Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor in pediatrics and young adolescents (1). Furthermore, the standard care for
patients with OS, which includes surgical resection in combination
with systemic chemotherapy, has a 5-year overall survival rate of
70% (2). Treatment of OS often
fails due to the development of chemotherapy resistance and
metastasis (3). It has been
previously reported that sex hormones are involved in the
occurrence and development of human OS, thus suggesting that novel
endocrine therapy may be development for OS using clinically
available estrogen inhibitors (4).
Plant-derived phytoestrogens and mammalian estrogens
have similar structures and functions, and can cause anti-estrogen
or estrogen-like effects (5). For
this reason, plant-derived phytoestrogens are a current topic in
research (6). Phytoestrogenic
compounds are widely found in nature and can be divided into four
categories: i) Isoflavones; ii), stilbene; iii), coumarins; and iv)
lignans (7). Calycosin, a
bioactive phytoestrogen isoflavone that is extracted from
Trifolium pratense (red clover), has been shown to inhibit
proliferation and induce apoptosis in cancer types (8,9). In
addition, calycosin has been shown to induce apoptosis in human
estrogen receptor (ER)-positive OS cells, but has no effect on
ER-negative OS cells, suggesting that the inhibition of calycosin
on ER-positive OS cells may be achieved by increasing ER expression
(10).
ER belongs to the steroid hormone receptor family
and consists of two subtypes, ERα and ERβ (11). A high ERα:ERβ ratio leads to
increased cell proliferation, whereas a higher level of ERβ than
ERα leads to decreased proliferation (12,13).
Since the expression of ERβ has been shown to decrease during tumor
progression, ERβ has been considered a potential tumor suppressor
and therapeutic target in various types of cancer, including breast
cancer and renal cell carcinoma (14,15).
Moreover, ERβ agonists may be novel potential therapeutic
candidates for OS endocrine therapy (16). Therefore, it was hypothesized that
upregulation of ERβ may inhibit tumor development and
progression.
The PI3K/Akt signaling pathway, an important
regulator of cellular functions, has been found to be frequently
hyperactivated in OS and contributes to tumorigenesis,
proliferation, invasion, cell cycle progression and inhibition of
apoptosis (17). Thus, suppressing
this signaling pathway could inhibit disease initiation and
development (18). Moreover, ERβ
has an anti-tumor effect on OS cells by regulating the PI3K/Akt
signaling pathway (16). In
addition, ERβ can mediate inhibition of proliferation and
activation of apoptosis in various types of cancer, including
breast cancer and colorectal cancer following treatment with
calycosin, which is shown to regulate the PI3K/Akt pathway
(8,9). Therefore, the present study
investigated whether calycosin had anti-tumor effects on OS MG-63
cells by mediating the ERβ-dependent PI3K/Akt signaling
pathway.
Collectively, along with the anti-proliferative
effect of calycosin on OS MG-63 cells, the present study examined
the role of the ERβ-mediated PI3K/Akt pathway in OS MG-63 cells to
help facilitate the current understanding of the molecular
mechanism underlying calycosin functions.
Materials and methods
Calycosin
Calycosin (purity 98%; Tianjin JAHE Science and
Technology Co. Ltd.) solution was diluted into a 250 µg/ml stock
solution with DMSO (Sigma-Aldrich; Merck KGaA).
Cell culture
Human OS cells (MG-63) and human fetal osteoblast
cells (hFOB1.19; Shanghai Institute of Biochemistry and Cell
Biology) were incubated in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and antibiotics (100 U/ml penicillin and 100 µg/ml
streptomycin) in a humidified incubator containing 5%
CO2 at 37°C. The medium was changed every 48 h.
MTT assay
Cell viability was determined by MTT (Sigma-Aldrich;
Merck KGaA) assay. Cells were harvested using 0.25% trypsin and
seeded into 96-well plates at a density of 3×104
cells/well at 37°C for 24 h. Then, cells were treated with
calycosin (at concentrations of 0, 25, 50 or 100 µM) at 37°C for
24, 48 and 72 h, or with 100 µM calycosin in the presence or
absence of the ERβ inhibitor PHTPP (50 µM; MedChemExpress) at 37°C
for 48 h. In total, 20 µl MTT (5 mg/ml) was added to cells for 4 h
at 37°C. Following incubation, DMSO (100 µl) was added to dissolve
the formazan crystals and shaken at room temperature for 10 min.
