Introduction
The lens of the eye contains high concentrations of
antioxidant compounds, such as reduced glutathione (GSH) and
ascorbic acid (AsA), which prevent oxidative stress caused by
ultraviolet light or reactive oxygen species (ROS) (1). In addition to GSH and AsA, catalase
(CAT) is well known to protect the lens from damage induced by
hydrogen peroxide, by decomposing it to water and oxygen (2). Approximately 80% of the information
we get daily is through the eye, and the lens is necessary to
maintain transparency. Opacification of the lens causes lack of
vision, which is called cataract. Given that lens opacity is a
direct result of oxidative stress, GSH and AsA levels and CAT
activities are frequently used as markers of cataract formation for
both human and in animal models (3,4).
It has been reported that the levels of oxidative
stress markers in the blood are increased in patients with cataract
(5). Oxidative stress in the blood
causes cataract and other life-style related diseases due to an
imbalance in the ratio of ROS and antioxidants (6). Superoxide dismutase (SOD) is a
reaction enzyme that catalyzes the dismutation of superoxide into
oxygen and hydrogen peroxide and maintains the body redox state in
coordination with CAT (7). In the
current study, we measured the plasma SOD and CAT activities to
assess the redox state of the body and lens by antioxidant compound
consumption.
We previously reported that a subcutaneous injection
of hesperetin (Hst; Fig. 1A) can
prevent or delay the onset of cataracts, as assessed using
selenite-induced animal cataract models (8,9).
Hst, which is an abundant and inexpensive plant flavanone largely
derived from citrus species, has a flavanone backbone structure and
strong antioxidant activity. It is the aglycone of hesperidin (Hsd;
Fig. 1B). Hst and Hsd are called
bioflavonoids and were previously called Vitamin P because of their
various biological activities, including anti-inflammatory,
anti-oxidative, anti-diabetic, anti-hypertensive, improvement of
very low-density lipoprotein (VLDL) metabolic abnormality, and
their ability to decrease the capillary permeability (10–12).
However, the bioavailability of Hst and Hsd after oral consumption
is reported to be slow and irregular because of their poor
solubility. To address these weaknesses, Hijiya and Miyake
(13) created α-glucosyl Hsd
(G-Hsd; Fig. 1C); the water
solubility of G-Hsd was about 10,000 times higher than that of Hsd.
High water solubility is a good advantage for creation of oral
drugs and/or healthy food product. Thus, for the in vivo
experiment in this paper, we used orally administered G-Hsd to
assess the anti-cataract activity of Hst.
Lens epithelial cells form a monolayer at the
anterior surface, and the fiber cells are differentiated from the
epithelial cells at the equatorial surface, are elongated, and
compose the bulk of the lens. During their differentiation to
become mature fiber cells, all cytoplasmic organelles, such as
nucleus and mitochondria, are degraded (14). For these reasons, ROS are easier to
generate in lens epithelial cells; the generated ROS are diffused
within the fiber cells, inducing protein aggregation and causing
cataract. Therefore, investigate the molecular mechanisms using
lens epithelial cell lines is a useful tool for finding an
anti-cataract drug; however, there are no reports about the effect
of Hst on the lens epithelial cells. In the current study, we
investigated the anti-cataract activity of G-Hsd oral consumption
using both in vivo and in vitro experiments.
Materials and methods
Materials
G-Hsd (including >80% α-glucosyl Hsd) was
provided by Hayashibara Co. Sprague-Dawley (SD) rats were obtained
from Japan SLC Inc., and balanced chow for rats (CE-2) was obtained
from Clea Japan Inc. Isoflurane, sodium selenite, GSH, AsA, and
metaphosphoric acid were purchased from Wako Pure Chemical
industries, Ltd. Dithionitrobenzene (DTNB), trichloroacetic acid
Penicillin-Streptomycin antibiotics mixture, and Annexin V-FITC
apoptosis detection kit were purchased from Nakalai Tesque Inc. CAT
assay kit was obtained from Cayman Chemical Inc. SOD assay kit-WST
was purchased from Dojindo molecular Technologies, Inc. GlutaMAX
and Dulbecco's modified Eagle's medium/Nutrient Mixture F-12
(DMEM/F-12) were obtained from Gibco; Thermo Fisher Scientific
Inc.
