Introduction
Sepsis is defined as life-threatening organ
dysfunction caused by an abnormal host response to infection, and
septic shock is a subset of sepsis (1). Clinically, organ dysfunction can be
represented by an increase in the sepsis-related organ failure
assessment (SOFA) score of 2 points or more; septic shock can be
identified by a vasopressor requirement to maintain mean arterial
pressure ≥65 mmHg and a serum lactate level >2 mmol/l in the
absence of hypovolemia (2). Sepsis
has a high mortality rate and is one of the main causes of death in
intensive care units (ICUs) worldwide (3). Despite recent progress in the
development of anti-infective drugs and life support treatments,
the mortality associated with sepsis, particularly septic shock, is
still high (4). The pathogenesis
of sepsis is complicated and has not yet been fully elucidated
(5). The major pathophysiological
mechanisms underlying sepsis include systemic inflammation, immune
imbalance, multiple organ dysfunction and gene polymorphism
(6). Early diagnosis and
intervention are the most effective methods to reduce the mortality
rates of patients with sepsis (7).
The severity and prognosis of sepsis are determined by
procalcitonin (PCT) and lactate (Lac) levels, SOFA (2), and acute physiology and chronic
health evaluation II (APACHE II) (8). However, there is still a lack of
precise biomarkers and intervention targets for sepsis.
Advances in human genome-wide analysis have revealed
that ~2% of the genome is composed of protein-coding genes, whereas
the remaining 98% of genome transcripts serve no protein-coding
functions (9). Long non-coding
RNAs (lncRNAs) are a class of non-protein-coding RNAs with a length
of >200 nucleotides that are widely involved in cell
proliferation, differentiation and apoptosis through regulating
target gene expression (10). The
lncRNA metastasis-associated lung adenocarcinoma transcript 1
(MALAT1) was first identified in non-small cell lung cancer
(11) and is among the most
studied lncRNAs (12). MALAT1 is
involved in a variety of physiological and pathological processes,
including neurodevelopment (13),
skeletal myogenesis (14),
angiogenesis (15), cancer
(16), and cardiovascular
(17) and neurological (18) diseases. Recent studies have
reported that MALAT1 is also associated with infection and
immune-mediated inflammatory diseases, such as sepsis (19), systemic lupus erythematosus
(20), multiple sclerosis
(21) and rheumatoid arthritis
(22), by regulating the
inflammatory response through multiple target genes and signaling
pathways. However, the clinical value of MALAT1 in the diagnosis,
severity and prognosis of sepsis has not been reported. Therefore,
the aim of the present study was to determine the expression levels
of plasma MALAT1 in patients with sepsis and to evaluate whether
MALAT1 may serve as an independent predictive biomarker for
patients with sepsis.
Materials and methods
Participants
A total of 120 patients with sepsis were admitted to
the ICU of The First People's Hospital of Yancheng between June
2016 and June 2018, and were consecutively recruited for this
prospective cohort study. The management of sepsis and septic shock
was performed in accordance with the Surviving Sepsis Campaign:
International Guidelines for Management of Sepsis and Septic Shock
(1). The inclusion criteria for
the present study were as follows: i) Patients who met the
aforementioned diagnostic criteria for sepsis and/or septic shock;
ii) patients aged between 18 and 80 years; and iii) the primary
causes of sepsis and septic shock were pulmonary infection,
abdominal infection, urinary tract infection and/or bacteremia.
Patients <18 or >80 years, pregnant and/or lactating
patients, those with tumors, blood disease, organ dysfunction and
immune-mediated inflammatory disease were excluded. During the same
period, 60 healthy volunteers with matching age and sex were
recruited as healthy controls (HCs). The inclusion criteria were as
follows: i) Subjects aged between 18 and 80 years; and ii) subjects
with good physical and mental health. HCs with a history of
immune-mediated inflammatory disease, severe infection, organ
dysfunction, cancer, or immunosuppressive therapy were excluded.
HCs who were pregnant and/or lactating at the time of recruitment
were also excluded.
This study was approved by the Ethics Committee of
The First People's Hospital of Yancheng and complied with the
ethics standards of the Declaration of Helsinki. All participants
or their statutory guardians provided written informed consent.
Study design
Firstly, the expression levels of MALAT1 in plasma
samples from HCs and patients with sepsis were analyzed and
compared between patients with sepsis and HCs, patients with and
without septic shock, as well as survivors and non-survivors.
Subsequently, the correlation between MALAT1 expression levels and
conventional evaluation indicators of sepsis, including PCT and Lac
levels, as well as SOFA and APACHE II scores, was evaluated.
Multivariate logistic regression was used to analyze whether MALAT1
may serve as an independent risk factor for the diagnosis, severity
and prognosis of sepsis. Finally, the predictive values of MALAT1
in distinguishing patients with sepsis from HCs, patients with
septic shock from those without septic shock, and non-survivors
from survivors were evaluated.