Subsequently, cell viability was assessed by measuring the
absorbance at 570 nm using a microplate reader (Thermo Fisher
Scientific, Inc.). Proliferation rate (%) was calculated as
follows: Optical density (OD) treatment group/OD control ×100%.
Flow cytometry assay
Flow cytometry was used to study the effects of
calycosin treatment on apoptosis of the OS cells using the FITC
Annexin V Apoptosis Detection Kit I (BD Biosciences), according to
the manufacturer's protocol. MG-63 cells were treated with
calycosin (0, 25, 50 or 100 µM), or calycosin (100 µM) in the
presence or absence of PHTPP at 37°C for 48 h. Cells were harvested
and washed with PBS. Apoptotic cells were identified by double
staining with 5 µl FITC-conjugated Annexin V and 5 µl PI. Data were
obtained and analyzed using a FACS-Canto flow cytometer (Beckman
Coulter, Inc.) with Cell Quest software (version 5.1; BD
Biosciences). Cells stained positive for Annexin V-FITC and
negative for PI were considered early apoptotic, and cells stained
positive for Annexin V-FITC and positive for PI were considered in
late apoptosis.
Western blot analysis
After being treated with calycosin (0, 25, 50 and
100 µM), or calycosin (100 µM) in the presence or absence of PHTPP
for 48 h, MG-63 cells were harvested with ice-cold PBS and lysed on
ice in lysis buffer (Beyotime Institute of Biotechnology) for 30
min. The lysates were centrifuged at 4°C at 700 × g for 10 min and
collected. Then, the total protein was measured with a
bicinchoninic protein assay kit (Tiangen Biotech Co., Ltd.). The
samples (20 µg) were separated via 12% SDS-PAGE and then
transferred to PVDF membranes (EMD Millipore). The membranes were
blocked with 5% non-fat dried milk in TBST (0.2% Tween-20) buffer
for 1 h at room temperature, and then incubated with the following
primary antibodies overnight at 4°C: ERβ (cat. no. sc8974; 1:500),
PI3K (cat. no. 4255; 1:1,000), phosphorylated (p)-PI3K (cat. no.
17366; 1:1,000), Akt (cat. no. 9271; 1:1,000), p-Akt (cat. no.
9611; 1:2,000), cleaved caspase-3 (cat. no. 9661; 1:1,000), cleaved
poly (ADP-ribose) polymerase 1 (cleaved PARP-1; cat. no. 9185;
1:1,000) and β-actin (cat. no. 7077; 1:1,000). After three washes
with TBST, the membranes were subsequently incubated with a
horseradish peroxidase-conjugated secondary antibody (cat. no.
7074; 1:5,000) for 1 h at room temperature. The protein signal was
detected via electrochemiluminescence with an ECL-Plus kit
(Beyotime Institute of Biotechnology) and analyzed using ImageJ
software (National Institutes of Health). Anti-ERβ was purchased
from Santa Cruz Biotechnology, Inc. The other antibodies were
purchased from Cell Signaling Technology, Inc.
Statistical analysis
Data were obtained from ≥3 independent experiments
and are presented as the mean ± SD relative to the control value.
Statistical analysis for multiple comparisons was performed using
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhibition of proliferation and
induction of apoptosis in OS MG-63 cells by calycosin
To evaluate the anti-proliferative effect of
calycosin, MG-63 and hFOB1.19 cells were incubated with different
concentrations of calycosin for 24, 48 and 72 h. It was found that
calycosin caused a time- and concentration-dependent inhibition on
the proliferation of MG-63 cells (Fig.
1A). However, the inhibitory effect of calycosin on the
proliferation of hFOB1.19 cells was not significant (Fig. 1B), thus suggesting that the effect
of calycosin was negligible on healthy osteoblasts.
Consistent with the aforementioned results, flow
cytometry assay results demonstrated that calycosin induced MG-63
cell apoptosis in a concentration-dependent manner, and induced a
significant increase in the percentage of early and late apoptotic
cells (Fig. 1C and D).
