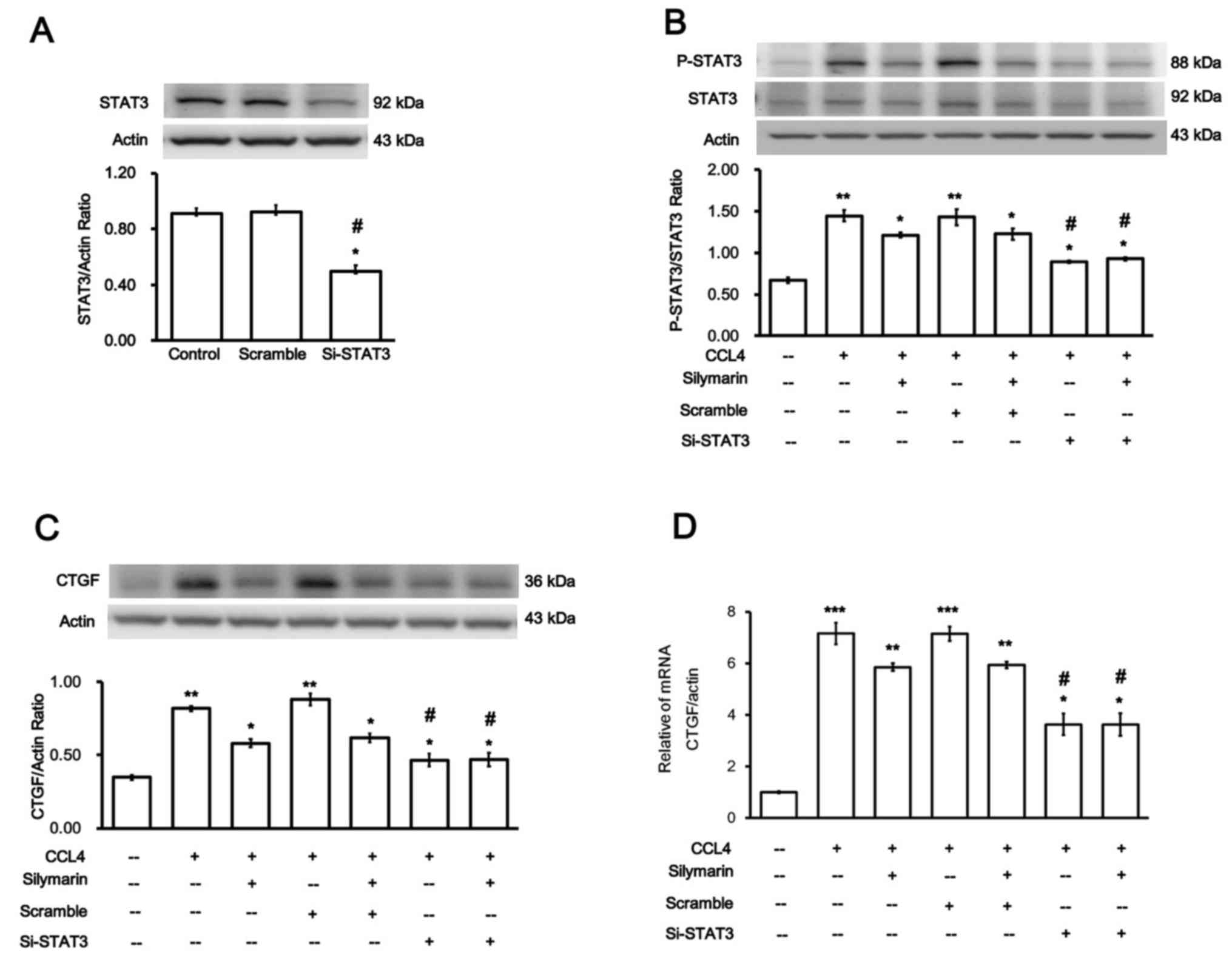
Role of STAT3 in the increase in CTGF
expression induced by CCl4 in hepatocytes
Whether the increase in CTGF expression by
CCl4 was mediated by STAT3 was investigated. Firstly,
siRNA was used to silence STAT3 expression; silencing was
subsequently confirmed (Fig. 5A).