Blood sample preparation
The blood samples of all subjects were collected
within 24 h of admission. Elbow venous blood (10 ml/subject) was
divided into two parts: One (5 ml) was collected into a vacutainer
tube containing EDTA (Becton, Dickinson and Company) for plasma
separation; the other part (5 ml) was collected into a vacutainer
tube containing clot activator and polymer gel (Becton, Dickinson
and Company) for serum separation. To obtain plasma/serum, the
whole blood was centrifuged at 2,500 × g for 15 min at 4°C. All
samples were stored at −80°C until further processing.
Data collection
The clinical data of patients with sepsis were
collected within 24 h following admission, and included age, sex,
serum PCT levels, arterial Lac levels, SOFA and APACHE II scores.
Patients were assessed by a senior physician for sepsis severity
(septic shock or no shock). The prognosis of patients with sepsis
for 28 days following admission (survival or non-survival) was also
recorded. Age, sex and PCT levels were recorded in HCs. The serum
PCT levels were detected by chemiluminescent immunoassay (Wuhan
EasyDiagnosis Biomedicine Co., Ltd.; cat. no. ED75440-Hu) according
to the manufacturer's protocol. Lac levels were detected by
arterial blood gas analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from isolated plasma using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and was reverse transcribed into cDNA using the PrimeScript™
RT reagent kit (Takara Bio, Inc.), according to the manufacturer's
protocol. Subsequently, the expression levels of MALAT1 were
determined using SYBR Premix Ex Taq™ II (Takara Bio, Inc.) on the
ABI Prism 7300 RT-qPCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). PCR was performed under the following
conditions: Denaturation at 50°C for 2 min, followed by 40 cycles
of 95°C for 15 sec and 60°C for 1 min. The relative expression
levels of MALAT1 were calculated using the 2−ΔΔCq method
(23) and GAPDH was used as
internal reference. The primer sequences used were as follows:
MALAT1, forward 5′-GTGATGCGAGTTGTTCTCCG-3′ reverse
5′-CTGGCTGCCTCAATGCCTAC-3′; and GAPDH, forward
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse
5′-TTGATTTTGGAGGGATCTCG-3′.
Statistical analysis
Data are expressed as the mean ± standard deviation,
median and interquartile range or count. PASW Statistics 18.0 (SPSS
Inc.) and GraphPad Prism 8.0 (GraphPad Software, Inc.) were used
for statistical analysis. The comparisons between two groups were
performed using unpaired Student's t-test, Mann-Whitney test or
χ2 test as appropriate. Spearman's correlation was used
to analyze the correlation between plasma MALAT1 and PCT, Lac, SOFA
and APACHE II scores. Multivariate logistic regression and receiver
operating characteristic (ROC) curve analyses were performed to
determine the predictive value of independent risk factors for the
diagnosis, severity and prognosis of sepsis. Risk factors with
P<0.15 from the univariate analyses (Student's t-test,
Mann-Whitney test and χ2 test) were included in the
multivariate logistic regression analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
MALAT1 as an independent risk factor
for sepsis
Univariate analysis revealed that age (P=0.085) and
sex (P=0.082) were not significantly different between patients
with sepsis and HCs (Table I).
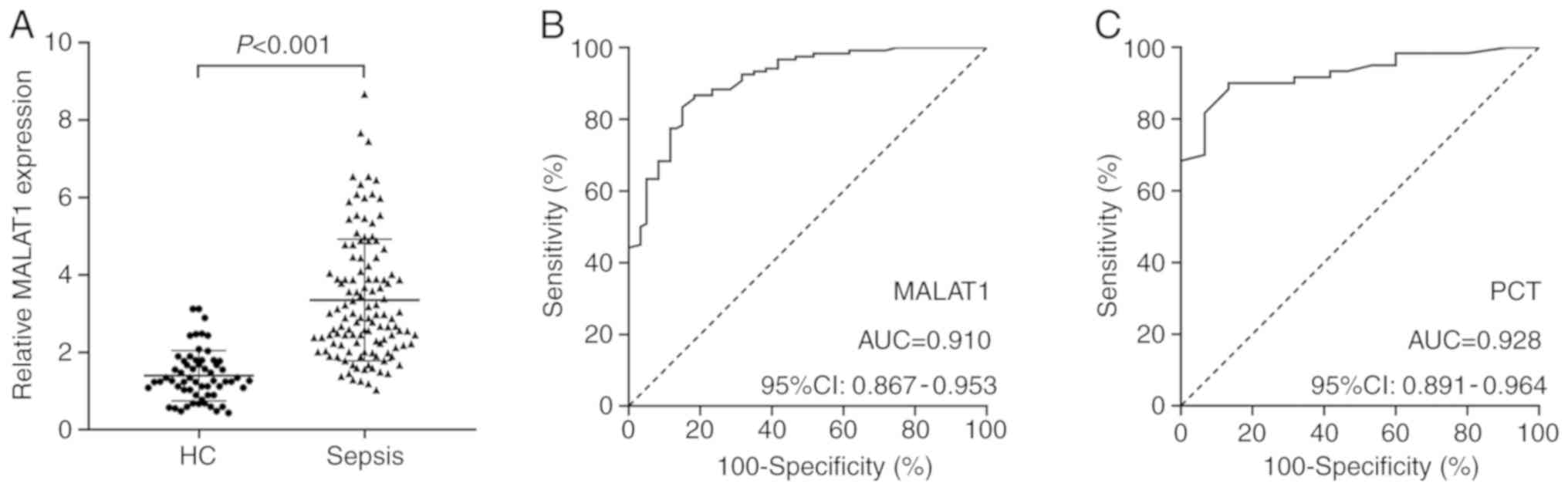
However, PCT (P<0.001) and MALAT1 (P<0.001) levels were
significantly higher in patients with sepsis compared with those in
HCs (Fig. 1A). In addition,
multivariate logistic regression analysis revealed that high MALAT1
expression (P<0.001) and PCT (P=0.007) levels were independent
predictive factors for sepsis risk, whereas, age (P=0.161) and sex
(P=0.084) exhibited no association with sepsis risk (Table II).