Upregulation of ERβ in OS MG-63 cells
by calycosin
ERβ is a traditional estrogen receptor, whose
activity is inversely related to the occurrence and development of
tumors (19). Therefore, the
expression of ERβ was examined in MG-63 cells following treatment
with calycosin. It was demonstrated that ERβ protein expression was
increased significantly in a dose-dependent manner after calycosin
treatment (Fig. 2A and B).
Moreover, MG-63 cells were treated with 100 µM calycosin in the
presence or absence of PHTPP, and then the expression of ERβ was
assessed. It was found that calycosin in combination with PHTPP
significantly decreased ERβ expression (P<0.01; Fig. 2C and D). Therefore, the present
results suggested that the inhibition of calycosin on MG-63 cells
occurred via the regulation of ERβ.
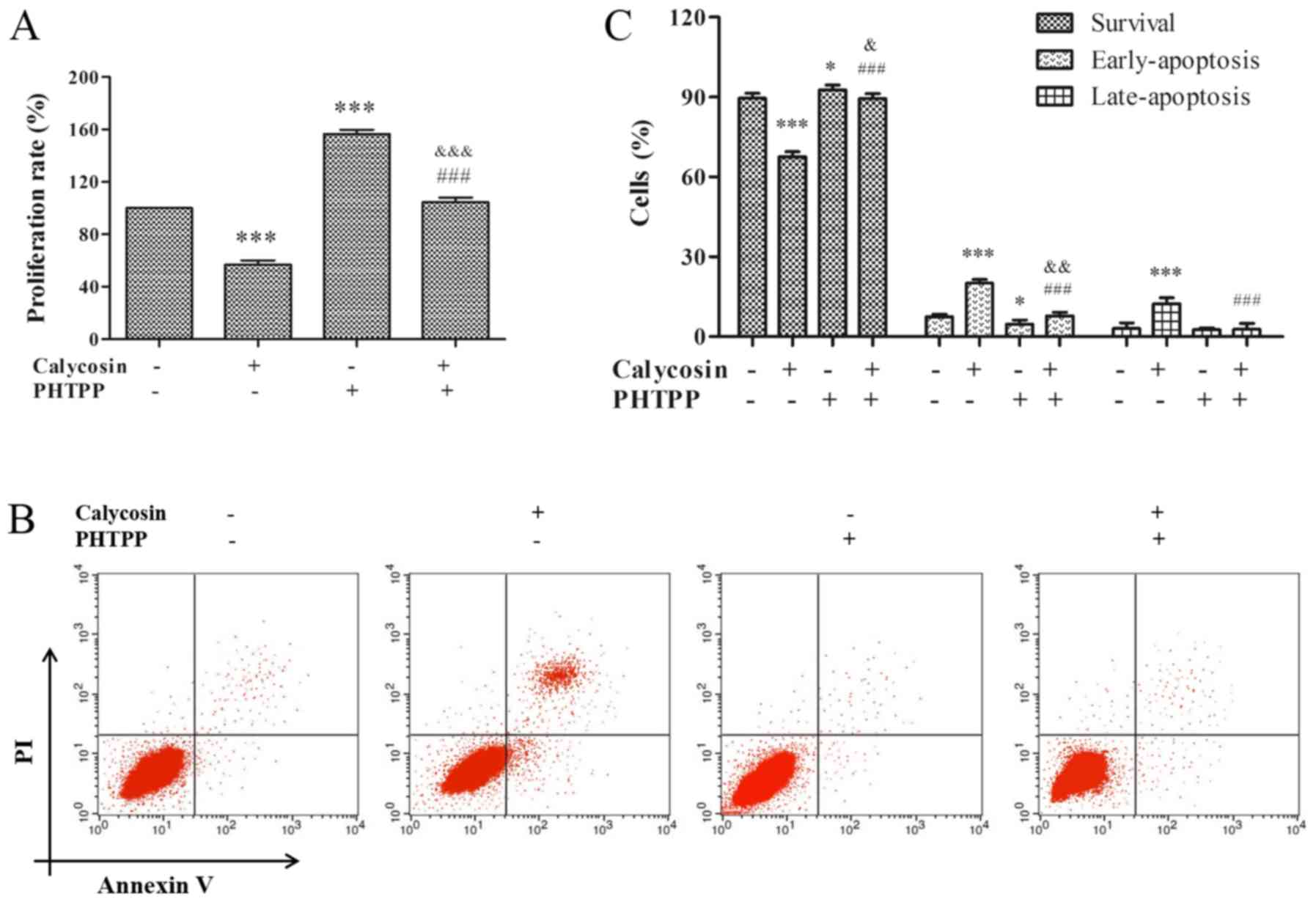
ERβ-mediated inhibition of
proliferation and activity of apoptosis in MG-63 cells by
calycosin
Previous studies have shown that ERβ is a key
negative mediator of cell proliferation, and positive regulator of
apoptosis in cancer (8,9,16).
