Introduction
Melanin is synthesized by melanocytes and determines
the color of fur, hair and skin. The melanosome, in which melanin
is synthesized and stored, is a functionally specialized
membrane-encased organelle (1).
The melanosome maturation process involves a number of proteins,
including tyrosinase (Tyr), pre-melanosomal protein (Pmel),
tyrosinase-related protein 1 (Tyrp1), Tyrp2 and ocular albinism
type 1 protein (2–4). Among these proteins, Tyr is a crucial
catalytic enzyme component and it is irreplaceable in melanogenesis
(5). Pmel is one of the structural
proteins that serves a key role in the formation of intraluminal
fibrils, eventually leading to the deposition of melanin in
melanosomes (6). Tyr and Pmel play
an important role in melanogenesis, and their expression is
regulated by microphthalmia-associated transcription factor (MITF)
(7).
Syntenin, also known as melanoma
differentiation-related gene-9 (mda-9), is an evolutionarily
conserved intracellular adaptor protein involved in many important
physiological and pathological aspects, such as development,
immunity and cancer metastasis (8). Syntenin was first identified in
melanoma research and previous studies have demonstrated that it is
expressed in a number of normal and tumoral tissues, as well as
normal melanocytes (9–11); however, to the best of our
knowledge, the effects of syntenin on melanin synthesis and
corresponding molecular mechanisms have not yet been reported.
Therefore, the present study aimed to clarify the
role of syntenin in melanogenesis, the effect of depletion of
syntenin on melanin production and expression of melanogenic
molecules Tyr, Pmel and MITF in melanocytes. In addition, the
effect of syntenin on the phosphorylation of p38 mitogen-activated
protein kinase (p38 MAPK) was also determined.
Materials and methods
Antibodies and reagents
Rabbit anti-syntenin (cat. no. ab133267), anti-Pmel
(cat. no. ab137078), anti-MITF (cat. no. ab20663) and anti-β-actin
(cat. no. ab8227) antibodies were purchased from Abcam. Horseradish
peroxidase (HRP)-conjugated goat anti-mouse IgG (cat. no. ab205719)
and HRP-conjugated goat anti-rabbit IgG (cat. no. ab205718)
secondary antibodies were purchased from Abcam. The mouse anti-Tyr
monoclonal antibody (cat. no. AT4426a) was purchased from Abgent,
Inc. The rabbit anti-p38 (cat. no. 9212) and anti-phosphorylated
p38 (p-p38; cat. no. 9211) monoclonal antibodies were purchased
from Cell Signaling Technology, Inc. SB203580 (p38 MAPK inhibitor)
was purchased from Beyotime Institute of Biotechnology.
L-3,4-dihydroxyphenylalanine (L-DOPA) was purchased from
MedChemExpress.
Cell culture
The immortalized human melanocyte cell line PIG1 was
a gift from Professor Caroline Le Poole (Department of Dermatology,
University of Cincinnati, USA). Cells were maintained in Medium 254
supplemented with 5% fetal bovine serum and human melanocyte growth
supplement (cat. no. S-002-5), all from Gibco (Thermo Fisher
Scientific, Inc.), at 37°C in a humidified atmosphere of 5%
CO2.
Following syntenin-siRNA transfection for 24 h,
co-treatment with 10 µM p38 MAPK inhibitor SB203580 at 37°C for 2
h, the melanin content and Tyr activity of the cells were
detected.
Transfection of small interfering
(si)RNAs
For the targeted knockdown of syntenin, a mixture of
three pairs of syntenin-siRNAs were designed and synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.), according to a
previously described method (12).