 | Table I.Univariate analysis of
clinicopathological characteristics between HCs and patients with
sepsis. |
Table I.
Univariate analysis of
clinicopathological characteristics between HCs and patients with
sepsis.
| Factor | HCs (n=60) | Sepsis (n=120) | P-value |
|---|
| Age, years | 47.68±10.25 | 50.35±8.47 | 0.085a |
| Sex, n |
|
| 0.082b |
|
Male | 36 | 54 |
|
|
Female | 24 | 66 |
|
| PCT, ng/ml | 0.30
(0.14–0.40) | 5.65
(0.68–12.28) |
<0.001c |
| Lac, mmol/l | – | 3.39
(2.22–5.83) | – |
| SOFA score | – | 7.38±3.89 | – |
| APACHE II
score | – | 15.21±4.69 | – |
 | Table II.Multivariate logistic regression of
risk factors for sepsis. |
Table II.
Multivariate logistic regression of
risk factors for sepsis.
| Factor | P-value | OR | 95% CI |
|---|
| Age | 0.161 | 1.050 | 0.981–1.123 |
| Sex (male vs.
female) | 0.084 | 3.009 | 0.861–10.514 |
| MALAT1 | <0.001 | 7.446 | 3.259–17.012 |
| PCT | 0.007 | 12.233 | 1.960–76.353 |
Diagnostic value of MALAT1 for
sepsis
ROC curve analysis revealed that MALAT1 had
significant diagnostic value for sepsis with an area under curve
(AUC) of 0.910 (Fig. 1B); however,
the diagnostic value of MALAT1 was lower compared with that of PCT
(AUC=0.928; Fig. 1C). The optimal
cut-off point for the level of MALAT1 that distinguished patients
with sepsis from HCs was >1.895, and the specificity and
sensitivity were 85 and 83.33%, respectively. In addition, the
optimal cut-off point for PCT level that distinguished patients
with sepsis from HCs was <0.5, and the specificity and
sensitivity were 93.33 and 81.67%, respectively.
Correlation between plasma MALAT1
levels and conventional evaluation indicators of sepsis
Spearman's correlation analysis revealed that MALAT1
plasma levels exhibited weak positive correlations with PCT levels
(r=0.253; P=0.005), Lac levels (r=0.488; P<0.001), SOFA scores
(r=0.566; P<0.001) and APACHE II scores (r=0.517; P<0.001) in
patients with sepsis (Fig. 2).
MALAT1 is an independent risk factor
for septic shock
As presented in Table
III, univariate analysis revealed that age (P=0.124), sex