Animals
SD rats had unlimited access to balanced chow CE-2
and drinking water and were housed in a temperature-controlled
(23°C±5°C) environment with a 12-h regular light/dark cycle. Rats
were sacrificed with isoflurane (5% inhalation). The Keio
University Animal Research Committee approved all animal procedures
performed in this study [12048-(4)]. All animals in this work were treated
according to the National Institutes of Health guide for the care
and use of laboratory animals.
Selenite-induced cataract and G-Hsd
treatment
Rats were randomized into 4 groups (Table I). Group 1: PBS treatment group
(control group: G1). Group 2: G-Hsd treatment group (G2). Group 3:
Sodium selenite treatment group (G3). Group 4: Sodium selenite and
G-Hsd treatment group (G4).
 | Table I.Experimental groups. |
Table I.
Experimental groups.
| Group | Challenge | Test compound | Administration
route |
|---|
| 1 | PBS | Vehicle | P.O |
| 2 | PBS | G-Hsd | P.O |
| 3 | Sodium
selenite | Vehicle | P.O |
| 4 | Sodium
selenite | G-Hsd | P.O |
Rats in each group received 0.2 ml
phosphate-buffered saline (PBS: 130 mM NaCl, 3 mM KCl, 10 mM
Na2HPO4, 2 mM KH2PO4;
pH 7.4) or an equal volume of 200 mg/kg G-Hsd dissolved in PBS via
a feeding tube 4 h before the sodium selenite injection, then once
a day for two days (total of 3 days). G-Hsd (>80%) was
administered in the rats in groups G2 and G4. After G-Hsd oral
administration, G-Hsd is hydrolysed by α-glucosidase in the
intestine from G-Hsd to Hsd. In the blood, all Hsd-related
compounds are Hsd or its aglycone, Hst Sodium selenite in a dose of
20 µmol/kg body weight was administered to the rats in groups G3
and G4, while rats in groups G1 and G2 received PBS as control.
After euthanization on day 6 (19-days old), enucleated eyes were
analyzed for GSH and AsA levels and lens CAT activities.
Cataract classification
Rats eyes were photographed on day 6 and the opacity
area was measured using ImageJ software. Cataract classification
was performed as previously described (15).
Measurement of GSH, AsA and catalase
activities in the lens
Levels of lens GSH and AsA were determined according
to a previously described method (16,17).
For the measurement of lens GSH, lenses were homogenized in 0.1 M
sodium phosphate buffer (pH 8.0) and centrifuged. The supernatant
fraction was deproteinized with trichloroacetic acid and
centrifuged. The supernatant sample was mixed with DTNB and
incubated at room temperature in dark. Absorbance at 412 nm was
measured using infinite M200 microplate reader after 30 min of
incubation, (Tecan Ltd.).
For AsA measurement, lenses were homogenized in 0.1
M phosphate buffered saline (PBS: pH 7.4) and mixed with
metaphosphoric acid to deproteinize. After centrifugation, the
supernatant sample was titrated with DCPIP. Absorbance at 540 nm
was measured in a microplate reader infinite M1000 (Tecan
Ltd.).
CAT activity was measured using the catalase assay
kit (Cayman Chemical) following the manufacturer's protocol.
Briefly, the lenses were homogenized in ice-cold 50 mM potassium
phosphate buffer (pH 7.0) containing 1 mM EDTA and were
centrifuged. The supernatant was mixed with
H2O2, potassium hydroxide, and the catalase
purpald. After 10-min incubation at room temperature, absorbance at
540 nm was measured using the infinite M1000 (Tecan Ltd.). The
standard curve of catalase was determined using a preparation with
formaldehyde.
Measurement of plasma SOD and CAT
activities
Under 5% isoflurane inhalation, blood samples were
immediately collected from the vena cava. Plasma samples and
erythrocytes were separated by centrifugation of the whole blood
with heparin. For SOD measurement, erythrocyte fractions were
re-suspended in Millipore purification water to cause hemolysis and
then an ethanol/chloroform mixture was added. After shaking,
samples were centrifuged and the water/ethanol fraction was
collected to measure the erythrocyte SOD activity. The SOD activity
of erythrocytes and plasma were measured using the SOD assay
kit-WST (Dojindo) with commercial methods. CAT activity in the
plasma was measured following the manufacturer's protocol. Briefly,
the plasma was mixed with H2O2, potassium
hydroxide, and the catalase purpald. After 10-minute incubation at
room temperature, absorbance at 540 nm was measured using the
infinite M1000. The standard curve of catalase was determined using
a preparation with formaldehyde.