The nucleotide sequences of the siRNAs were as follows:
Syntenin-homo-612, sense 5′-GGGACCAAGUACUUCAGAUTT-3′, antisense
5′-AUCUGAAGUACUUGGUCCCTT-3′; Syntenin-homo-398, sense
5′-GCAAGACCUUCCAGUAUAATT-3′, antisense 5′-UUAUACUGGAAGGUCUUGCTT-3′;
Syntenin-homo-839, sense 5′-GGUCUUCUCACGGAACAUATT-3′, antisense
5′-UAUGUUCCGUGAGAAGACCTT-3′, respectively. Negative control
(NC)-siRNAs with the nucleotide sequences of sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′ were also used. A total of
5×104 melanocytes were seeded in 24-well plates and
grown for one day to reach 30–50% confluence. A total of 15 pmol of
the three aforementioned syntenin-siRNA pairs or 15 pmol NC-siRNA
were then transfected into the cells using 1.5 µl
Lipofectamine® RNAi-MAX (Invitrogen; Thermo Fisher
Scientific, Inc.). Untreated cells were used as blank. Cells were
cultured in Medium 254 at 37°C with 5% CO2. At 24 and 48
h post-transfection, transfection efficiency was determined by
western blotting.
Cell viability assay
The cell viability assay was performed using a Cell
Titer-Blue H Cell Viability assay kit (Promega Corporation)
according to the manufacturer's instructions. Cells were seeded at
a density of 5×103 cells/well in 96-well plates and
incubated over night at 37°C. After 24 and 48 h of
syntenin-targeted siRNA transfection, cell viability was determined
by adding cell titer blue (20 µl/well) as an indicator and further
incubated at 37°C for 4 h, fluorescence was subsequently measured
at a wavelength of 560/590 nm using a Gen™ spectrophotometer (•
BioTek Instruments, Inc.).
Western blotting
Whole cell protein extracts prepared using the RIPA
buffer (Beyotime Institute of Biotechnology) and quantified using
the BCA Protein assay kit (Beyotime Institute of Biotechnology).
Protein (20 µg per lane) was separated by 12% SDS-PAGE and then
transferred to a nitrocellulose membrane (Pharmacia; GE
Healthcare). After blocking with 5% skimmed milk at room
temperature for 1 h, the membrane was incubated with diluted
primary antibody at 4°C overnight. The primary antibodies used were
anti-Pmel (1:300), anti-syntenin (1:500), anti-Tyr (1:200),
anti-MITF (1:500), anti-β-actin (1:1,000), anti-p38 (1:500) and
p-p38 (1:500). Then the membrane was incubated with HRP-labeled
secondary antibodies at room temperature for 2 h. Protein bands
were visualized using ECL reagents (Roche Diagnostics GmbH).
Protein expression levels were semi-quantified using Gel-Pro
Analyzer software (version 4.0; Media Cybernetics, Inc.) with
β-actin as the loading control.
Melanin content determination
The total content of melanin was measured as
previously described by Hosoi et al (13). Cells were seeded (3×105
cells/well) in 6 wells plate and incubated over night at 37°C.
After 24 or 48 h of transfection with syntenin-siRNA, cells were
washed twice with PBS and the cell pellets were dissolved in 1 N
NaOH (1 ml) at 100°C for 30 min and centrifuged at 16,000 × g for
20 min at room temperature. The optical density of the supernatant
was measured at 405 nm using an ELISA microplate reader.
Tyr activity assay
Tyr activity was estimated using a modification of a
previously reported method (14).
Cells were seeded (5×103 cells/well) in 96 wells plate
and incubated overnight at 37°C. After 24 or 48 h of syntenin-siRNA
transfection, cells were washed twice with PBS and homogenized in
200 µl of 0.1 M sodium phosphate buffer (pH 6.8) containing 1 M
phenylmethylsulfonyl fluoride and 1% Triton X-100. A total of 50 µl
of the supernatant, 50 µl of 0.1% L-DOPA and 100 µl of 0.1 M sodium
phosphate buffer (pH 6.8) were combined and incubated at 37°C for
15 min. The formation of dopachrome was monitored by detection of
the absorbance at a wavelength of 475 nm with an ELISA microplate
reader. Each treatment was repeated three times.
