Introduction
Intestinal ischemia reperfusion (IIR) injury always
occurs following intestinal obstruction, shock, intestinal torsion
and mesenteric artery occlusion (1–3). IIR
frequently causes lung injury, including acute lung injury (ALI)
and acute respiratory distress syndrome (ARDS) (4,5).
Oxidative stress serves important functions in lung injury induced
by IIR, as free radicals are able to attack a number of cell
constituents (6) and activate the
processes of inflammation through transcription factors (7,8).
Hence, antioxidant therapy against lung injury during IIR is
imperative.
Dexmedetomidine (DEX) is primarily administered
during intensive care and anesthesia due to its sedative and
analgesic effects (9). It has been
demonstrated that DEX is able to suppress oxidative stress in
lipopolysaccharide-induced liver injury by exerting its effects on
α2 adrenoreceptors (10),
suggesting a potential protective effect for diseases associated
with oxidative stress. The transcription factor nuclear
factor-erythroid 2 related factor 2 (Nrf2) is able to bind to
antioxidant response elements, including heme oxygenase 1 (HO-1),
which antagonize reactive oxygen species (ROS)-associated oxidative
stress (11,12). Activation of Nrf2 has been
confirmed to rescue signaling pathways in order to inhibit
oxidative pulmonary injury and abnormal inflammatory response to
protect against lung injury in Staphylococcus aureus
pneumonia (13,14). Hence, the aim of the present study
was to investigate the effect of DEX on IIR-induced lung injury and
determine whether the protective function depended on the Nrf2/HO-1
pathway.
Materials and methods
Animals
The present study was approved by the animal welfare
committee of Fudan University (Shanghai, China). All experimental
procedures of the present study were performed in compliance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (15). Adult
male Sprague-Dawley (SD) rats (8–12 weeks old, 200–250 g) were
provided by the Animal Experimental Center of Fudan University. The
animals were housed in a room with a 12 h light-dark cycle under
controlled environmental conditions with a temperature of 22±1°C
and a relative humidity of 55±5%. Water and food were available
ad libitum. All rats were acclimatized to these conditions
for 1 week prior to the study. There were 110 rats used in total in
the present study.
IIR protocol and animal groups
Rats were anesthetized using pentobarbital (50
mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) by
intraperitoneal injection. Once the midline abdominal incision was
performed, the superior mesenteric artery (SMA) was isolated and
occluded for 60 min with an atraumatic microvascular clamp
(16,17). Then, the clip was then gently
release and the bowel perfusion was controlled by the presence of a
pulse. The abdominal wall was sutured prior to recovering from
anesthesia. After a 2 h reperfusion period, blood samples (5 ml
each sample) from each animal were collected from the arterial
line. A certain quantity of heparinized blood was used for the
measurement of arterial blood gas with automated blood gas analyzer
(ABL80 FLEX, Radiometer). Other samples were centrifuged at 3,500 ×
g for 15 min at 4°C to separate the serum. Serum samples were
stored at −80°C for further analysis. Meanwhile, lung tissues were
collected for histopathologic and biochemical analyses. Then, the
rats were decapitated under deep anesthesia. If the rats went into
shock or the IIR model was aborted, the animals were euthanized.
The duration of the experiment lasted ~4 h.
In the present study two experiments were performed.
Experiment 1 was designed to test the effects of DEX (Jiangsu
Hengrui Medicine Co., Ltd., Jiangsu, China) pretreatment on
pathological damage to the lung during IIR and to select the
optimal drug dose. At present, the majority of researchers have
selected 25–50 µg/kg DEX (injected intraperitoneally) in order to
study its protection against ischemia reperfusion injury (18–20).
Intravenous injection of DEX always requires small doses ranging
from 1 to 10 µg/kg (21,22). Hence, in the present study, three
different doses were assessed to select the optimal dose
intraperitoneally. A total of 60 SD male rats were randomly divided
into 6 groups (n=10 per group), as follows: Control group, IIR
group, IIR + normal saline (NS) group, IIR + DEX (10 µg/kg) group,
IIR + DEX (30 µg/kg) group and IIR + DEX (90 µg/kg) group. Rats in
the control group only underwent laparotomy without an
intraperitoneal operation. In the IIR + DEX groups, DEX was
intraperitoneally injected into the rats prior to releasing the
clamp. If DEX was able to protect against lung injury during IIR,
the best dose of DEX was selected based on if it altered the
pathological lesions substantially without obvious side effects.
The same volume of normal saline was selected to be the
control.