Therefore, to study the effect of ERβ on proliferation and
apoptosis, MG-63 cells were pretreated with 100 µM calycosin in the
presence or absence of PHTPP for 48 h. It was identified that
calycosin + PHTPP reversed the calycosin-mediated inhibition of
cell proliferation (P<0.001; Fig.
3A). In addition, calycosin + PHTPP abolished calycosin-induced
apoptosis (P<0.001, Fig. 3B and
C). Consistent with the findings of previous studies,
calycosin-induced cytotoxicity and apoptosis were found to be
mediated by the ERβ-signaling pathway.
Regulation of ERβ-mediated PI3K/Akt
signaling in OS MG-63 cells by calycosin
It has been previously shown that inhibiting the
PI3K/Akt signaling pathway may be a novel treatment strategy for OS
(17). Therefore, the PI3K/Akt
signaling pathway was examined in the present study. After a 48 h
exposure to calycosin (0, 25, 50 or 100 µM) in MG-63 cells, PI3K
phosphorylation and Akt expression were found to be downregulated
in a concentration-dependent manner (Fig. 4A and B). Thus, the present results
indicated that calycosin inactivated the PI3K/Akt signaling pathway
in MG-63 cells. In addition, to assess the relationship between ERβ
and the PI3K/Akt signaling pathway, MG-63 cells were treated with
calycosin in the presence or absence of PHTPP. It was demonstrated
that the combination of calycosin + PHTPP reversed the decrease in
the phosphorylation levels of PI3K and Akt (P<0.01; Fig. 4C and D), while no changes were
observed in the expression levels of total Akt and total PI3K.
Therefore, the present results suggested that the ERβ-mediated
decrease in PI3K/Akt activity was involved in calycosin-induced
cell death.
ERβ-mediated increase in the
expression levels of apoptotic-associated protein in OS MG-63 cells
treated with calycosin
Apoptosis is often associated with the cleavage of
specific substrates, such as cleaved PARP-1 and caspase-3, whose
excessive activation can promote apoptosis (20). When MG-63 cells were treated with
0, 25, 50 and 100 µM calycosin for 48 h, it was found that the
protein expression levels of PARP-1 and cleaved caspase-3 increased
in a time- and dose-dependent manner (Fig. 5A and B). This suggested that
activation of PARP-1 and cleavage of caspase-3 may be involved in
calycosin-mediated cell apoptosis. In addition, calycosin + PHTPP
treatment reduced PARP-1 and caspase-3 cleavage protein expression
levels (P<0.05; Fig. 5C and D).
Collectively, the present results indicated that PARP-1 and
caspase-3 may be downstream targets of the ERβ-mediated PI3K/Akt
pathway.
Discussion
OS usually occurs in adolescence when the synthesis
of sex hormones, such as estrogen or androgen, peaks, thus
indicating that sex steroids and their receptors may be involved in
the development of OS (21).
Phytoestrogens have recently received increased attention due to
their ability to bind to ERs. Previous studies have suggested that
certain phytoestrogens exhibit antiestrogenic activity via
ER-mediated signaling pathways (22,23).
Moreover, calycosin is a phytoestrogen isoflavone that exhibits
estrogenic activity and anti-tumor effects on several cancer types,
by inducing apoptosis of tumor cells in vitro and in
vivo (8,9). Furthermore, in vitro and in
vivo studies have also shown that calycosin has antiapoptotic
and antimetastatic activities against OS (10,24).