(P=0.912) and PCT level (P=0.257) were not significantly associated
with the occurrence of septic shock. However, Lac levels
(P<0.001), SOFA scores (P<0.001), APACHE II scores
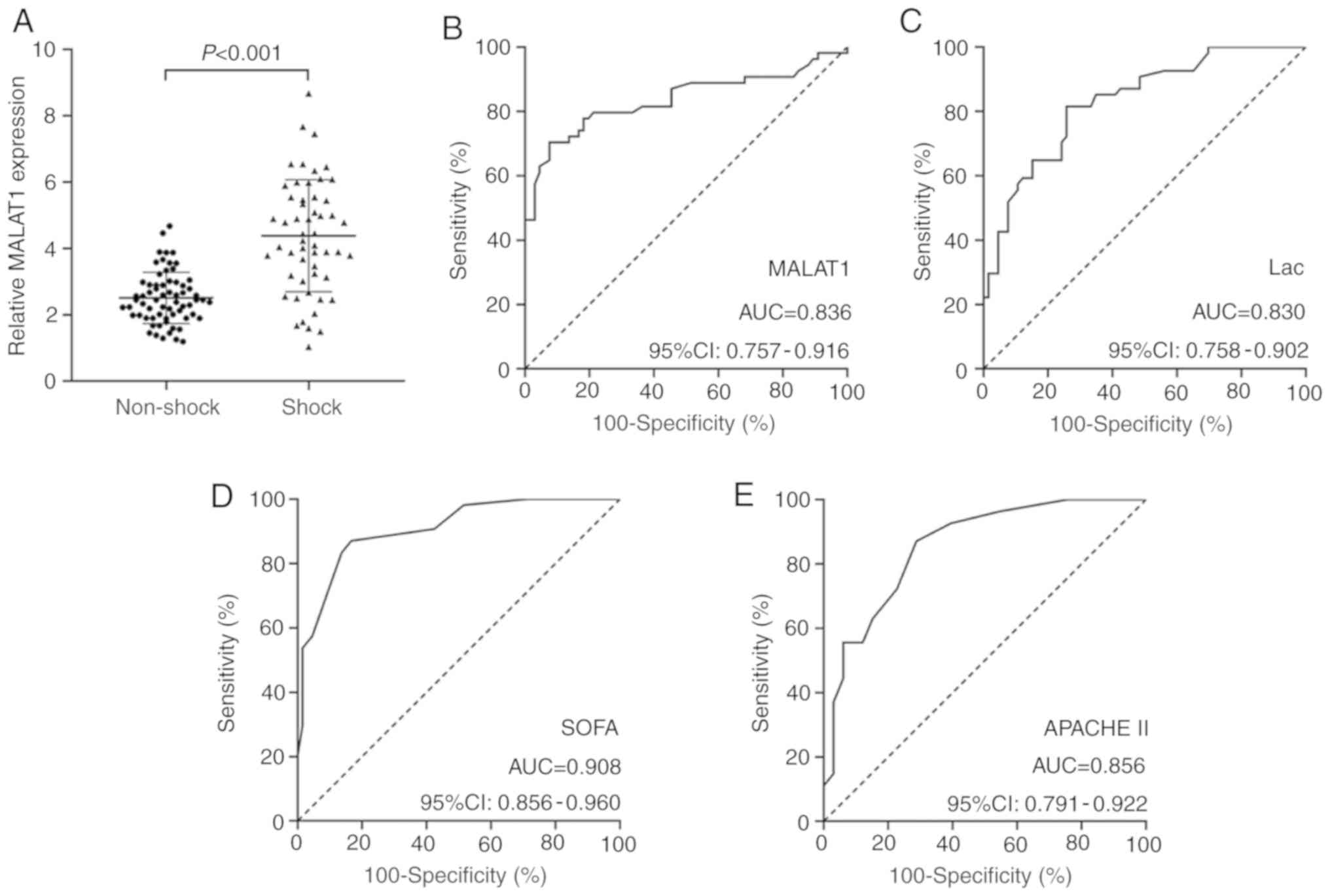
(P<0.001) and MALAT1 levels (P<0.001; Fig. 3A) were significantly higher in
patients with septic shock compared with in those without septic
shock. In addition, multivariate logistic regression analysis
demonstrated that high MALAT1 (P=0.030) or Lac (P=0.027) levels,
and SOFA (P=0.001) and APACHE II (P=0.011) scores were independent
risk factors for septic shock, whereas age (P=0.368) exhibited no
association with the severity of sepsis (Table IV).
 | Table III.Univariate analysis of
clinicopathological characteristics between patients with and
without septic shock. |
Table III.
Univariate analysis of
clinicopathological characteristics between patients with and
without septic shock.
| Factor | No septic shock
(n=66) | Septic shock
(n=54) | P-value |
|---|
| Age, years | 49.27±8.90 | 51.67±7.80 | 0.124a |
| Sex, n |
|
| 0.912b |
| Male | 30 | 24 |
|
| Female | 36 | 30 |
|
| PCT, ng/ml | 5.90
(2.00–10.40) | 5.50
(0.50–14.88) | 0.257c |
| Lac, mmol/l | 2.34
(1.72–3.72) | 5.07
(3.54–8.98) |
<0.001c |
| SOFA score | 4.97±2.13 | 10.31±3.51 |
<0.001c |
| APACHE II
score | 12.70±3.13 | 18.28±4.57 |
<0.001c |
 | Table IV.Multivariate logistic regression of
risk factors for septic shock. |
Table IV.
Multivariate logistic regression of
risk factors for septic shock.
| Factor | P-value | OR | 95% CI |
|---|
| Age, years | 0.368 | 1.036 | 0.959–1.119 |
| MALAT1 | 0.030 | 2.030 | 1.070–3.851 |
| Lac | 0.027 | 1.405 | 1.039–1.901 |
| SOFA | 0.001 | 1.580 | 1.196–2.086 |
| APACHE II | 0.011 | 1.268 | 1.057–1.522 |
Diagnostic value of MALAT1 level for
septic shock
ROC curve analysis exhibited significant predictive
value for MALAT1 (AUC=0.836) in distinguishing patients with septic
shock from those without septic shock (Fig. 3B). This value was higher compared
with that for Lac (AUC=0.830; Fig.