Changes in the cells transfected with siSTAT3 were evaluated
through comparison with cells transfected with Sc (negative control
transfection). However, the expression levels of STAT3 in the cells
transfected with Sc did not differ from those in the normal
hepatocytes.
p-STAT3/STAT3 expression levels were significantly
decreased in the cells transfected with siSTAT3 and treated with
CCL4 (Fig. 5B).
Notably, CTGF protein (Fig. 5C)
and mRNA (Fig. 5D) expression
levels were significantly reduced in the CCl4 + siSTAT3
group. These findings suggested that the attenuation of
CCl4-induced liver injury by silymarin occurs via
suppression of STAT3 signaling.
Effects of STAT3 inhibitor on CTGF
expression in CCl4-treated hepatocytes
According to a previously reported protocol
(38), stattic, a pharmacological
inhibitor of STAT3, was used. In the presence of stattic, the
expression levels of p-STAT3/STAT3 were markedly decreased
(Fig. 6A). Similarly, expression
levels of CTGF protein (Fig. 6B)
and mRNA (Fig. 6C) were
significantly attenuated compared with the CCl4 group.
This provided additional data to support the hypothesis that the
decrease in STAT3 activation results in the reduction of CTGF
expression in the hepatocytes.
Discussion
The results of the present study indicated that
CCl4 may activate STAT3 to promote CTGF expression in
hepatocytes. CCl4-induced hepatic fibrosis was simulated
in rats using previously described methods (28,30,31).
A marked increase in the levels of plasma biomarkers indicated the
success of induction of hepatic fibrosis in the rats using
CCl4. Silymarin alleviated hepatic fibrosis; this
reagent has been widely used as a positive control in studies on
antifibrotic agents (22,39). Similar to a previous study
(22), CCl4 treatment
in rats enhanced the expression of CTGF in the liver, whereas this
was reversed by silymarin. STAT3 activation is undoubtedly required
for CTGF induction in activated HSCs (40).
CTGF expression and STAT3 activation were enhanced
in hepatocytes by direct exposure to CCl4 in a
dose-dependent manner. However, CCl4 did not induce
damage of the cultured hepatocytes when administered at an
effective dose (20 mM). Silymarin attenuated the
CCl4-induced increase in CTGF expression and STAT3
activation in the hepatocytes. The toxic effect of CCl4
has been attributed to the production of ROS and free radicals
(41). A number of
hepatoprotective agents, including silymarin (42) and other natural products (43), have been documented to counteract
oxidative stress-mediated tissue damage through their antioxidant
ability and/or ability to scavenge free radicals. The present study
applied another antioxidant, tiron (44), to confirm the antioxidant activity
of silymarin. Tiron is known to inhibit oxidative stress (37). Notably, tiron inhibited the
increase in CTGF expression and STAT3 activation in the cultured
hepatocytes. An increase in STAT3 phosphorylation via oxidative
stress has been established (45).
Therefore, these findings indicated that the
CCl4-induced increases in CTGF expression and STAT3
activation in the AML-12 cells may be associated with oxidative
stress.
In the present study, the association between STAT3
and CTGF expression was investigated in the cultured hepatocytes.
Using siSTAT3, the expression of STAT3 in AML-12 cells was
silenced. Consequently, the promotion of CTGF expression induced by
CCl4 was not observed in the cells transfected with
siSTAT3. Silymarin also inhibited CCl4-induced CTGF
expression. These findings suggested that CCl4 induced
STAT3 activation to enhance CTGF expression in hepatocytes.
Stattic, the pharmacological inhibitor of STAT3 (38), effectively inhibited STAT3
activation and expression in AML-12 cells in the present study,
which was consistent with a previous study (46). Stattic has also been reported to be
effective at reversing the expression of CTGF in type 1-like
diabetic rats (47). The
specificity of stattic has been challenged (48,49);
however, it has been widely used as a specific STAT3 inhibitor in a
number of studies (46,47,50,51).