Experiment 2 was designed to study the effects of
DEX pretreatment on the Nrf2/HO-1 signaling pathway and whether its
action depended on an α2-adrenergic receptor. Oxidative damage and
inflammatory response were also detected. A total of 50 SD male
rats were randomly divided into 5 groups (n=10 per group), as
follows: Control group, IIR group, IIR + NS group, IIR + DEX group
and IIR + DEX + atipamezole (ATI; α2-adrenergic antagonist;
Sigma-Aldrich; Merck KGaA) group. Once the optimal dose of DEX was
determined, the corresponding dose of α2-adrenergic receptor
antagonist ATI was selected.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of the lung tissues was isolated using
TRIZOL® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
cDNA was synthesized using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) with the following
reaction conditions: 25°C for 10 min, 42°C for 1 h, 72°C for 10 min
and a 4°C hold. PCR was performed according to the following
thermocycling conditions: Predenaturation for 2 min at 95°C for a
cycle, denaturation for 15 sec at 95°C, annealing for 15 sec at
60°C and extension for 1 min at 72°C with 40 cycles from
denaturation to extension. RT-qPCR was performed using the SYBR
green method (FastStart Universal SYBR Master; Roche Diagnostics,
Basel, Switzerland) and a MiniOpticon RT-qPCR detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The method
2−ΔΔCq was used to determine the relative quantification
of the target gene expression (23). β-actin was selected as the
house-keeping gene. The following PCR primers (Sangon Biotech Co.,
Ltd. Shanghai, China) were used: Nrf2 forward,
5′-CACAGTGCTCCTATGCGTGA-3′ and reverse, 5′-TTCTGGGCGGCGACTTTATT-3′;
HO-1 forward, TGATGGCCTCCTTGTACC-3′ and reverse,
5′-GTGGGGCATAGACTGGGTTC-3′; β-actin forward,
5′-GGAAATCGTGCGTGACATTAAAG-3′ and reverse,
5′-CGGCAGTGGCCATCTCTT-3′.
Western blotting
T-PER protein extraction reagents (Thermo Fisher
Scientific Inc.) were used to extract the proteins of lung tissues.
Subsequently, the protein concentration in each sample was
determined using a BCA protein assay (Bio-Rad Laboratories, Inc.).
Equal amounts of proteins (30 µg per sample) were separated using
10% SDS-PAGE gel, transferred to polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc.). blocked with 5% nonfat dry milk at
room temperature for 1 h and incubated with primary antibodies
against HO-1 (1:200; cat. no. sc-136960; Santa Cruz Biotechnology,
Inc.), Nrf2 (1:200; cat. no. sc-365949; Santa Cruz Biotechnology,
Inc.) and GAPDH (1:1,000; cat. no. sc-365062; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. Then, the membranes were
washed and incubated with anti-rabbit immunoglobulin G (IgG)-HRP
(sc-2357) or anti-mouse IgG-HRP (sc-2005) secondary antibodies
(1:2,000; Santa Cruz Biotechnology, Inc.) for 2 h at 37°C. Proteins
were visualized using enhanced chemiluminescent reagents (Beyotime
Institute of Biotechnology, Jiangsu, China). The expression of
GAPDH was used as an internal control. The optical density of the
bands was measured using a densitometer (Syngene Europe) together
with Genesnap 4.0 and Genetools 4.0 software (Syngene Europe).
Biochemical analysis of lung tissues
and plasma
The lung tissues and plasma were used to evaluate
the malondialdehyde (MDA) levels and the myeloperoxidase (MPO) and
the superoxide dismutase (SOD) activities using a MDA detection kit
(A003-1-2), a MPO detection kit (A044-1-1) and a SOD detection kit
(A001-3-2) (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China), respectively, according to the manufacturer's protocol.
Enzyme-linked immunosorbent assay
(ELISA)
Whole blood was centrifuged for 15 min at 1,000 × g
at 4°C subsequent to collection. Plasma was removed immediately for
further analysis. Lung tissues were rinsed with ice-cold phosphate
buffered saline (PBS; 0.01 M, pH=7.4) to remove excess blood
thoroughly. Tissue pieces were weighed and then minced into small
pieces which were homogenized in PBS with a glass homogenizer on
ice. The homogenates were then centrifuged for 15 min at 4,000 × g
at −20°C to obtain the supernatant. The plasma and supernatant were
collected for ELISA. Interleukin-1β (IL-1β, F15810) and tumor
necrosis factor-α (TNF-α, F16960) concentrations in the plasma and
supernatant were measured using the ELISA kits (Shanghai Westang
Bio-Tech Co., Ltd.) in accordance with the manufacturer's
protocols.
Histopathology assessment
Lung tissues were harvested 2 h following IIR and
were fixed in 4% paraformaldehyde in PBS at room temperature for 2
h. Sections were stained with 0.45% hematoxylin for 10 min and 0.5%
eosin for 2 min at room temperature and observed under light
microscopy (magnification, ×40) to detect lung injury. Severity of
lung injury was evaluated as described from 0 to 5 grades (24): 0, normal tissue; 1, minimal
inflammatory change; 2, mild to moderate inflammatory changes (no
obvious damage to the lung architecture); 3, moderate inflammatory
injury (thickening of the alveolar septae); 4, moderate to severe
inflammatory injury (formation of nodules or areas of pneumonitis
that distorted the normal architecture); and 5, severe inflammatory
injury with total obliteration of the field.
Statistical analysis
All data were analyzed using SPSS 20.0 software (IBM
Corp., Armonk, NY, USA). Difference was assessed using a one-way
analysis of variance. Dunnett's test was used for multiple
comparisons. Data are expressed as the mean ± standard error of the
mean. P<0.05 was considered to indicate a statistically
significant difference.