Cell culture
Induced human lens epithelial cells (ihLECs) were
established from human lens epithelial cells transfected with SV40
large T antigen, using the immortalized cell preparation method
developed by Yamamoto et al (18). ihLECs were grown in DMEM/F-12 with
10% fetal bovine serum (FBS; Biosera), 4 mM glutamine (GlutaMAX:
Gibco), and penicillin-streptomycin antibiotic mixture (100 U/ml
and 100 mg/ml, respectively), under standard culture conditions of
5% CO2 at 37°C. Cells were plated in a DMEM/F-12 medium
24 h before the experiment. Four hours before the sodium selenite
or PBS treatment (10 µM), Hst dissolved in DMSO or vehicle was
added to the ihLECs at the concentrations of 50 or 100 mM,
respectively. Twenty-four hours after the Hst treatment, cells were
harvested and used for the following experiments.
Cell viability and cytotoxicity
assay
Cell viability and cytotoxicity was measured by
water-soluble tetrazolium (WST) dye. Briefly, cells were cultured
in a 96-well plate treated with Hst and/or sodium selenite, and 24
h after Hst or PBS treatment, Cell Count Reagent SF (Nacalai
Tesque) was added to each well and cells were incubated in normal
culture conditions for 1 h. After incubation, absorbances at
450/490 nm were measured using the microplate reader, Infinite 200
Pro.
Annexin V-FITC apoptosis
detection
Apoptotic cells were also analyzed utilizing an
Annexin V-FITC apoptosis detection kit (Nakalai Tesque). Briefly,
cells incubated with Hst and/or sodium selenite were harvested,
washed with ice-cold PBS, and stained with Annexin V-FITC and PI
according to the manufacturer's instructions. The resulting
fluorescence was detected by BD FACS LSR II (BD Biosciences).
Cell cycle parameter analysis
Cell cycles were analyzed using propidium iodide
(PI). Briefly, the cells treated with Hst and/or sodium selenite
were harvested using rubber- police man and fixed using pre-cold
70% (v/v) ethanol at −20°C. After an overnight incubation, cells
were centrifuged and treated with PBS containing 10 µg/ml RNAase A
(Nakalai Tesque). Subsequently, 100 µg/ml PI were added and the
cell cycle parameter was measured using FASC Caliber (BD
Bioscience).
DNA fragmentation assay
DNA fragmentation assay was performed using DNA
electrophoresis. Genomic DNA was prepared for electrophoresis on 1%
(w/v) agarose gel and was visualized by staining with ethidium
bromide after the electrophoresis.
Statistical analysis
All data are reported as the mean ± standard error.
Statistical analysis was performed using one-way analysis of
variance (ANOVA) with a post-hoc Tukey's multiple comparison test
with SPSS software, version 24 (IBM corporation). P-values less
than 0.05 indicated statistical significance.
Results
Effect of G-Hsd on selenite-induced
cataract formation in rats
First, we assessed the effect of G-Hsd on
selenite-induced cataract formation in rats. Thirteen-day-old SD
rats were randomly divided into two groups and injected with either
PBS (control group) or sodium selenite (cataract group). Each group
was further divided into two subgroups and received either PBS or
G-Hsd (200 mg/kg body weight) once a day for three days. In the
control group, there were no rats that had cataract regardless of
the G-Hsd treatment (Fig. 2A and
B). Among the selenite treatment group, rats that were given
PBS developed mature or premature nuclear cataracts; the cataract
was classified as grade 6 in 44%, grade 5 in 50%, and grade 4 in 6%
of the rats. Fig. 2C presents a
grade 6 mature cataract in the selenite cataract group (G3). In
contrast, rats treated with G-Hsd had delayed cataract development
(cataract grade 3 in this group: Fig.
2D). The rats treated with selenite and G-Hsd did not have
central opacity. Their cataract grades were as follows: Grade 5 in
7%, grade 4 in 14%, grade 3 in 29%, grade 2 in 36%, and grade 1 in
14% of the rats (Fig. 2E). These
results indicated that G-Hsd had an anti-cataract effect in the
selenite-induced cataract experimental model.
G-Hsd ameliorates the reduction of
GSH, AsA, and CAT activity induced by sodium selenite
Thereafter, we measured the lens antioxidant
compound levels (GSH and AsA) because ROS is thought to be a major
cause of selenite-induced cataract. There were no changes in the
lens GSH concentrations between the control group and the G-Hsd
treated group (G1 vs. G2). The GSH concentrations in the rat lenses
treated with sodium selenite were significantly decreased (G3);
however, this reduction was reversed in the sodium selenite with
G-Hsd treated group (G4) (Fig.