STAT3 is known as a cytoplasmic transcription factor
that transmits extracellular signals to the nucleus (52). Cytokine receptor-dependent Janus
kinase (JAK)/STAT activation is generally introduced as the main
source of STAT3, whereas an intracellular regulation serves a role
in activating STAT3 (40).
JAK/STAT activation may be the source of STAT3 that was activated
by CCl4 in cultured hepatocytes. Activated STAT3 in the
nucleus binds to specific DNA promoter sequences to regulate gene
expression (53). Therefore,
CCl4 promotes CTGF expression via STAT3 activation in
hepatocytes. The STAT3 inhibitor S3I-201 has been demonstrated to
inhibit the development of liver fibrosis (54). Sorafenib is known as a protein
kinase inhibitor and is used in the treatment of liver fibrosis
(55). Early antifibrotic
treatment with sorafenib reduces the levels of hepatic p-STAT3,
however, p-STAT3 increases in response to the dynamic regulation of
IL-6 signaling in Kupffer cells (56). Therefore, a combination therapy
co-targeting the receptor tyrosine kinases and STAT3 pathway
constitutes a promising strategy for improving clinical prognosis
(54). However, additional
evidence is required from future clinical trials. The main
limitation of the present study was that the oxidative stress was
not measured, and this requires further investigation in future
studies. One concern may be considered as a limitation of the
present study: Direct assessment of oxidative stress produced by
CCl4 in AML-12 cells may strengthen the role of
oxidative stress in the activation of STAT3. This will be the
subject of future studies.
In conclusion, the results of the present study
suggested that CCl4 may activate STAT3 through oxidative
stress to promote CTGF expression in hepatocytes. Therefore, it
underscores the requirement for the further development of STAT3
inhibitors alone or in combination with an established drug, such
as sorafenib, for patients with liver fibrosis.
Acknowledgements
The authors would like to thank Miss Yang-Lien Yen
(Department of Medical Research, Chi-Mei Medical Center, Taiwan)
and Mr Yi-Zhi Chen (Department of Medical Research, Chi-Mei Medical
Center, Taiwan) for their technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC and YL were responsible for the conception and
design of the current study, analysis, interpretation of the data
and drafting of the manuscript. CTH contributed to the analysis and
statistical analysis. KCC contributed to the acquisition of
molecular data. CSN contributed to the interpretation of data and
submission of the manuscript. WHP and HSN designed the study,
revised the manuscript and gave final approval of the version to be
published.. All authors discussed, revised and approved the
manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Institutional Animal Ethics Committee (2017-047) of China Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AML-12
|
α mouse liver 12
|
|
CCl4
|
carbon tetrachloride
|
|
CTGF
|
connective tissue growth factor
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Pari L and Sankaranarayanan C: Beneficial
effects of thymoquinone on hepatic key enzymes in
streptozotocin-nicotinamide induced diabetic rats. Life Sci.
85:830–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen-Lefebvre AT and Horuzsko A: Kupffer
cell metabolism and function. J Enzymol Metab. 1(pii):
1012015.PubMed/NCBI
|
|
3
|
Mello T, Zanieri F, Ceni E and Galli A:
Oxidative stress in the healthy and wounded hepatocyte: A cellular
organelles perspective. Oxid Med Cell Longev. 2016:83274102016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gressner OA, Weiskirchen R and Gressner
AM: Biomarkers of hepatic fibrosis, fibrogenesis and genetic
pre-disposition pending between fiction and reality. J Cell Mol
Med. 11:1031–1051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higuchi H and Gores GJ: Mechanisms of
liver injury: An overview. Curr Mol Med. 3:483–490. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Canbay A, Friedman S and Gores GJ:
Apoptosis: The nexus of liver injury and fibrosis. Hepatology.