3C), but lower compared with those for SOFA (AUC=0.908;
Fig. 3D) and APACHE II (AUC=0.856;
Fig. 3E) scores. At the optimal
cut-off point of >3.665 for MALAT1 levels, the specificity and
sensitivity were 92.42 and 70.37%, respectively. At the optimal
cut-off point of >3.215 for Lac levels, the specificity and
sensitivity were 74.24 and 81.48%, respectively. At the optimal
cut-off point of >6 for SOFA scores, the specificity and
sensitivity were 83.33 and 87.04%, respectively. At the optimal
cut-off point of >13 for APACHE II scores, the specificity and
sensitivity were 71.21 and 87.04%, respectively.
MALAT1 is an independent risk factor
for poor prognosis of sepsis
As presented in Table
V, univariate analysis revealed that age (P=0.496) and sex
(P=0.939) were not significantly different between survivors and
non-survivors. However, septic shock (P<0.001), PCT levels
(P=0.038), Lac levels (P<0.001), and SOFA (P<0.001) and
APACHE II (P<0.001) scores, as well as MALAT1 plasma levels
(Fig. 4A; P<0.001) were
significantly increased in non-survivors compared with survivors.
Further multivariate logistic regression analysis demonstrated that
high MALAT1 (P=0.015) and Lac (P=0.023) levels, SOFA (P=0.011) and
APACHE II (P=0.028) scores, and septic shock (P=0.043) were all
independent predictive factors for poor prognosis of sepsis,
whereas PCT levels (P=0.156) were not associated with prognosis in
patients with sepsis (Table
VI).
 | Table V.Univariate analysis of
clinicopathological characteristics between survivors and
non-survivors. |
Table V.
Univariate analysis of
clinicopathological characteristics between survivors and
non-survivors.
| Factor | Survivors
(n=76) | Non-survivors
(n=44) | P-value |
|---|
| Age, years | 49.95±7.97 | 51.05±9.33 | 0.496a |
| Sex, n |
|
| 0.939b |
|
Male | 34 | 20 |
|
|
Female | 42 | 24 |
|
| Septic shock,
n |
|
|
<0.001b |
| No | 59 | 7 |
|
|
Yes | 17 | 37 |
|
| PCT, ng/ml | 5.20
(0.70–9.40) | 8.25
(0.67–20.20) | 0.038c |
| Lac, mmol/l | 2.37
(1.77–3.70) | 6.11
(3.97–11.60) |
<0.001c |
| SOFA score | 5.18±2.15 | 11.16±3.25 |
<0.001c |
| APACHE II
score | 13.03±3.31 | 18.98±4.33 |
<0.001c |
 | Table VI.Multivariate logistic regression of
mortality risk factors for sepsis. |
Table VI.
Multivariate logistic regression of
mortality risk factors for sepsis.
| Factor | P-value | OR | 95% CI |
|---|
| Septic shock (yes
vs. no) | 0.043 | 50.144 |
1.133–2,219.541 |
| MALAT1 | 0.015 | 3.819 | 1.303–11.189 |
| PCT | 0.156 | 1.133 | 0.954–1.345 |
| Lac | 0.023 | 1.933 | 1.096–3.410 |
| SOFA | 0.011 | 4.054 | 1.379–11.917 |
| APACHE II | 0.028 | 1.719 | 1.062–2.782 |
Prognostic value of MALAT1 level for
sepsis
ROC curve analysis exhibited significant predictive
value for MALAT1 levels (AUC=0.886) at distinguishing non-survivors
from survivors (Fig. 4B), which
was higher compared with Lac levels (AUC=0.868; Fig. 4C) and APACHE II scores (AUC=0.868;
Fig. 4E), but lower compared with
SOFA scores (AUC=0.943; Fig. 4D).
At the optimal cut-off point of >3.62 for MALAT1 levels, the
specificity and sensitivity were 89.47 and 81.82%, respectively. At
the optimal cut-off point of >3.51 for Lac levels, the
specificity and sensitivity were 75 and 88.64%, respectively. At
the optimal cut-off point of >6 for SOFA scores, the specificity
and sensitivity were 77.63 and 93.18%, respectively. At the optimal
cut-off point of >15 for APACHE II scores, the specificity and
sensitivity were 86.84 and 77.27%, respectively.
Discussion
MALAT1 is ubiquitously expressed in the majority of
human tissues and body fluids, which suggests that MALAT1 may be a
potential biomarker for disease diagnosis, prognosis and treatment
(24). A large proportion of
current studies focus on the underlying molecular mechanisms of
MALAT1 function in the regulation of various pathophysiological
processes (12,25,26);
however, few studies have been conducted to investigate the
clinical application of MALAT1 as a biomarker (21,27).
To the best of our knowledge, the present study is the first to
evaluate the independent risk and predictive value of MALAT1 as a
biomarker for the diagnosis, severity and prognosis of sepsis.