Results
DEX alleviates IIR-induced lung
injury
Lung tissue in the IIR group was markedly damaged
with aberrant alveolar structures, notable cell infiltration,
alveolar thickening and diffuse interstitial edema (Fig. 1A). The alveolar structures were
integral in the control group. An intraperitoneal injection of DEX
at a concentration of 10 µg/kg did not decrease the pulmonary
damage induced by IIR (Fig. 1A).
However, 30 and 90 µg/kg DEX substantially alleviated
IIR-associated lung injury to a similar degree (Fig. 1A). The histopathological injury
scores in all groups are presented in Fig. 1B. Arterial blood gas levels were
also detected following reperfusion for 2 h. PaO2 and pH
were significantly lower in the IIR group compared with the control
group (Table I). Although 30 and
90 µg/kg DEX significantly increased oxygenation compared with the
IIR + NS group (P<0.01), rats with 90 µg/kg DEX exhibited more
adverse drug reactions, including a longer sedation time and
greater urinary volume. Hence, 30 µg/kg DEX was selected for
further experiments in order to determine the mechanism of its
protective effect on lung injury induced by IIR.
 | Figure 1.Histopathological changes in the lung
tissue of control, IIR, IIR + NS, IIR + DEX (10 µg/kg), IIR + DEX
(30 µg/kg) and IIR + DEX (90 µg/kg) groups. (A) Tissue sections of
the lung tissue stained with hematoxylin and eosin in different
groups. Magnification, ×40. (B) Pathological damage scores of the
lung tissue in the control, IIR + NS, IIR + DEX (10 µg/kg), IIR +
DEX (30 µg/kg) and IIR + DEX (90 µg/kg) groups. Data are presented
as the mean ± standard error of the mean. #P<0.05 vs.
control group; ##P<0.01 vs. control group; *P<0.05
vs. IIR + NS group; **P<0.01 vs. IIR + NS group. IIR, intestinal
ischemia reperfusion; DEX, dexmedetomidine; NS, normal saline. |
 | Table I.Arterial blood gas levels (n=10). |
Table I.
Arterial blood gas levels (n=10).
| Item | Control group | IIR group | IIR+NS group | IIR+DEX (10 µg/kg)
group | IIR+DEX (30 µg/kg)
group | IIR+DEX (90 µg/kg)
group |
|---|
| pH | 7.36+0.019 |
7.22+0.012a |
7.21+0.033a | 7.24+0.009
a |
7.31+0.022b,c |
7.29+0.013a |
|
PaO2 | 97.34+2.76 |
74.33+1.63a |
74.05+1.24a |
76.42+1.72a |
88.46+1.56a,c,e |
82.74+1.20a,c,e |
|
PaCO2 | 37.04+0.84 |
45.15+1.00a |
44.22+1.78a |
42.91+0.88a |
39.79+1.00d |
40.01+1.04d |
DEX decreases oxidative stress in lung
tissue and plasma
In order to evaluate oxidative damage, the present
study detected the MDA levels and SOD activity (25,26).
There was a significant increase in MDA levels in the lung tissue
and plasma of the IIR group compared with the control group
(P<0.05 in the plasma; P<0.01 in the lung; Table II). Pretreatment with DEX
significantly decreased the increase in MDA level induced by IIR in
the lung tissue and plasma (P<0.05; Table II). In the IIR group, SOD activity
was significantly lower compared with that in the control group
(P<0.01). DEX pretreatment significantly improved SOD activity
compared with normal saline (P<0.01; Table II).
 | Table II.Expression levels of SOD and MDA in
the lung and plasma of different groups (n=10). |
Table II.
Expression levels of SOD and MDA in
the lung and plasma of different groups (n=10).
| Item | Control group | IIR group | IIR + NS group | IIR + DEX
group |
|---|
| Pulmonary SOD
(U/ml) | 37.19±1.14 |
31.21±1.16b |
32.08±0.58b |
33.41±0.93d |
| Pulmonary MDA
(nmol/ml) | 2.61±0.08 |
3.16±0.06b |
3.15±0.08b |
2.90±0.06d |
| Plasma SOD
(U/ml) | 43.77±0.91 |
33.27±1.45b |
33.00±1.36b |
40.30±0.69a,d |
| Plasma MDA
(nmol/ml) | 2.92±0.12 |
3.45±0.04b |
3.40±0.06b |
3.13±0.06c |
DEX decreases the inflammatory
response
Activation and infiltration of polymorphonuclear
(PMN) cells are key factors resulting in IIR (27). As a marker enzyme of PMN, MPO is
able to reflect the degree of tissue PMN infiltration (28). There was a significant increase in
MPO concentration in the lung tissue and plasma of the IIR group
compared with the control group (P<0.01; Table III). Pretreatment with DEX
significantly suppressed the increase in MPO concentration induced
by IIR in the lung tissue and plasma (P<0.01; Table III). The expression of IL-1β and
TNF-α in lung tissue and plasma was determined via ELISA. IIR
caused the significantly increased release of inflammatory factors
in the lung tissue and plasma compared with the control group
(P<0.01; Table IV). DEX
significantly inhibited the IL-1β and TNF-α expression levels
induced by IIR (P<0.05 in the lung; P<0.01 in the plasma;
Table IV).
 | Table III.Expression levels of MPO in the lung
and plasma of different groups (n=10). |
Table III.