39:273–278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X and Wu B: Critical issues in the
diagnosis and treatment of liver cirrhosis. Gastroenterol Rep
(Oxf). 7:227–230. 2019.PubMed/NCBI
|
|
8
|
Pinzani M and Rombouts K: Liver fibrosis:
From the bench to clinical targets. Dig Liver Dis. 36:231–242.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gressner AM, Weiskirchen R, Breitkopf K
and Dooley S: Roles of TGF-beta in hepatic fibrosis. Front Biosci.
7:d793–d807. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sugimoto H, Yang C, LeBleu VS, Soubasakos
MA, Giraldo M, Zeisberg M and Kalluri R: BMP-7 functions as a novel
hormone to facilitate liver regeneration. FASEB J. 21:256–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schuster N and Krieglstein K: Mechanisms
of TGF-beta-mediated apoptosis. Cell Tissue Res. 307:1–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arnott JA, Lambi AG, Mundy C, Hendesi H,
Pixley RA, Owen TA, Safadi FF and Popoff SN: The role of connective
tissue growth factor (CTGF/CCN2) in skeletogenesis. Crit Rev
Eukaryot Gene Expr. 21:43–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abreu JG, Ketpura NI, Reversade B and De
Robertis EM: Connective-tissue growth factor (CTGF) modulates cell
signalling by BMP and TGF-beta. Nat Cell Biol. 4:599–604. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gressner OA, Lahme B, Demirci I, Gressner
AM and Weiskirchen R: Differential effects of TGF-beta on
connective tissue growth factor (CTGF/CCN2) expression in hepatic
stellate cells and hepatocytes. J Hepatol. 47:699–710. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gressner AM, Yagmur E, Lahme B, Gressner O
and Stanzel S: Connective tissue growth factor in serum as a new
candidate test for assessment of hepatic fibrosis. Clin Chem.
52:1815–1817. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meindl-Beinker NM and Dooley S:
Transforming growth factor-beta and hepatocyte transdifferentiation
in liver fibrogenesis. J Gastroenterol Hepatol. 23 (Suppl
1):S122–S127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Williams AT and Burk RF: Carbon
tetrachloride hepatotoxicity: An example of free radical-mediated
injury. Semin Liver Dis. 10:279–284. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manibusan MK, Odin M and Eastmond DA:
Postulated carbon tetrachloride mode of action: A review. J Environ
Sci Health C Environ Carcinog Ecotoxicol Rev. 25:185–209. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Planaguma A, Claria J, Miquel R,
López-Parra M, Titos E, Masferrer JL, Arroyo V and Rodés J: The
selective cyclooxygenase-2 inhibitor SC-236 reduces liver fibrosis
by mechanisms involving non-parenchymal cell apoptosis and
PPARgamma activation. FASEB J. 19:1120–1122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Badr G, Sayed EA, Waly H, Hassan KA,
Mahmoud MH and Selamoglu Z: The therapeutic mechanisms of Propolis
against CCl4-mediated liver injury by mediating
apoptosis of activated hepatic stellate cells and improving the
hepatic architecture through PI3K/AKT/mTOR, TGF-beta/Smad2,
Bcl2/BAX/P53 and iNOS signaling pathways. Cell Physiol Biochem.
53:301–322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong S, Chen QL, Song YN, Sun Y, Wei B, Li
XY, Hu YY, Liu P and Su SB: Mechanisms of CCl4-induced
liver fibrosis with combined transcriptomic and proteomic analysis.