MALAT1 is located on human chromosome 11q13
(28), and serves pivotal roles in
the inflammatory regulation of sepsis and septic organ dysfunction;
for instance, the downregulation of MALAT1 attenuated cardiac
inflammation and microvascular endothelial cell injury in rats with
sepsis by inhibiting the inflammatory response and apoptosis
(29,30). The knockdown of MALAT1 suppressed
lipopolysaccharide (LPS)-induced acute kidney and lung injury by
alleviating the inflammatory response through the
microRNA-146a/NF-κB pathway (31,32).
In addition, MALAT1 has been demonstrated to be upregulated in
LPS-induced macrophages and chondrocytes, whereas the knockdown of
MALAT1 enhanced LPS-induced inflammatory injury by increasing the
expression levels of the tumor necrosis factor (TNF)-α and
interleukin (IL)-6 through negative regulation of the NF-κB pathway
(33–35). These results indicated that MALAT1
may be a pro- or anti-inflammatory regulator in different tissues
and cells induced by LPS, which may be associated with the
imbalance in the immune system during septic injury. Similarly, the
results of the present study demonstrated that MALAT1 plasma levels
were significantly increased in patients with sepsis. Higher
expression levels of MALAT1 were also observed in patients with
septic shock and those who succumbed to sepsis compared with the
respective control groups.
To determine whether MALAT1 was associated with the
diagnosis, severity and prognosis of sepsis, the correlation
between MALAT1 levels and conventional indicators of sepsis,
including PCT and Lac levels, as well as SOFA and APACHE II scores,
was further evaluated. PCT is ubiquitously expressed in parathyroid
glands during bacterial infection and is likely to be induced by
TNF-α and IL-6 (36). PCT has been
used in the diagnosis of sepsis for decades; in the present study,
PCT levels were identified as an independent risk and diagnostic
factor for sepsis, and MALAT1 levels exhibited a weak positive
correlation with PCT levels. Dahaba and Metzler (37) reported that PCT levels were closely
correlated with the severity of sepsis and exhibited a significant
predictive value for the prognosis of sepsis on day 6 following
admission. However, PCT levels had no independent predictive value
for the severity and prognosis of sepsis in the present study,
which may be due to the smaller sample size and earlier time of PCT
level analysis (within 24 h of admission). Arterial Lac levels,
SOFA and APACHE II scores are commonly used to assess the severity
and prognosis of sepsis (2,8). In
accordance with previous studies, the results of the present study
demonstrated that high Lac levels, SOFA and APACHE II scores were
independent predictive factors for the severity and mortality of
sepsis. In addition, MALAT1 levels exhibited a weak positive
correlation with Lac levels, SOFA and APACHE II scores. These
results suggested that plasma MALAT1 expression may be associated
with the diagnosis, severity and prognosis of sepsis.
The diagnostic and prognostic value of MALAT1 has
been investigated in various types of cancer and diabetic
retinopathy (27,38). Recently, two studies reported the
predictive values of the plasma lncRNAs nuclear enriched abundant
transcript 1 and intersectin 1–2 for the diagnosis and prognosis of
sepsis (39,40). In the present study, multivariate
logistic regression analysis revealed that MALAT1 was an
independent risk biomarker for the diagnosis, severity and poor
prognosis of sepsis. The results of the ROC curve analysis revealed
significant predictive value for MALAT1 in differentiating patients
with sepsis from HCs, patients with septic shock from those without
septic shock, and non-survivors from survivors. These results
indicated that MALAT1 might serve as a biomarker for the diagnosis,
severity and prognosis of sepsis. It could be speculated that
MALAT1 may regulate the inflammatory response to infection by
targeting various genes and signaling pathways, inducing the
development and progression of sepsis and increasing the risk of
mortality.
There were certain limitations in this study.
Firstly, this study was performed in a single center and the sample
size was relatively small. Secondly, as a diagnostic biomarker,
only the predictive value of MALAT1 in the diagnosis of sepsis was
evaluated, whereas detailed mechanistic studies were not performed.
The role and mechanism of MALAT1 in the pathogenesis of sepsis
require further investigation. Thirdly, the 28-day follow-up period
was relatively short, and MALAT1 expression was measured only once
for each participant. A longer follow-up period, in addition to the
continuous measurements of MALAT1, may yield more persuasive
results regarding MALAT1 and sepsis-associated mortality. Finally,
a systemic inflammatory response syndrome (SIRS) category was not
set in this study. Sepsis was initially defined as an infection
with at least two of the four SIRS criteria (41); since SIRS criteria do not reflect
poor prognosis, sepsis is currently defined as a dysregulated host
response to infection with a SOFA score ≥2 points (2). The emphasis of this new definition is
placed on mortality prediction rather than early diagnosis. Thus,
the distinction between sepsis and SIRS still presents difficulty
in early diagnosis of sepsis. Further mechanistic studies are
necessary to determine whether MALAT1 may be a useful biomarker in
distinguishing sepsis from SIRS.
In conclusion, the results of the present study
demonstrated that plasma MALAT1 was upregulated in patients with
sepsis, and may serve as a potential biomarker for the diagnosis,
severity and prognosis of sepsis. Further investigations with
larger sample sizes from multiple centers and detailed mechanistic
studies are required to evaluate the clinical application of MALAT1
as a biomarker and therapeutic target for sepsis.