Expression levels of MPO in the lung
and plasma of different groups (n=10).
| Item | Control group | IIR group | IIR + NS group | IIR + DEX
group |
|---|
| Pulmonary MPO
(U/l) | 113.28±2.79 |
131.42±3.14b |
132.02±1.05b |
118.75±2.13c |
| Plasma MPO
(U/l) | 124.10±3.06 |
150.98±3.44b |
153.11±2.52b |
135.09±2.48a,c |
 | Table IV.Expression levels of IL-1β and TNF-α
in the lung and plasma of different groups (n=10). |
Table IV.
Expression levels of IL-1β and TNF-α
in the lung and plasma of different groups (n=10).
| Item | Control group | IIR group | IIR + NS group | IIR + DEX
group |
|---|
| Pulmonary IL-1β
(pg/ml) | 7.10±0.21 |
8.56±0.24b |
7.78±0.20b |
7.78±0.32a,c |
| Pulmonary TNF-α
(pg/ml) | 62.26±2.15 |
72.95±1.99b |
72.77±0.69b |
66.52±1.91c |
| Plasma IL-1β
(pg/ml) | 7.28±0.20 |
9.19±0.21b |
9.33±0.28b |
8.13±0.25a,d |
| Plasma TNF-α
(pg/ml) | 64.64±2.27 |
86.37±1.90b |
85.56±1.82b |
71.28±1.39a,d |
DEX enhances the expression of the
Nrf2/HO-1 signaling pathway in the IIR model
In order to assess whether the Nrf2/HO-1 signaling
pathway serves a function in the protective effect of DEX, the
present study measured the gene and protein levels of Nrf2 and
HO-1. RT-qPCR revealed that the gene levels of Nrf2 and HO-1 were
significantly downregulated in the lung tissue of IIR rats compared
with the control groups (P<0.01; Fig. 2). DEX at 30 µg/kg significantly
prevented the decrease in Nrf2 and HO-1 induced by IIR (P<0.01;
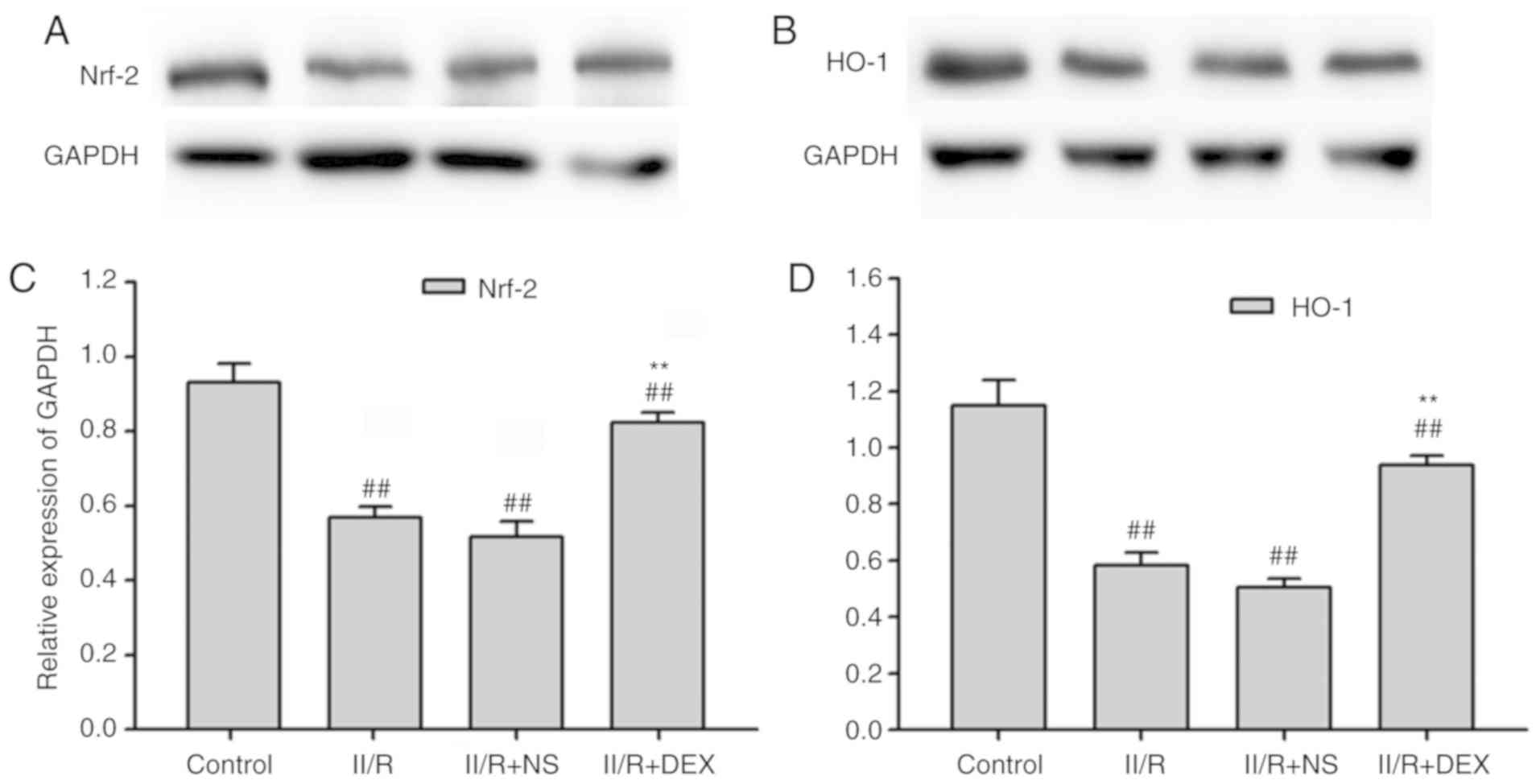
Fig. 2). Protein levels of Nrf2
and HO-1 were measured via western blotting in order to investigate
the effect of DEX on the IIR-induced inactivation of Nrf2/HO-1
signaling (Fig. 3A-D). Nrf2 and
HO-1 protein levels in the IIR group were significantly lower
compared with those in the control group (P<0.01). Pretreatment
with DEX significantly alleviated the IIR-induced inactivation of
Nrf2 and HO-1 in the lungs (P<0.01; Fig. 3). These results indicated that the
Nrf2/HO-1 signaling pathway served an important role in oxidative
stress. Hence, the results revealed that the antioxidant effect of
DEX may be associated with the Nrf2/HO-1 signaling pathway.