3A). Similarly, the lens AsA concentrations were not changed
with or without G-Hsd treatment. These concentrations were
significantly reduced in the rat lenses treated with sodium
selenite. The G-Hsd treatment ameliorated the Se-induced AsA
reduction (Fig. 3B). These results
indicated that G-Hsd consumption inhibits the decrease in GSH and
AsA concentrations in selenite-induced cataract. We also measured
the CAT activity in the lenses of rats administered G-Hsd and/or
sodium selenite. In the control groups (G1 and G2), CAT activities
were not changed regardless of the G-Hsd treatment. In the groups
of sodium selenite treatment, the CAT activity was significantly
decreased compared with that in the controls; however, it was
reversed with the G-Hsd co-treatment (Fig. 3C). These results suggested that
G-Hsd consumption could prevent the onset of cataract due to
maintaining a reduced state in the lens.
G-Hsd treatment reverses the reduction
of antioxidant enzyme activities in blood
We measured the total-SOD activity (Cu/Mn SOD) in
erythrocytes and plasma using the SOD assay kit-WST (Dojindo). SOD
activity in the erythrocytes was not changed in the control and
G-Hsd treatment groups rats; however, it was significantly
decreased in the rats treated with sodium selenite, but this
reduction could be reversed by the co-treatment with G-Hsd and
sodium selenite (Fig. 4A).
Similarly, the SOD activity in the plasma was not different between
the control group rats (G1) and G-Hsd treatment group rats (G2),
and it was reduced in the sodium selenite group rats (G3); however,
the SOD activity was recovered by the G-Hsd treatment in the sodium
selenite cataract rats (G4) (Fig.
4B). Thereafter, we measured the plasma CAT activity in each
group. CAT activity in plasma was significantly decreased in the
rats treated with sodium selenite (G3); however, this phenotype was
canceled with the G-Hsd oral consumption (Fig. 4C). These results suggested that
G-Hsd administration could reverse the reduction of SOD and CAT
activities in the blood.
Hst cancels the cell death induced by
sodium selenite
From the above data, it is suggested that the
anti-cataract activity of G-Hsd could be induced by the inhibition
of lens epithelial cell damage. Therefore, we tested whether Hst,
since cells do not have α-glucosidase and Hst is an active
ingredient of G-Hsd, could prevent the cell death from sodium
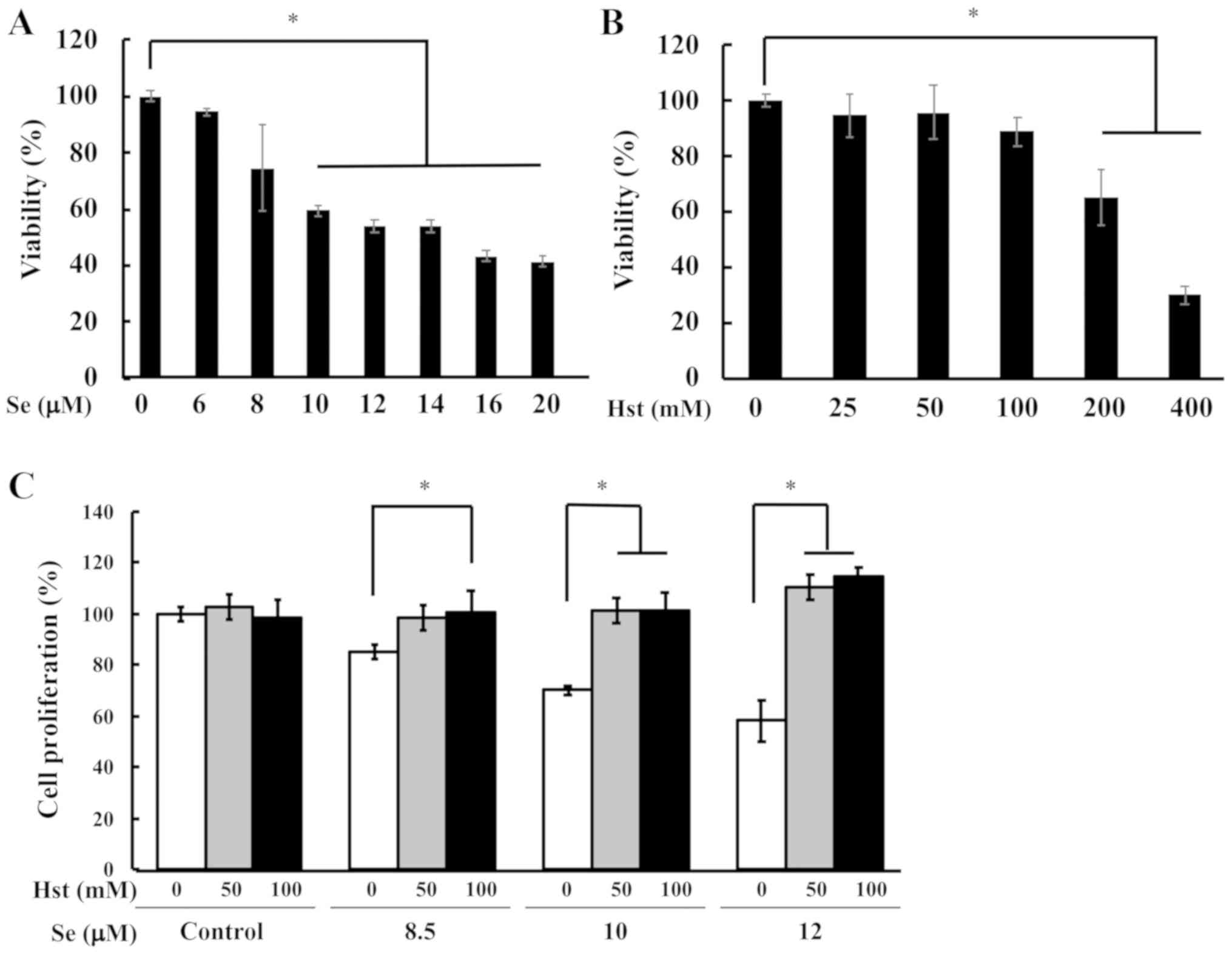
selenite using the ihLEC line. First, we checked the cell toxicity
of sodium selenite in ihLECs using WST-1 dye assay. The cell
proliferation was significantly decreased in the cells that were