Acknowledgements
The authors would like to thank Dr Yuan Xue and Dr
Xinxin Li (Department of Intensive Care Medicine, The First
People's Hospital of Yancheng) for their technical assistance in
plasma/serum samples preparation.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS and JC designed and coordinated the study. JC, YH
and YD collected the samples and data. LS, LZ and YD performed the
statistical analyses and produced graphs. YH and LZ drafted the
manuscript. LS and YD revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First People's Hospital of Yancheng and complied with the
ethics standards of the Declaration of Helsinki. All participants
or their statutory guardians provided written informed consent. All
identifying information of each participant was removed from this
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rhodes A, Evans LE, Alhazzani W, Levy MM,
Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally
ME, et al: Surviving sepsis campaign: International guidelines for
management of sepsis and septic shock: 2016. Intensive Care Med.
43:304–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K;
International Forum of Acute Care Trialists, : Assessment of global
incidence and mortality of hospital-treated sepsis. Current
estimates and limitations. Am J Respir Crit Care Med. 193:259–272.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Angus DC and van der Poll T: Severe sepsis
and septic shock. N Engl J Med. 369:20632013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mira JC, Gentile LF, Mathias BJ, Efron PA,
Brakenridge SC, Mohr AM, Moore FA and Moldawer LL: Sepsis
pathophysiology, chronic critical illness, and persistent
inflammation-immunosuppression and catabolism syndrome. Crit Care
Med. 45:253–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Poll T, van de Veerdonk FL,
Scicluna BP and Netea MG: The immunopathology of sepsis and
potential therapeutic targets. Nat Rev Immunol. 17:407–420. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rivers E, Nguyen B, Havstad S, Ressler J,
Muzzin A, Knoblich B, Peterson E and Tomlanovich M; Early
Goal-Directed Therapy Collaborative Group, : Early goal-directed
therapy in the treatment of severe sepsis and septic shock. N Engl
J Med. 345:1368–1377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Godinjak A, Iglica A, Rama A, Tančica I,
Jusufović S, Ajanović A and Kukuljac A: Predictive value of SAPS II
and APACHE II scoring systems for patient outcome in a medical
intensive care unit. Acta Med Acad. 45:97–103. 2016.PubMed/NCBI
|
|
9
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Hamblin MH and Yin KJ: The long
noncoding RNA Malat1: Its physiological and pathophysiological
functions. RNA Biol. 14:1705–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lipovich L, Dachet F, Cai J, Bagla S,
Balan K, Jia H and Loeb JA: Activity-dependent human brain
coding/noncoding gene regulatory networks. Genetics. 192:1133–1148.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watts R, Johnsen VL, Shearer J and Hittel
DS: Myostatin-induced inhibition of the long noncoding RNA Malat1
is associated with decreased myogenesis. Am J Physiol Cell Physiol.
304:C995–C1001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michalik KM, You X, Manavski Y,
Doddaballapur A, Zörnig M, Braun T, John D, Ponomareva Y, Chen W,
Uchida S, et al: Long noncoding RNA MALAT1 regulates endothelial
cell function and vessel growth. Circ Res. 114:1389–1397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshimoto R, Mayeda A, Yoshida M and
Nakagawa S: MALAT1 long non-coding RNA in cancer. Biochim Biophys
Acta. 1859:192–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uchida S and Dimmeler S: Long noncoding
RNAs in cardiovascular diseases. Circ Res. 116:737–750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Tang X, Liu K, Hamblin MH and Yin
KJ: Long noncoding RNA malat1 regulates cerebrovascular pathologies
in ischemic stroke. J Neurosci. 37:1797–1806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vergadi E, Vaporidi K and Tsatsanis C:
Regulation of endotoxin tolerance and compensatory
anti-inflammatory response syndrome by Non-coding RNAs. Front
Immunol. 9:27052018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang H, Liang N, Wang M, Fei Y, Sun J, Li
Z, Xu Y, Guo C, Cao Z, Li S and Jiao Y: Long noncoding RNA MALAT-1
is a novel inflammatory regulator in human systemic lupus
erythematosus. Oncotarget. 8:77400–77406. 2017.PubMed/NCBI
|
|
21
|
Shaker OG, Mahmoud RH, Abdelaleem OO,
Ibrahem EG, Mohamed AA, Zaki OM, Abdelghaffar NK, Ahmed TI, Hemeda
NF, Ahmed NA and Mansour DF: LncRNAs, MALAT1 and lnc-DC as
potential biomarkers for multiple sclerosis diagnosis. Biosci Rep.
39:BSR201813352019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan F, Zhu L, Lv H and Pei C: Quercetin
promotes the apoptosis of fibroblast-like synoviocytes in
rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med.