Consistent with these previous studies, the present results
suggested that calycosin effectively inhibited cell proliferation
and induced apoptosis in a time- and dose-dependent manner in OS
MG-63 cells. In addition, calycosin had a low cytotoxicity in
osteoblast hFOB1.19 cells.
Several previous studies have shown that calycosin
inhibits tumorigenesis and tumor progression by regulating ERβ
expression (8,9,25).
Moreover, the downregulation of ERβ has been observed in various
types of cancer (8,9). In the present study, ERβ protein
expression in OS cells was found to be decreased, while its
expression increased significantly in a dose-dependent manner after
calycosin treatment. A previous study showed that estrogen
extracted from Astragalus membranaceus could inhibit
etoposide-induced apoptosis of human OS cells by activating ERβ
(26). Furthermore, despite the
reduction in ERβ expression, which may be related to different
subtypes of OS cells, the estrogen inhibitor fulvestrant exhibits
significant anti-OS activity in OS 143B cells (27). The present results suggested that
the ERβ inhibitor PHTPP reversed the increase in the protein
expression of ERβ, and significantly reversed the cytotoxicity and
apoptosis detected following calycosin treatment in MG-63 cells.
Therefore, the present results indicated that the anti-tumor
effects of calycosin were mediated by the ERβ signaling
pathway.
In addition, the mechanism underlying the anti-tumor
effect of ERβ was examined in the present study. The association
between calycosin and ERβ was investigated, and calycosin was found
to reduce OS cell proliferation by inhibiting the ERβ signaling
pathway, in particular a potential downstream effector. The
PI3K/Akt signaling pathway is frequently hyperactivated in OS and
has been shown to be involved in tumorigenesis, proliferation,
invasion, cell cycle progression and inhibition of apoptosis
(17). Furthermore, ERβ can
independently predict the prognosis of triple-negative breast
cancer by interacting with the PI3K/Akt pathway (19). Estrogen can activate the PI3K/Akt
pathway via ERβ in breast cancer (28). Moreover, ERβ has a significant
anti-tumor effect on OS U2-OS cells by regulating the PI3K/Akt
signal pathway (16). It has been
shown that the inhibitory effects of calycosin on OS MG-63 cells
are mediated by the PI3K/Akt pathway (10). In the present study, it was
demonstrated that calycosin inactivated the PI3K/Akt signaling
pathway in OS MG-63 cells, whereas the ERβ inhibitor PHTPP enhanced
the phosphorylation of PI3K and Akt. Thus, the present results
suggested that the anti-tumor effects of ERβ were associated with
the PI3K/Akt signaling pathway.
Defects in apoptosis play an important role in tumor
pathogenesis, and the cytotoxic effects of many antineoplastic
drugs are usually accompanied by an increase in apoptosis (29). Experimental studies of OS cells and
143B-harbored nude mice have shown that calycosin possesses an
anti-osteosarcoma effect, and the underlying mechanism is
associated with the activation of apoptosis (30). In the present study, calycosin was
found to induce apoptosis in MG-63 cells, as indicated by
morphological changes, and the activation of caspase-3 and PARP-1.
Caspases are known for their role as initiators and executors of
apoptosis (31). Activation of
different caspase cascades plays an important role in apoptosis by
cleaving key factors involved in cellular function and viability
(32). Moreover, the apoptotic
executor factor caspase-3 can cleave the caspase substrate PARP-1
into two specific fragments, thus contributing to cell death
(33). Therefore, cleaved PARP-1
and caspase-3 are considered as apoptotic markers. The present
results indicated that there were increased expression levels of
cleaved PARP-1 and caspase-3 following treatment with calycosin in
a concentration-dependent manner, thus indicating the involvement
of PARP-1 and caspase-3 in the effects of calycosin. Moreover, the
PHTPP inactivated PARP-1 and caspase-3 cleavage, indicating that
ERβ mediated PARP-1 and caspase-3 activity in calycosin-induced
apoptosis.