DEX protects against lung injury in
IIR rats via an α2-adrenoceptor
ATI is an α2-adrenoceptor antagonist. Co-treatment
with ATI (300 µg/kg) reversed the protective effect of 30 µg/kg DEX
on lung injury in IIR rats. The histopathological injury score in
the IIR + DEX + ATI group was higher compared with that in IIR +
DEX group (P<0.01; Fig. 4A and
B). A total of 300 µg/kg ATI also significantly inhibited the
effect of DEX on the expression levels of the Nrf2/HO-1 signaling
pathway (P<0.01; Fig. 4C and D)
and MDA levels and SOD activity in the lung tissue (P<0.05;
Fig. 4E and F). DEX significantly
decreased the MPO concentration, IL-1β and TNF-α expression levels
in the lung tissue of IIR rats compared with the NS group
(P<0.01), which was also significantly alleviated by ATI
(P<0.05; Fig. 4G-I).
 | Figure 4.Protective effects of DEX on lung
injury depend on α2-adrenergic receptors. (A) Tissue sections and
(B) pathological damage scores of the lung tissue stained with
hematoxylin and eosin in IIR + NS, IIR + DEX and IIR + DEX + ATI
groups. Magnification, ×40. (C) Western blotting of Nrf2/HO-1
expression in different groups. (D) Quantified western blotting
results. Expression levels of (E) SOD and (F) MDA in the lung of
different groups. Expression levels of (G) MPO, (H) IL-1β and (I)
TNF-α in the lungs of different groups. ##P<0.01 vs.
IIR + NS group; *P<0.05 vs. IIR + DEX group; **P<0.01 vs. IIR
+ DEX group. IIR, intestinal ischemia reperfusion; DEX,
dexmedetomidine; NS, normal saline; ATI; Nrf2, nuclear
factor-erythroid 2 related factor 2; HO-1, heme oxygenase 1; MPO,
myeloperoxidase; SOD, superoxide dismutase; MDA, malondialdehyde;
IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α. |
Discussion
The present study demonstrated that pretreatment
with DEX had a protective effect on IIR-associated acute lung
injury via the α2 adrenoreceptor. DEX may be activating the
Nrf2/HO-1 signaling pathway in order to decrease the oxidative
stress and inflammatory reaction, thus alleviating the lung injury
induced by IIR.
DEX was approved by the US Food and Drug
Administration in 1999 and has been used for analgesia and sedation
in intensive care units (29). In
animal studies, it has been demonstrated to inhibit oxidative
stress and inflammatory responses to protect the kidneys, brain,
intestine and heart from ischemia-reperfusion injury (16,30–34).
However, to the best of our knowledge, few studies have focused on
DEX on IIR-induced lung injury. The present study revealed that DEX
decreased the release of TNF-α and IL-6. In other models of
ischemia reperfusion, DEX was revealed to attenuate the apoptosis
of lung alveolar epithelial cells induced by oxidative stress by
inhibiting ROS generation (35).
Pretreatment with DEX decreased the MDA concentration and enhanced
SOD activity in patients with lung cancer during one-lung
ventilation (36). The present
study investigated the effect of DEX on lung injury during IIR, and
further identified that intraperitoneal DEX significantly
suppressed IIR-induced oxidation products, including MDA (P<0.01
in the lung and P<0.05 in the plasma), and enhanced SOD activity
(P<0.01 in the lung tissue and plasma). Since free radicals may
further promote the release of inflammatory cytokines (37), the present study also revealed that
a significant increase in the release of inflammatory cytokines
IL-1β (P<0.05 in the lung and P<0.01 in the plasma) and TNF-α
(P<0.05 in the lung and P<0.01 in the plasma) in the lung
during IIR was also inhibited by DEX. Among the oxidation products,
MDA (38) and SOD (39) are always used to assess the degree
of oxidative stress.