J Toxicol Sci. 41:561–572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tzeng JI, Chen MF, Chung HH and Cheng JT:
Silymarin decreases connective tissue growth factor to improve
liver fibrosis in rats treated with carbon tetrachloride. Phytother
Res. 27:1023–1028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Lafdil F, Kong X and Gao B: Signal
transducer and activator of transcription 3 in liver diseases: A
novel therapeutic target. Int J Biol Sci. 7:536–550. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao B, Wang H, Lafdil F and Feng D: STAT
proteins-key regulators of anti-viral responses, inflammation, and
tumorigenesis in the liver. J Hepatol. 57:430–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nieto N: Oxidative-stress and IL-6 mediate
the fibrogenic effects of [corrected] Kupffer cells on stellate
cells. Hepatology. 44:1487–1501. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su TH, Shiau CW, Jao P, Liu CH, Liu CJ,
Tai WT, Jeng YM, Yang HC, Tseng TC, Huang HP, et al: Sorafenib and
its derivative SC-1 exhibit antifibrotic effects through signal
transducer and activator of transcription 3 inhibition. Proc Natl
Acad Sci USA. 112:7243–7248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hui J, Gao J, Wang Y, Zhang J, Han Y, Wei
L, Liu Xiaochuang and Wu J: Panax notoginseng saponins ameliorates
experimental hepatic fibrosis and hepatic stellate cell
proliferation by inhibiting the Jak2/Stat3 pathways. J Tradit Chin
Med. 36:217–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qu W, Huang H, Li K and Qin C:
Danshensu-mediated protective effect against hepatic fibrosis
induced by carbon tetrachloride in rats. Pathol Biol (Paris).
62:348–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Surai PF: Silymarin as a natural
antioxidant: An overview of the current evidence and perspectives.
Antioxidants (Basel). 4:204–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen X, Ying X, Zhang W, Chen Y, Shi C,
Hou Y and Zhang Y: The hepatoprotective effect of fraxetin on
carbon tetrachloride induced hepatic fibrosis by antioxidative
activities in rats. Int Immunopharmacol. 17:543–547. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abdel-Moneim AM, Al-Kahtani MA, El-Kersh
MA and Al-Omair MA: Free Radical-scavenging, Anti-
inflammatory/Anti-Fibrotic and Hepatoprotective Actions of Taurine
and silymarin against CCl4 induced rat liver damage.
PLoS One. 10:e01445092015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuo SC, Li Y, Cheng KC, Niu CS, Cheng JT
and Niu HS: Increase in renal erythropoietin receptors in diabetic
rats is mainly mediated by hyperglycemia associated with the
STAT3/GATA-1 signaling pathway. Biomed Pharmacother. 96:1094–1102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang R, Wang J, Song F, Li S and Yuan Y:
Tanshinol ameliorates CCl4-induced liver fibrosis in
rats through the regulation of Nrf2/HO-1 and NF-κB/IκBα signaling
pathway. Drug Des Devel Ther. 12:1281–1292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsai JH, Liu JY, Wu TT, Ho PC, Huang CY,
Shyu JC, Hsieh YS, Tsai CC and Liu YC: Effects of silymarin on the
resolution of liver fibrosis induced by carbon tetrachloride in
rats. J Viral Hepat. 15:508–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Wen PH, Zhang XX, Dai Y and He Q:
Breviscapine ameliorates CCl4-induced liver injury in
mice through inhibiting inflammatory apoptotic response and ROS
generation. Int J Mol Med. 42:755–768. 2018.PubMed/NCBI
|
|
37
|
Morgan A, Ibrahim MA, Galal MK, Ogaly HA
and Abd-Elsalam RM: Innovative perception on using Tiron to
modulate the hepatotoxicity induced by titanium dioxide
nanoparticles in male rats. Biomed Pharmacother. 103:553–561. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schust J, Sperl B, Hollis A, Mayer TU and
Berg T: Stattic: A small-molecule inhibitor of STAT3 activation and
dimerization. Chem Biol. 13:1235–1242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
El-Lakkany NM, Hammam OA, El-Maadawy WH,
Badawy AA, Ain-Shoka AA and Ebeid FA:
Anti-inflammatory/anti-fibrotic effects of the hepatoprotective
silymarin and the schistosomicide praziquantel against Schistosoma
mansoni-induced liver fibrosis. Parasit Vectors. 5:92012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Liu H, Meyer C, Li J, Nadalin S,
Königsrainer A, Weng H, Dooley S and ten Dijke P: Transforming
growth factor-β (TGF-β)-mediated connective tissue growth factor
(CTGF) expression in hepatic stellate cells requires Stat3
signaling activation. J Biol Chem. 288:30708–30719. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ha HL, Shin HJ, Feitelson MA and Yu DY:
Oxidative stress and antioxidants in hepatic pathogenesis. World J
Gastroenterol. 16:6035–6043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shaker E, Mahmoud H and Mnaa S: Silymarin,
the antioxidant component and Silybum marianum extracts prevent
liver damage. Food Chem Toxicol. 48:803–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huo HZ, Wang B, Liang YK, Bao YY and Gu Y:
Hepatoprotective and antioxidant effects of licorice extract
against CCl(4)-induced oxidative damage in rats. Int J Mol Sci.