Statistical analysis
Data were analyzed using the SPSS 19.0 statistical
software (IBM Corp). Data are expressed as the mean ± SEM. All
experiments were repeated at least three times. One-way ANOVA
followed by the Tukey's post hoc test was used for multiple
comparison tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
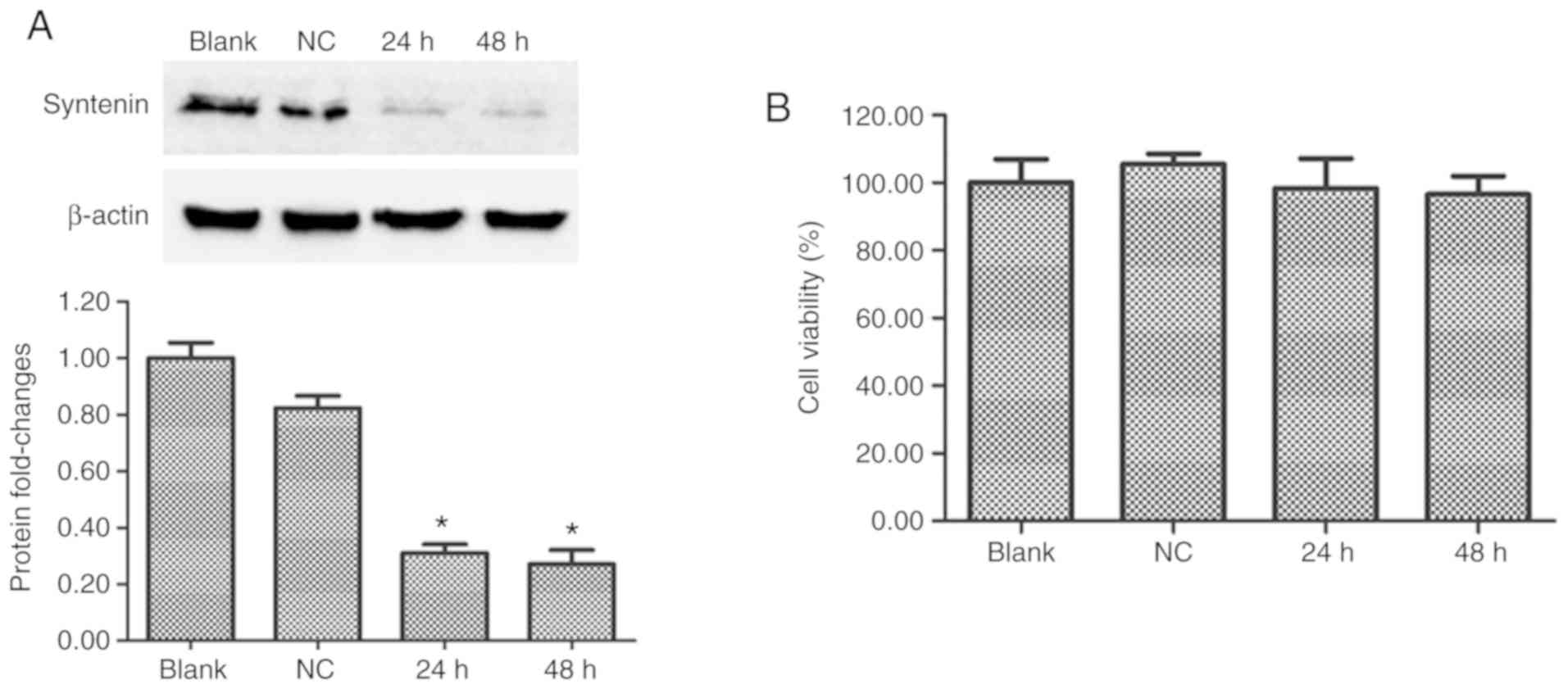
Syntenin-siRNA significantly
downregulates syntenin protein levels in immortalized human
melanocytes
Western blot analysis was used to determine whether
the syntenin siRNA transfections successfully inhibited the
expression of syntenin protein in PIG1 cells. At 24 and 48 h after
transfection, the expression of syntenin protein was effectively
reduced in syntenin-siRNA-transfected melanocytes compared with
NC-siRNA transfected melanocytes (Fig.