The affinity of DEX binding to α2-adrenergic
receptors is 8-fold greater compared with that of clonidine, and
DEX has a half-life of 6 min and an elimination half-life of 2 h
(29,40). Via α2-adrenergic receptors, DEX has
demonstrated sedative, analgesic, anti-anxiety and diuresis effects
(40,41). In addition, the anti-apoptosis
effect of DEX was inhibited by the α2-adrenoceptor antagonist ATI
in liver ischemia-reperfusion injury and renal ischemia-reperfusion
injury (10,18). The present study further
demonstrated that the antioxidant and anti-inflammatory action of
DEX in lung injury during IIR was also acting on the
α2-adrenoceptor.
Excessive ROS, produced during IIR, may overwhelm
the antioxidant capacity and result in the oxidative stress of lung
tissues (42,43). Nrf2 has been demonstrated to serve
an important role in the protection against oxidant-associated
lung, including ischemia reperfusion lung injury,
ventilation-induced lung injury and sepsis-induced lung injury
(44). Following activation, the
conformation of the Nrf2 complex changes, which inhibits the
proteasomal degradation of Nrf2 and facilitates Nrf2 translocation
into the nucleus to bind with antioxidant response element
sequences in the promoter regions (43–46).
Yan et al (47) reported
that DEX alleviates LPS-induced lung injury via activating
Nrf2/Keap1 signaling and inhibiting the inflammatory response and
oxidative stress in rats. However, the molecular mechanisms
underlying ALI/ARDS induced by IIR or LPS are different. IIR occurs
due to the transient obliteration of the SMA and reperfusion of the
ischemic bowel (48). As a common
complication of IIR, lung injury is caused by a systemic
inflammatory response due to the proinflammatory cytokines and
bacteria-derived endotoxins released from the damaged intestine
(49–51). Since the intestinal flora is
complex, lung injury induced by IIR may be further complicated by
gram-negative bacterial infections (49,52,53).
Pathological damage is more serious with alveolar exudation,
bleeding and invasion of immune cells, in addition to the release
of abundant cytokines and enzymes (53). Lipopolysaccharides (LPS) are a core
constituent of gram-negative bacterial cell walls. Hence,
LPS-induced lung injury is always used to study gram-negative
bacterial-associated lung injury (54). The protective mechanisms of DEX on
lung injury induced by IIR or LPS may not exactly be the same,
since the activation of the Nrf2/HO-1 pathway has been reported to
decrease oxidation products and improve intestinal mucosal injury
in an IIR model (55). HO-1 is
also a stress-responsive enzyme that produces antioxidants and
anti-inflammatory agents (56).
Therefore, the present study detected the effect of DEX on the
Nrf2/HO-1 signaling pathway and revealed that pretreatment with DEX
may activate the Nrf2/HO-1 signaling pathway in the pulmonary
tissue of IIR rats. Through this method, DEX may decrease the
oxidation products, inflammatory cytokines and pathological changes
induced by IIR.
In conclusion, pretreatment with DEX exhibited
potent antioxidant and anti-inflammatory properties in lung injury
induced by IIR. The Nrf2/HO-1 signaling pathway may serve a
function in the protective effect of DEX.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanghai
Jinshan Grants of National Health and Family Planning Commission,
Shanghai, China (grant no. JSKJ-KTQN-2016-09).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BX designed the study. YC performed the experiments
and analyzed the data. WB constructed the animal models, analyzed
part of the data, wrote the manuscript and created the figures.
Ethics approval and consent to
participate
All experimental procedures of the present study
were performed in compliance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen LW, Egan L, Li ZW, Greten FR, Kagnoff
MF and Karin M: The two faces of IKK and NF-kappaB inhibition:
Prevention of systemic inflammation but increased local injury
following intestinal ischemia-reperfusion. Nat Med. 9:575–581.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mallick IH, Yang W, Winslet MC and
Seifalian AM: Ischemia-reperfusion injury of the intestine and
protective strategies against injury. Dig Dis Sci. 49:1359–1377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Wen S, Yao X, Liu W, Shen J, Deng W,
Tang J, Li C and Liu K: MicroRNA-378 protects against intestinal
ischemia/reperfusion injury via a mechanism involving the
inhibition of intestinal mucosal cell apoptosis. Cell Death Dis.
8:e31272017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tadros T, Traber DL, Heggers JP and
Herndon DN: Effects of interleukin-1alpha administration on
intestinal ischemia and reperfusion injury, mucosal permeability,
and bacterial translocation in burn and sepsis. Ann Surg.
237:101–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higuchi S, Wu R, Zhou M, Marini CP,
Ravikumar TS and Wang P: Gut hyperpermiability after ischemia and
reperfusion: Attenuation with adrenomedullin and its binding
protein treatment. Int J Clin Exp Pathol. 1:409–418.
2008.PubMed/NCBI
|
|
6
|
Isik A, Peker K, Gursul C, Sayar I, Firat
D, Yilmaz I and Demiryilmaz I: The effect of ozone and naringin on
intestinal ischemia/reperfusion injury in an experimental model.
Int J Surg. 21:38–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiang CH, Pai HI and Liu SL:
Ventilator-induced lung injury (VILI) promotes ischemia/reperfusion
lung injury (I/R) and NF-kappaB antibody attenuates both injuries.