12:6529–6543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han YH and Park WH: Tiron, a ROS
scavenger, protects human lung cancer Calu-6 cells against
antimycin A-induced cell death. Oncol Rep. 21:253–261.
2009.PubMed/NCBI
|
|
45
|
Chiu YH, Ku PM, Cheng YZ, Li Y, Cheng JT
and Niu HS: Phosphorylation of signal transducer and activator of
transcription 3 induced by hyperglycemia is different with that
induced by lipopolysaccharide or erythropoietin via receptorcoupled
signaling in cardiac cells. Mol Med Rep. 17:1311–1320.
2018.PubMed/NCBI
|
|
46
|
Pan Y, Zhou F, Zhang R and Claret FX:
Stat3 inhibitor Stattic exhibits potent antitumor activity and
induces chemo- and radio-sensitivity in nasopharyngeal carcinoma.
PLoS One. 8:e545652013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang CM, Hsu CT, Niu HS, Chang CH, Cheng
JT and Shieh JM: Lung damage induced by hyperglycemia in diabetic
rats: The role of signal transducer and activator of transcription
3 (STAT3). J Diabetes Complications. 30:1426–1433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heidelberger S, Zinzalla G, Antonow D,
Essex S, Basu BP, Palmer J, Husby J, Jackson PJ, Rahman KM,
Wilderspin AF, et al: Investigation of the protein alkylation sites
of the STAT3:STAT3 inhibitor Stattic by mass spectrometry. Bioorg
Med Chem Lett. 23:4719–4722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Szelag M, Sikorski K, Czerwoniec A,
Szatkowska K, Wesoly J and Bluyssen HA: In silico simulations of
STAT1 and STAT3 inhibitors predict SH2 domain cross-binding
specificity. Eur J Pharmacol. 720:38–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Boengler K, Ungefug E, Heusch G and Schulz
R: The STAT3 inhibitor stattic impairs cardiomyocyte mitochondrial
function through increased reactive oxygen species formation. Curr
Pharm Des. 19:6890–6895. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sanseverino I, Purificato C, Gauzzi MC and
Gessani S: Revisiting the specificity of small molecule inhibitors:
The example of stattic in dendritic cells. Chem Biol. 19:1213–1216.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miller AM, Wang H, Bertola A, Park O,
Horiguchi N, Ki SH, Yin S, Lafdil F and Gao B:
Inflammation-associated interleukin-6/signal transducer and
activator of transcription 3 activation ameliorates alcoholic and
nonalcoholic fatty liver diseases in interleukin-10-deficient mice.
Hepatology. 54:846–856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jung JE, Lee HG, Cho IH, Chung DH, Yoon
SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK and Chung MH: STAT3 is
a potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Z, Li J, Xiao W, Long J and Zhang H:
The STAT3 inhibitor S3I-201 suppresses fibrogenesis and
angiogenesis in liver fibrosis. Lab Invest. 98:1600–1613. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Y, Gao J, Zhang D, Zhang J, Ma J and
Jiang H: New insights into the antifibrotic effects of sorafenib on
hepatic stellate cells and liver fibrosis. J Hepatol. 53:132–144.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deng YR, Ma HD, Tsuneyama K, Yang W, Wang
YH, Lu FT, Liu CH, Liu P, He XS, Diehl AM, et al: STAT3-mediated
attenuation of CCl4-induced mouse liver fibrosis by the
protein kinase inhibitor sorafenib. J Autoimmun. 46:25–34. 2013.
View Article : Google Scholar : PubMed/NCBI
|