38:1507–1514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tano K, Mizuno R, Okada T, Rakwal R,
Shibato J, Masuo Y, Ijiri K and Akimitsu N: MALAT-1 enhances cell
motility of lung adenocarcinoma cells by influencing the expression
of motility-related genes. FEBS Lett. 584:4575–4580. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang C, Han J, Wu Y, Li S, Wang Q, Lin W
and Zhu J: Exosomal MALAT1 derived from oxidized low-density
lipoprotein-treated endothelial cells promotes M2 macrophage
polarization. Mol Med Rep. 18:509–515. 2018.PubMed/NCBI
|
|
26
|
Gao F, Chen R, Xi Y, Zhao Q and Gao H:
Long noncoding RNA MALAT1 regulates sepsis in patients with burns
by modulating miR-214 with TLR5. Mol Med Rep. 19:3756–3766.
2019.PubMed/NCBI
|
|
27
|
Shaker OG, Abdelaleem OO, Mahmoud RH,
Abdelghaffar NK, Ahmed TI, Said OM and Zaki OM: Diagnostic and
prognostic role of serum miR-20b, miR-17-3p, HOTAIR, and MALAT1 in
diabetic retinopathy. IUBMB Life. 71:310–320. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang B, Arun G, Mao YS, Lazar Z, Hung G,
Bhattacharjee G, Xiao X, Booth CJ, Wu J, Zhang C and Spector DL:
The lncRNA Malat1 is dispensable for mouse development but its
transcription plays a cis-regulatory role in the adult. Cell Rep.
2:111–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen H, Wang X, Yan X, Cheng X, He X and
Zheng W: LncRNA MALAT1 regulates sepsis-induced cardiac
inflammation and dysfunction via interaction with miR-125b and p38
MAPK/NFkB. Int Immunopharmacol. 55:69–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu Z, Rayile A, Zhang X, Li Y and Zhao Q:
Ulinastatin protects against lipopolysaccharide-induced cardiac
microvascular endothelial cell dysfunction via downregulation of
lncRNA MALAT1 and EZH2 in sepsis. Int J Mol Med. 39:1269–1276.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai L, Zhang G, Cheng Z, Wang X, Jia L,
Jing X, Wang H, Zhang R, Liu M, Jiang T, et al: Knockdown of LncRNA
MALAT1 contributes to the suppression of inflammatory responses by
up-regulating miR-146a in LPS-induced acute lung injury. Connect
Tissue Res. 59:581–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding Y, Guo F, Zhu T, Li J, Gu D, Jiang W,
Lu Y and Zhou D: Mechanism of long non-coding RNA MALAT1 in
lipopolysaccharide-induced acute kidney injury is mediated by the
miR-146a/NF-κB signaling pathway. Int J Mol Med. 41:446–454.
2018.PubMed/NCBI
|
|
33
|
Pan L, Liu D, Zhao L, Wang L, Xin M and Li
X: Long noncoding RNA MALAT1 alleviates lipopolysaccharide-induced
inflammatory injury by upregulating microRNA-19b in murine
chondrogenic ATDC5 cells. J Cell Biochem. 119:10165–10175. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gast M, Rauch BH, Nakagawa S, Haghikia A,
Jasina A, Haas J, Nath N, Jensen L, Stroux A, Böhm A, et al: Immune
system-mediated atherosclerosis caused by deficiency of long
non-coding RNA MALAT1 in ApoE-/-mice. Cardiovasc Res. 115:302–314.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao G, Su Z, Song D, Mao Y and Mao X: The
long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced
inflammatory response through its interaction with NF-κB. FEBS
Lett. 590:2884–2895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castelli GP, Pognani C, Cita M, Stuani A,
Sgarbi L and Paladini R: Procalcitonin, C-reactive protein, white
blood cells and SOFA score in ICU: Diagnosis and monitoring of
sepsis. Minerva Anestesiol. 72:69–80. 2006.PubMed/NCBI
|
|
37
|
Dahaba AA and Metzler H: Procalcitonin's
role in the sepsis cascade. Is procalcitonin a sepsis marker or
mediator? Minerva Anestesiol. 75:447–452. 2009.PubMed/NCBI
|
|
38
|
Tian X and Xu G: Clinical value of lncRNA
MALAT1 as a prognostic marker in human cancer: Systematic review
and meta-analysis. BMJ Open. 5:e0086532015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zeng Q, Wu J and Yang S: Circulating
lncRNA ITSN1-2 is upregulated, and its high expression correlates
with increased disease severity, elevated inflammation, and poor
survival in sepsis patients. J Clin Lab Anal. 33:e228362019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang Q, Huang C, Luo Y, He F and Zhang R:
Circulating lncRNA NEAT1 correlates with increased risk, elevated
severity and unfavorable prognosis in sepsis patients. Am J Emerg
Med. 36:1659–1663. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
American college of chest
physicians/society of critical care medicine consensus conference,
. Definitions for sepsis and organ failure and guidelines for the
use of innovative therapies in sepsis. Crit Care Med. 20:864–874.
1992. View Article : Google Scholar : PubMed/NCBI
|