exposed to 10 µM of sodium selenite, which was used for further
current study (Fig. 5A). We also
tested the cell toxicity of Hst in ihLECs ranging from 25 to 400 mM
(Fig. 5B). Thereafter, we tested
the cell proliferation ability for ihLEC co-treatment with Hst and
sodium selenite. After 24 h Hst treatment, cells were collected and
measured for cell toxicity. The cell proliferation ability was
significantly decreased in the ihLECs treated with sodium selenite,
but it could be reversed in the cells treated with Hst (Fig. 5C). These results suggested that Hst
could ameliorate cell death of ihLECs induced by sodium selenite in
a dose-dependent manner. From these data, we have decided to use 10
µM sodium selenite and 50 or 100 mM Hst for further studies.
Hst cancels the cell death induced by
sodium selenite
To identify the effect of Hst on cell death in
ihLECs, cells were treated with Annexin V and PI to measure the
percentage of apoptotic cells using flow cytometry. The cells
treated with PBS or Hst alone did not show apoptosis (Fig. 6A and B), and the number of
apoptotic cells increased with exposure to sodium selenite
(Fig. 6C); however, these were
rescued by co-treatment with sodium selenite and Hst (Fig. 6D). The percentages of apoptotic
cells are listed in Table II.
Thereafter, to clarify the role of sodium selenite in ihLEC cell
death, we measured the percentage of cells in the sub-G1 phase
treated with sodium selenite and/or Hst using PI staining. This was
found to be significantly increased after the sodium selenite
treatment without Hst; however, Hst treatment inhibited the sub-G1
population in a dose-dependent manner (Fig. 6E). Next, we performed a genomic DNA
fragmentation assay, which identified DNA fragmentation in the
sodium selenite treatment cells, but it was weakened with Hst
treatment in a dose-dependent manner (Fig. 6F). These results suggested that
sodium selenite could induce cell death in lens epithelial cells,
but Hst treatment could neutralize this action.
 | Table II.Percentage of apoptotic cells of
induced human lens epithelial cells. |
Table II.
Percentage of apoptotic cells of
induced human lens epithelial cells.
| Group | Apoptotic
cells |
|---|
| 1 | 8.03±1.33 |
| 2 | 3.00±2.48 |
| 3 | 49.03±1.46 |
| 4 | 7.17±2.46 |
Discussion
The aim of the current study was to evaluate the
preventive effect of G-Hsd on cataract formation on
selenite-induced cataract in vivo and in vitro, which
is a classical and widely accepted method because of its
effectiveness, the reproducibility of cataract formation, and its
similarity to human senile cataract characteristics (8,9).