Resuscitation. 79:147–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahman I, Marwick J and Kirkham P: Redox
modulation of chromatin remodeling: Impact on histone acetylation
and deacetylation, NF-kappaB and pro-inflammatory gene expression.
Biochem Pharmacol. 68:1255–1267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li A, Yuen VM, Goulay-Dufay S and Kwok PC:
Pharmacokinetics and pharmacodynamics of dexmedetomidine. Drug Dev
Ind Pharm. 42:1917–1927. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sha J, Zhang H, Zhao Y, Feng X, Hu X, Wang
C, Song M and Fan H: Dexmedetomidine attenuates
lipopolysaccharide-induced liver oxidative stress and cell
apoptosis in rats by increasing GSK-3β/MKP-1/Nrf2 pathway activity
via the a2 adrenergic receptor. Toxicol Appl Pharmacol.
364:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han J, Wang M, Jing X, Shi H, Ren M and
Lou H: (−)-Epigallocatechin gallate protects against cerebral
ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem
Res. 39:1292–1299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Dong H, Song E, Xu X, Liu L and Song
Y: Nrf2/ARE pathway activation, HO-1 and NQO1 induction by
polychlorinated biphenyl quinone is associated with reactive oxygen
species and PI3K/AKT signaling. Chem Biol Interact. 209:56–67.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sies H, Berndt C and Jones DP: Oxidative
atress. Annu Rev Biochem. 86:715–748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Athale J, Ulrich A, MacGarvey NC, Bartz
RR, Welty-Wolf KE, Suliman HB and Piantadosi CA: Nrf2 promotes
alveolar mitochondrial biogenesis and resolution of lung injury in
Staphylococcus aureus pneumonia in mice. Free Radic Biol Med.
53:1584–1294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the Care and Use of Laboratory Animals. 8th.
Washington (DC): National Academies Press (US); 2011
|
|
16
|
Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao
X, Huang WQ and Liu KX: Dexmedetomidine administration before, but
not after, ischemia attenuates intestinal injury induced by
intestinal ischemia-reperfusion in rats. Anesthesiology.
116:1035–1046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stefanutti G, Pierro A, Parkinson EJ,
Smith VV and Eaton S: Moderate hypothermia as a rescue therapy
against intestinal ischemia and reperfusion injury in the rat. Crit
Care Med. 36:1564–1572. 2018. View Article : Google Scholar
|
|
18
|
Li J, Chen Q, He X, Alam A, Ning J, Yi B,
Lu K and Gu J: Dexmedetomidine attenuates lung apoptosis induced by
renal ischemia-reperfusion injury through a2AR/PI3K/Akt pathway. J
Transl Med. 16:782018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu C, Dai X, Yang Y, Lin M, Cai Y and Cai
S: Dexmedetomidine attenuates lipopolysaccharide-induced acute lung
injury by inhibiting oxidative stress, mitochondrial dysfunction
and apoptosis in rats. Mol Med Rep. 15:131–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue BB, Chen BH, Tang YN, Weng CW and Lin
LN: Dexmedetomidine protects against lung injury induced by limb
ischemia-reperfusion via the TLR4/MyD88/NF-κB pathway. Kaohsiung J
Med Sci. 35:672–678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W and Zhang J: Dexmedetomidine
preconditioning protects against lung injury induced by
ischemia-reperfusion through inhibition of autophagy. Exp Ther Med.
14:973–980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang S, Wang Y and Liu Y: Dexmedetomidine
alleviates lung ischemia-reperfusion injury in rats by activating
PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 23:370–377.
2019.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong WW, Liu YJ, Lv Z, Mao YF, Wang YW,
Zhu XY and Jiang L: Lung endothelial barrier protection by
resveratrol involves inhibition of HMGB1 release and HMGB1-induced
mitochondrial oxidative damage via an Nrf2-dependent mechanism.
Free Radic Biol Med. 88:404–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pryor WA and Stanley JP: Letter: A
suggested mechanism for the production of malonaldehyde during the
autoxidation of polyunsaturated fatty acids. Nonenzymatic
production of prostaglandin endoperoxides during autoxidation. J
Org Chem. 40:3615–3617. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayyan M, Hashim MA and AlNashef IM:
Superoxide Ion: Generation and chemical implications. Chem Rev.
116:3029–3085. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toth B, Alexander M, Daniel T, Chaudry IH,
Hubbard WJ and Schwacha MG: The role of gammadelta T cells in the
regulation of neutrophil-mediated tissue damage after thermal
injury. J Leukoc Biol. 76:545–552. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coursin DB, Coursin DB and Maccioli GA:
Dexmedetomidine. Curr Opin Crit Care. 7:221–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Z, Ding T and Ma CG: Dexmedetomidine
(DEX) protects against hepatic ischemia/reperfusion (I/R) injury by
suppressing inflammation and oxidative stress in NLRC5 deficient
mice. Biochem Biophys Res Commun. 493:1143–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Si Y, Bao H, Han L, Chen L, Zeng L, Jing
L, Xing Y and Geng Y: Dexmedetomidine attenuation of renal
ischaemia-reperfusion injury requires sirtuin 3 activation. Br J
Anaesth. 121:1260–1271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang B, Li XQ, Bi B, Tan WF, Liu G, Zhang
Y and Ma H: Dexmedetomidine attenuates blood-spinal cord barrier
disruption induced by spinal cord ischemia reperfusion injury in
rats. Cell Physiol Biochem. 36:373–383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan F, Fu H, Sun K, Wu S and Dong T:
Effect of dexmedetomidine on cerebral ischemia-reperfusion rats by
activating mitochondrial ATP-sensitive potassium channel. Metab
Brain Dis. 32:539–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng X, Hu J, Wang Y, Ye H, Li X, Gao Q
and Li Z: Effects of dexmedetomidine postconditioning on myocardial
Ischemia/Reperfusion injury in diabetic rats: Role of the
PI3K/Akt-dependent signaling pathway. J Diabetes Res.