1A). Syntenin-siRNA transfection had no significant effect on
cell viability over 48 h (Fig.
1B). These results demonstrated that syntenin-siRNA
transfection effectively depleted expression of syntenin.
siRNA-induced silencing of syntenin
increases melanin content and Tyr activity
Melanin content and Tyr enzyme activity were
detected at 24 and 48 h after PIG1 cells were transfected with
syntenin-siRNA. Compared with the NC-siRNA-transfected group, the
syntenin-siRNA-transfected cells demonstrated a significant
increase in melanin content and Tyr activity (Fig. 2A and B, respectively). These
results indicated that depletion of syntenin induced melanogenesis
in immortalized human melanocytes.
Syntenin silencing increases the
expression of melanogenesis- related proteins in PIG1 cells
As the depletion of syntenin was found to increase
melanin production and Tyr activity, the study sought to determine
the effects of syntenin depletion on the expression of
melanogenesis-related proteins Tyr, Pmel and MITF. At 24 and 48 h
after syntenin-siRNA transfection, a significant increase in the
protein expression levels of Tyr, Pmel and MITF, alongside a
decreased expression of syntenin were observed compared with the
control group (Fig. 3).
Silencing of syntenin stimulates
melanin synthesis through activation of p38 MAPK
The phosphorylation of p38 MAPK was reported to be
one of the signaling pathways involved in hyperpigmentation
(15). Therefore, western blot
analysis was performed to determine the effects of syntenin on p38
phosphorylation. As shown in Fig.
4A, phosphorylation of p38 MAPK was significantly increased
after transfection of syntenin-siRNA in PIG1 cells compared with
the control group. To confirm that the p38 MAPK is involved in
syntenin-mediated melanogenesis, a melanin content assay and a
cellular Tyr activity assay were performed. After syntenin-siRNA
transfection for 24 h, co-treatment with 10 µM p38 MAPK inhibitor
SB203580 at 37°C for 2 h significantly reduced syntenin-siRNA
triggered melanin content and Tyr activity (Fig. 4B and C). These observations
revealed that the p38 MAPK signaling may be involved in the
melanogenesis pathway mediated by syntenin.
Discussion
Syntenin, also known as mda-9, was originally cloned
from human melanoma cells (9). As
a molecule containing a PDZ domain, syntenin recuits membrane
receptors and cytoplasmic signaling proteins into functional
complexes via its PDZ domains, allowing for fast and efficient
signal transduction and membrane transport processes in responses
to external stimuli. Thereby, syntenin serves a number of roles and
regulates a variety of physiological processes (8–10).
Syntenin has been identified as a melanosome protein, but its role
in melanin biosynthesis is still unclear (16). The present study focused on how
syntenin affected melanosome formation. The data demonstrated an
increase in melanogenesis after syntenin was silenced by siRNAs,.
The findings reveal a novel mechanism by which syntenin dysfunction
can regulate melanogenesis.
The synthesis of melanin is essential for the
research and treatment of pigmentary skin diseases (17). Tyr is a key enzyme that regulates
the synthesis of melanin; the expression and enzymatic activities
of Tyr affect melanogenesis and the total melanin content of cells
(5,18). Pmel is a melanosome-specific type 1
transmembrane glycoprotein that directly initiates the formation of
pro-melanosome proteins in polyvesicular bodies, which are
important for Tyr sorting into melanosomes and ultimately
pigmentation (19). The present
study results showed that syntenin gene silencing led to increased
expression of Tyr and the melanosome structural protein Pmel. These
results also demonstrated that melanin synthesis was increased
following depletion of syntenin, which was consistent with the Tyr
activity assay.