Using this animal model, we previously reported that Hst could
prevent or delay the onset of cataract by a subcutaneous injection.
To the best of our knowledge, this study was the first report that
orally administered G-Hsd could prevent the cataract formation. We
detected 1.33±0.23 nmol/g Hst in the eye after 1,000 mg/kg G-Hsd
for 5 days administration orally. Hst and Hsd were reported to
modulate expressions of apoptosis regulatory proteins such as Bax,
Bcl-XL, and cleaved caspase-3 (19,20).
These data suggested that orally administered G-Hsd had direct
effect for anti-cataract to modulate apoptosis regulatory proteins
expression in lens and indirect effect for anti-cataract to
maintain the body redox state in blood. It has been reported that
Hsd could permeate across the blood-brain barrier (BBB) (21,22).
We hypothesised that Hst and/or Hsd could reach the lens through
the vitreous body via the blood-retinal-barrier (BRB) or aqueous
humor via the blood-aqueous-barrier (BAB) because the
characteristics of BRB and BHB are almost the same as those of the
BBB. Further studies are needed to investigate the route of the Hst
from the blood to the lens.
It is vital to study the long-term safety of
pharmacological therapies for cataract patients because drug
therapeutics for these patients should be applied for a long period
of time to get the effect of delay or prevent the development of
cataract effectively. Furthermore, it would provide significant
health and economic benefits to identify and devel effective
anti-cataract agents in the human diet that can be consumed daily.
Several researchers have used this model for finding new compounds
with anti-cataract effect, such as garlic and curcumin extracts
(23,24). From our laboratory, we previously
reported that daily coffee consumption could prevent the onset of
selenite-induced cataract formation (15,17).
Hst has several general health benefits, such as
anti-inflammatory, anti-hypertensive, and improvement of very
low-density lipoprotein (VLDL) metabolic abnormality (10–12).
Many health supplements containing G-Hsd and its related compounds
are sold in Japan under the system of foods with functional claims.
These supplements are claimed as functional substances because of
their role in maintaining blood triglyceride levels, blood
pressure, and peripheral blood flow. In the USA and other
countries, some supplements contain Hsd for its anti-inflammatory
and anti-hypersusceptibility properties. However, currently, no
health supplement claims to have an anti-cataract effect. Further
studies are needed to understand the molecular mechanisms
underlying the anti-cataract activity of G-Hsd.
Beside the lens GSH and AsA levels, we measured the
SOD and CAT activities in plasma to indicate the redox state of the
body in this study. It is difficult to measure the lens SOD
activity because lens contains high concentrations of GSH and AsA,
which are high antioxidant activity, and these compounds interfere
with this assay system. In the current study, we were unable to
detect plasma GSH levels using DTNB and AsA level using DCPIP
because plasma GSH and AsA levels were below the detection limit.
However, GSH and AsA in lens are came from blood through the
vitreous body via the BRB and aqueous humor via BAB. So, it is
important to evaluate plasma GSH and AsA levels to prevent
cataractgenesis. Further studies are needed to detector or HPLC
system with greater sensitivity to measure the GSH and AsA levels
by G-Hsd consumption.
Selenium is an essential micronutrient involved in
several important intracellular process, and low doses of selenite
treatment are inversely correlated with the risk of cancer that
acts as a pro-oxidant to induce tumor cell apoptosis (25,26).
For some cancer cells, sodium selenite acts an antioxidant that
protect cells against oxidative damage (27,28),
however, it is also known to increase intracellular ROS levels. The
environment of the lens epithelial cells is known to be in high
redox state to prevent oxidative damage. For lens cell, sodium
selenite may act as pro-oxidant and induced cell death. Further
studies are needed to understand the function of sodium selenite in
the lens.
In this current study, we measured and percentage of
sub G1 phase population and Annexin V/PI double staining using flow
cytometer. These methods are widely used for detection of apoptotic
cells. The flow cytometric analysis and DNA ladder formation
analysis also indicated that sodium selenite exposure induced
apoptosis in ihLEC cells, and the apoptosis cells of ihLEC was
significantly decreased by Hst treatment. It has been reported that
apoptosis in lens epithelial cells occurs at an early stage and
accelerates during the formation of selenite-induced cataract
(29). Thus, to prevent the
apoptosis in lens epithelial cells is one of the best ways to delay
the onset of cataract. In the current study, we have found that Hst
treatment could inhibit cell death in lens epithelial cells induced
by sodium selenite.