Collectively, the present study provided further
evidence for the interaction between calycosin and ERβ. In
addition, the antiproliferative effects of calycosin were found to
be mediated by the ERβ-dependent regulation of the PI3K/Akt
pathways. Therefore, the present study provides a theoretical basis
for the potential use of calycosin as a therapeutic to treat
OS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WT performed the majority of the experiments and
drafted the manuscript. ZWW helped perform the experiments. BMY
analyzed the data and drafted the manuscript. YGB conceived the
study, supervised the experiments and edited the manuscript.
designed the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pingping B, Yuhong Z, Weiqi L, Chunxiao W,
Chunfang W, Yuanjue S, Chenping Z, Jianru X, Jiade L, Lin K, et al:
Incidence and mortality of sarcomas in Shanghai, China, during
2002–2014. Front Oncol. 9:6622019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin YH, Jewell BE, Gingold J, Lu L, Zhao
R, Wang LL and Lee DF: Osteosarcoma: Molecular pathogenesis and
iPSC modeling. Trends Mol Med. 23:737–755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Q, Li S, Chen C, Zhu M, Chen Y and Zhao
Z: 17β-estradiol treatment drives Sp1 to upregulate MALAT-1
expression and epigenetically affects physiological processes in
U2OS cells. Mol Med Rep. 15:1335–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li M, Zeng M, Zhang Z, Zhang J, Zhang B,
Zhao X, Zheng X and Feng W: Uridine derivatives from the seeds of
Lepidium apetalum Willd. And their estrogenic effects.
Phytochemistry. 155:45–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Duursen MB, Smeets EE, Rijk JC,
Nijmeijer SM and van den Berg M: Phytoestrogens in menopausal
supplements induce ER-dependent cell proliferation and overcome
breast cancer treatment in an in vitro breast cancer model. Toxicol
Appl Pharmacol. 269:132–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin AH, Li RW, Ho EY, Leung GP, Leung SW,
Vanhoutte PM and Man RY: Differential ligand binding affinities of
human estrogen receptor-α isoforms. PLoS One. 8:e631992013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao X, Li X, Ren Q, Tian J and Chen J:
Calycosin induces apoptosis in colorectal cancer cells, through
modulating the ERβ/MiR-95 and IGF-1R, PI3K/Akt signaling pathways.
Gene. 591:123–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Hou R, Zhang X, Ye Y, Wang Y and
Tian J: Calycosin suppresses breast cancer cell growth via
ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways.
PLoS One. 9:e912452014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun H, Yin M, Qian W and Yin H: Calycosin,
a Phytoestrogen isoflavone, induces apoptosis of estrogen
receptor-positive MG-63 osteosarcoma cells via the
phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of
rapamycin (mTOR) pathway. Med Sci Monit. 24:6178–6186. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Renoir JM: Estradiol receptors in breast
cancer cells: Associated co-factors as targets for new therapeutic
approaches. Steroids. 77:1249–1261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Wei F, Zhang J Hao L, Jiang J,
Dang L, Mei D, Fan S, Yu Y and Jiang L: Bisphenol A and estrogen
induce proliferation of human thyroid tumor cells via an
estrogen-receptor-dependent pathway. Arch Biochem Biophys.
633:29–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Treeck O, Diepolder E, Skrzypczak M,
Schüler-Toprak S and Ortmann O: Knockdown of estrogen receptor β
increases proliferation and affects the transcriptome of
endometrial adenocarcinoma cells. BMC Cancer. 19:7452019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Tu Z, An L, Qian Z, Achilefu S and
Gu Y: Inhibitory effects of ERβ on proliferation, invasion, and
tumor formation of MCF-7 breast cancer cells-prognostication for
the use of ERβ-selective therapy. Pharm Biol. 50:839–849. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu CP, Ho JY, Huang YT, Cha TL, Sun GH, Yu
DS, Chang FW, Chen SP and Hsu RJ: Estrogen inhibits renal cell
carcinoma cell progression through estrogen receptor-β activation.