2018:30719592018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cui J, Zhao H, Wang C, Sun JJ, Lu K and Ma
D: Dexmedetomidine attenuates oxidative stress induced lung
alveolar epithelial cell apoptosis in vitro. Oxid Med Cell Longev.
2015:3583962015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao S, Wang Y, Zhao J and Su A: Effects of
dexmedetomidine pretreatment on heme oxygenase-1 expression and
oxidative stress during one-lung ventilation. Int J Clin Exp
Pathol. 8:3144–3149. 2015.PubMed/NCBI
|
|
37
|
Ward PA: Oxidative stress: Acute and
progressive lung injury. Ann N Y Acad Sci. 1203:53–59. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lei J, Wei Y, Song P, Li Y, Zhang T, Feng
Q and Xu G: Cordycepin inhibits LPS-induced acute lung injury by
inhibiting inflammation and oxidative stress. Eur J Pharmacol.
818:110–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McCord JM and Edeas MA: SOD, oxidative
stress and human pathologies: A brief history and a future vision.
Biomed Pharmacother. 59:139–142. 2015. View Article : Google Scholar
|
|
40
|
Paris A and Tonner PH: Dexmedetomidine in
anaesthesia. Curr Opin Anaesthesiol. 18:412–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carollo DS, Nossaman BD and Ramadhyani U:
Dexmedetomidine: A review of clinical applications. Curr Opin
Anaesthesiol. 21:457–461. 2018. View Article : Google Scholar
|
|
42
|
Poljsak B, Suput D and Milisav I:
Achieving the balance between ROS and antioxidants: When to use the
synthetic antioxidants. Oxid Med Cell Longev. 2013:9567922013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao W, Gan X, Su G, Wanling G, Li S, Hei
Z, Yang C and Wang H: The interaction between oxidative stress and
mast cell activation plays a role in acute lung injuries induced by
intestinal ischemia-reperfusion. J Surg Res. 187:542–552. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hybertson BM and Gao B: Role of the Nrf2
signaling system in health and disease. Clin Genet. 86:447–452.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Roos B and Duthie GG: Role of dietary
pro-oxidants in the maintenance of health and resilience to
oxidative stress. Mol Nutr Food Res. 59:1229–1248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tkachev VO, Menshchikova EB and Zenkov NK:
Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry
(Mosc). 76:407–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan X, Cheng X, Zhou L, He X, Zheng W and
Chen H: Dexmedetomidine alleviates lipopolysaccharide-induced lung
injury in Wistar rats. Oncotarget. 8:44410–44417. 2017.PubMed/NCBI
|
|
48
|
Nadatani Y, Watanabe T, Shimada S, Otani
K, Tanigawa T and Fujiwara Y: Microbiome and intestinal
ischemia/reperfusion injury. J Clin Biochem Nutr. 63:26–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bellingan GJ: The pulmonary physician in
critical care* 6: The pathogenesis of ALI/ARDS. Thorax. 57:540–546.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Souza DG, Vieira AT, Soares AC, Pinho V,
Nicoli JR, Vieira LQ and Teixeira MM: The essential role of the
intestinal microbiota in facilitating acute inflammatory responses.
J Immunol. 173:4137–4146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tamion F, Richard V, Lyoumi S, Daveau M,
Bonmarchand G, Leroy J, Thuillez C and Lebreton JP: Gut ischemia
and mesenteric synthesis of inflammatory cytokines after
hemorrhagic or endotoxic shock. Am J Physiol. 273:G314–G321.
1997.PubMed/NCBI
|
|
52
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cui T, Miksa M, Wu R, Komura H, Zhou M,
Dong W, Wang Z, Higuchi S, Chaung W, Blau SA, et al: Milk fat
globule epidermal growth factor 8 attenuates acute lung injury in
mice after intestinal ischemia and reperfusion. Am J Respir Crit
Care Med. 181:238–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zu G, Zhou T, Che N and Zhang X:
Salvianolic acid A protects against oxidative stress and apoptosis
induced by intestinal ischemia-reperfusion injury through
activation of Nrf2/HO-1 pathways. J Biol Chem. 49:2320–2332.
2018.
|
|
56
|
Fan J, Xu G, Jiang T and Qin Y:
Pharmacologic induction of heme oxygenase-1 plays a protective role
in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci.
53:6541–6556. 2012. View Article : Google Scholar : PubMed/NCBI
|