In this study, we used the selenite-induced cataract
model that is widely used for screening assay for anti-cataract
agents. We have clearly shown that G-Hsd oral consumption can delay
the onset of selenite- induced cataract in vivo, due to
reversal of the selenite-induced cell death. After human clinical
trial for humans, G-Hsd will be the first supplements that claimed
for anti-cataract supplement in the near future.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Keio
Gijuku Fukuzawa Memorial Fund for the Advancement of Education and
Research, and from the Japan Health Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YN and HT defined the research theme. YN, NM, SE,
MFT and HT designed the methods. YN, MA, SI, NN and NY performed
the laboratory experiments. YN, NN, MFT and HT analyzed and
interpreted the data. YN was major contributor in the writing of
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Keio
University Animal Research Committee [approval no. 12048-(4)].
Patient consent for publication
Not applicable.
Competing interests
NM and SE are employees of Hayashibara Co., Ltd.
(Okayama, Japan), and Hayashibara Co., Ltd. provided the
alpha-glucosyl hesperidin (G-Hsd) for these experiments.
Glossary
Abbreviations
Abbreviations:
|
Hst
|
hesperetin
|
|
Hsd
|
hesperidin
|
|
G-Hsd
|
α-glucosyl hesperidin
|
|
GSH
|
reduced glutathione
|
|
AsA
|
ascorbic acid
|
|
ROS
|
reactive oxygen species
|
|
CAT
|
catalase
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Hegde KR and Varma SD: Protective effect
of ascorbate against oxidative stress in the mouse lens. Biochim
Biophys Acta. 1670:12–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zigman S, Reddan J, Schultz JB and
McDaniel T: Structural and functional changes in catalase induced
by near-UV radiation. Photochem Photobiol. 63:818–824. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan X, Liu X, Hao S, Wang B, Robinson ML
and Monnier VM: The LEGSKO mouse: A mouse model of age-related
nuclear cataract based on genetic suppression of lens glutathione
synthesis. PLoS One. 7:e508322012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grey AC, Demarais NJ, West BJ and
Donaldson PJ: A quantitative map of glutathione in the aging human
lens. Int J Mass Spectrom. 437:58–68. 2019. View Article : Google Scholar
|
|
5
|
Katta AV, Katkam RV and Geetha H: Lipid
peroxidation and the total antioxidant status in the pathogenesis
of age related and diabetic cataracts: A study on the lens and
blood. J Clin Diagn Res. 7:978–981. 2013.PubMed/NCBI
|
|
6
|
Kim JH, Baik HW, Yoon YS, Joung HJ, Park
JS, Park SJ, Jang EJ, Park SW, Kim SJ, Kim MJ, et al: Measurement
of antioxidant capacity using the biological antioxidant potential
test and its role as a predictive marker of metabolic syndrome.
Korean J Intern Med. 29:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fridovich I: Superoxide radical and
superoxide dismutases. Annu Rev Biochem. 64:97–112. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakazawa Y, Oka M, Bando M and Takehana M:
Hesperetin prevents selenite-induced cataract in rats. Mol Vis.
21:804–810. 2015.PubMed/NCBI
|
|
9
|
Nakazawa Y, Oka M, Tamura H and Takehana
M: Effect of hesperetin on chaperone activity in selenite-induced
cataract. Open Med (Wars). 11:183–189. 2016.PubMed/NCBI
|
|
10
|
Miwa Y, Mitsuzumi H, Sunayama T, Yamada M,
Okada K, Kubota M, Chaen H, Mishima Y and Kibata M: Glucosyl
hesperidin lowers serum triglyceride level in hypertriglyceridemic
subjects through the improvement of very low-density lipoprotein
metabolic abnormality. J Nutr Sci Vitaminol (Tokyo). 51:460–470.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akiyama S, Katsumata S, Suzuki K, Nakaya
Y, Ishimi Y and Uehara M: Hypoglycemic and hypolipidemic effects of
hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki
rats with type 2 diabetes. Biosci Biotechnol Biochem. 73:2779–2782.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alu'datt MH, Rababah T, Alhamad MN,
Al-Mahasneh MA, Ereifej K, Al-Karaki G, Al-Duais M, Andrade JE,
Tranchant CC, Kubow S and Ghozlan KA: Profiles of free and bound
phenolics extracted from Citrus fruits and their roles in
biological systems: Content, and antioxidant, anti-diabetic and
anti-hypertensive properties. Food Funct. 8:3187–3197. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hijiya H and Miyake T: α-glycosyl
hesperidin, and its preparation and uses. Eur Patent no.