PLoS One. 8:e566672013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang M, Liu B, Jin L, Tao H and Yang Z:
Estrogen receptor β exhibited anti-tumor effects on osteosarcoma
cells by regulating integrin, IAP, NF-kB/BCL-2 and PI3K/Akt signal
pathway. J Bone Oncol. 9:15–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Li N, Xiang R and Sun P: Emerging
roles of the p38 MAPK and PI3K/AKT/mTOR pathways in
oncogene-induced senescence. Trends Biochem Sci. 39:268–76. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Zhang C, Chen K, Tang H, Tang J,
Song C and Xie X: ERβ1 inversely correlates with PTEN/PI3K/AKT
pathway and predicts a favorable prognosis in triple-negative
breast cancer. Breast Cancer Res Treat. 152:255–269. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta S, Kass GE, Szegezdi E and Joseph B:
The mitochondrial death pathway: A promising therapeutic target in
diseases. J Cell Mol Med. 13:1004–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Botter SM, Neri D and Fuchs B: Recent
advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwang KA, Park MA, Kang NH, Yi BR, Hyun
SH, Jeung EB and Choi KC: Anticancer effect of genistein on BG-1
ovarian cancer growth induced by 17 β-estradiol or bisphenol A via
the suppression of the crosstalk between estrogen receptor α and
insulin-like growth factor-1 receptor signaling pathways. Toxicol
Appl Pharmacol. 272:637–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thelen P, Wuttke W and Seidlová-Wuttke D:
Phytoestrogens selective for the estrogen receptor beta exert
anti-androgenic effects in castration resistant prostate cancer. J
Steroid Biochem Mol Biol. 139:290–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu R, Li X, Qin K, Chen X, Wang R, Dai Y,
Deng L and Ye Y: Antimetastatic effects of calycosin on
osteosarcoma and the underlying mechanism. Biofactors. 45:975–982.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Zhao X, Ye Y, Wang Y and Tian J:
Estrogen receptor beta-mediated proliferative inhibition and
apoptosis in human breast cancer by calycosin and formononetin.
Cell Physiol Bioch. 32:1790–1797. 2013. View Article : Google Scholar
|
|
26
|
Zhou R, Chen H, Chen J, Chen X, Wen Y and
Xu L: Extract from Astragalus membranaceus inhibit breast
cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC
Complement Altern Med. 18:832018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gorska M, Wyszkowska RM, Kuban-Jankowska A
and Wozniak M: Impact of apparent antagonism of estrogen receptor β
by fulvestrant on anticancer activity of 2-methoxyestradiol.
Anticancer Res. 36:2217–2226. 2016.PubMed/NCBI
|
|
28
|
Kanaizumi H, Higashi C, Tanaka Y, Hamada
M, Shinzaki W, Azumi T, Hashimoto Y, Inui H, Houjou T and Komoike
Y: PI3K/Akt/mTOR signalling pathway activation in patients with
ER-positive, metachronous, contralateral breast cancer treated with
hormone therapy. Oncol Lett. 17:1962–1968. 2019.PubMed/NCBI
|
|
29
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiu R, Ma G, Zheng C, Qiu X, Li X, Li X,
Mo J, Li Z, Liu Y, Mo L, et al: Antineoplastic effect of calycosin
on osteosarcoma through inducing apoptosis showing in vitro and in
vivo investigations. Exp Mol Pathol. 97:17–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kopeina GS, Prokhorova EA, Lavrik IN and
Zhivotovsky B: Alterations in the nucleocytoplasmic transport in
apoptosis: Caspases lead the way. Cell Prolif. 51:e124672018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akanda MR, Kim MJ, Kim IS, Ahn D, Tae HJ,
Rahman MM, Park YG, Seol JW, Nam HH, Choo BK and Park BY:
Neuroprotective effects of sigesbeckia pubescens extract on
glutamate-induced oxidative stress in HT22 cells via downregulation
of MAPK/caspase-3 pathways. Cell Mol Neurobiol. 38:497–505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rogalska A, Bukowska B and Marczak A:
Metformin and epothilone A treatment up regulate pro-apoptotic
PARP-1, Casp-3 and H2AX genes and decrease of AKT kinase level to
control cell death of human hepatocellular carcinoma and ovary
adenocarcinoma cells. Toxicol In Vitro. 47:48–62. 2018. View Article : Google Scholar : PubMed/NCBI
|