EP0402049A2. Filed June 31, 1990; issued July 12, 1995.
|
|
14
|
Cvekl A and Ashery-Padan R: The cellular
and molecular mechanisms of vertebrate lens development.
Development. 141:4432–4447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishimori N, Oguchi J, Nakazawa Y, Kobata
K, Funakoshi-Tago M and Tamura H: Roasting enhances the
anti-cataract effect of coffee beans: Ameliorating selenite-induced
cataracts in rats. Curr Eye Res. 42:864–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakazawa Y, Pauze M, Fukuyama K, Nagai N,
Funakoshi-Tago M, Sugai T and Tamura H: Effect of hesperetin
derivatives on the development of selenite-induced cataracts in
rats. Mol Med Rep. 18:1043–1050. 2018.PubMed/NCBI
|
|
17
|
Nakazawa Y, Ishimori N, Oguchi J, Nagai N,
Kimura M, Funakoshi-Tago M and Tamura H: Coffee brew intake can
prevent the reduction of lens glutathione and ascorbic acid levels
in HFD-fed animals. Exp Ther Med. 17:1420–1425. 2019.PubMed/NCBI
|
|
18
|
Yamamoto N, Kato Y, Sato A, Hiramatsu N,
Yamashita H, Ohkuma M, Miyachi E, Horiguchi M, Hirano K and Kojima
H: Establishment of a new immortalized human corneal epithelial
cell line (iHCE-NY1) for use in evaluating eye irritancy by in
vitro test methods. In Vitro Cell Dev Biol Anim. 52:742–748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samie A, Sedaghat R, Baluchnejadmojarad T
and Roghani M: Hesperetin, a citrus flavonoid, attenuates
testicular damage in diabetic rats via inhibition of oxidative
stress, inflammation, and apoptosis. Life Sci. 210:132–139. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanchang W, Khamchan A, Wongmanee N and
Seedadee C: Hesperidin ameliorates pancreatic β-cell dysfunction
and apoptosis in streptozotocin-induced diabetic rat model. Life
Sci. 235:1168582019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Youdim KA, Dobbie MS, Kuhnle G,
Proteggente AR, Abbott NJ and Rice-Evans C: Interaction between
flavonoids and the blood-brain barrier: In vitro studies. J
Neurochem. 85:180–192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takumi H, Mukai R, Ishiduka S, Kometani T
and Terao J: Tissue distribution of hesperetin in rats after a
dietary intake. Biosci Biotechnol Biochem. 75:1608–1610. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Javadzadeh A, Ghorbanihaghjo A, Arami S,
Rashtchizadeh N, Mesgari M, Rafeey M and Omidi Y: Prevention of
selenite-induced cataractogenesis in Wistar albino rats by aqueous
extract of garlic. J Ocul Pharmacol Ther. 25:395–400. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao J, Wang T and Wang M: Investigation of
the anti-cataractogenic mechanisms of curcumin through in vivo and
in vitro studies. BMC Ophthalmol. 18:482018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schrauzer GN: Selenium and
selenium-antagonistic elements in nutritional cancer prevention.
Crit Rev Biotechnol. 29:10–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terry PD, Qin B, Camacho F, Moorman PG,
Alberg AJ, Barnholtz-Sloan JS, Bondy M, Cote ML, Funkhouser E,
Guertin KA, et al: Supplemental selenium may decrease ovarian
cancer risk in African-American Women. J Nutr. 147:621–627. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Woth G, Nagy B, Mérei Á, Ernyey B, Vincze
R, Kaurics Z, Lantos J, Bogár L and Mühl D: The effect of
Na-selenite treatment on the oxidative stress-antioxidants balance
of multiple organ failure. J Crit Care. 29:e7–e11. 2014. View Article : Google Scholar
|
|
28
|
lçe F, Gök G and Pandir D: Acute effects
of lipopolysaccharide (LPS) in kidney of rats and preventive role
of vitamin E and sodium selenite. Hum Exp Toxicol. 38:547–560.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tamada Y, Fukiage C, Nakamura Y, Azuma M,
Kim YH and Shearer TR: Evidence for apoptosis in the selenite rat
model of cataract. Biochem Biophys Res Commun. 275:300–306. 2000.
View Article : Google Scholar : PubMed/NCBI
|