The transcription factor MITF has an important role
in the formation and transport of melanosomes (20). MITF belongs to the family of
helix-loop-helix leucine zipper transcription factors, specifically
binding to M-box (AGTCATGTGCT) and E-box (CATGTG) motifs,
regulating the transcription of more than 25 pigment-related genes,
including enzyme components and structural protein components that
have key roles in melanogenesis (21). Tyr is mediated via activation of
MITF production (22). In
addition, decreased MITF expression induces downregulation of
melanocyte differentiation markers and inhibits melanogenesis
(23). The results of the present
study demonstrated that the protein expression levels of the key
enzyme component Tyr and the melanosome structural protein Pmel
were increased, whereas the expression level of MITF, which
regulates the expression of these two proteins was also increased;
this indicated that silencing of syntenin increased the expression
of melanogenic-related proteins that were dependent on MITF
transcription factors, and also suggested a possible mechanism for
syntenin-based silencing of melanin.
MAPKs are key signaling molecules involved in the
regulation of melanogenesis, including the stress-activated protein
kinase/JNK, extracellular signal-regulating kinase (ERK) and the
p38 MAPK signaling cascades (24).
Previous studies have shown that the JNK and ERK stress-activated
protein kinase pathways led to downregulated melanin synthesis
(25,26). By contrast, the phosphorylation of
p38 MAPK activates MITF to ultimately stimulate melanogenesis
(17,18). In the present study, silencing of
syntenin significantly promoted p38 phosphorylation in PIG1
melanocytes. To verify whether the p38 MAPK signaling molecules are
responsible for syntenin-induced melanogenesis, co-incubation of
the p38 MAPK inhibitor SB203580 with syntenin-siRNA significantly
abolished syntenin-stimulated tyrosinase activity and melanin
content. These results suggested that the p38 MAPK may be
responsible for the pigmentation process mediated by depletion of
syntenin in melanocytes among the upstream pathways involved in
melanogenesis.
In conclusion, syntenin has an effect on melanin
synthesis in PIG1 immortalized human melanocytes. Silencing of
syntenin enhanced melanogenesis through activation of the p38 MAPK
signaling pathways. In future studies, we aim to further verify the
function of syntenin in primary melanocytes instead of relying on
studies conducted in immortalized cell lines. This study has
demonstrated that syntenin may represent a promising candidate of
treatment for pigmented diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81903218).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS, XX and JH conceived and designed the study. LS,
CG and LY performed the experiments. HL and JS analyzed the data.
XH interpreted the data and critically revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
d'Ischia M, Wakamatsu K, Napolitano A,
Briganti S, Garcia-Borron JC, Kovacs D, Meredith P, Pezzella A,
Picardo M, Sarna T, et al: Melanins and melanogenesis: Methods,
standards, protocols. Pigment Cell Melanoma Res. 26:616–633. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olivares C and Solano F: New insights into
the active site structure and catalytic mechanism of tyrosinase and
its related proteins. Pigment Cell Melanoma Res. 22:750–760. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cortese K, Giordano F, Surace EM, Venturi
C, Ballabio A, Tacchetti C and Marigo V: The ocular albinism type 1
(OA1) gene controls melanosome maturation and size. Invest
Ophthalmol Vis Sci. 46:4358–4364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bissig C, Rochin L and van Niel G: PMEL
amyloid fibril formation: The bright steps of pigmentation. Int J
Mol Sci. 17(pii): E14382016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paterson EK, Fielder TJ, MacGregor GR, Ito
S, Wakamatsu K, Gillen DL, Eby V, Boissy RE and Ganesan AK:
Tyrosinase depletion prevents the maturation of melanosomes in the
mouse hair follicle. PLoS One. 10:e01437022015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berson JF, Harper DC, Tenza D, Raposo G
and Marks MS: Pmel17 initiates premelanosome morphogenesis within
multivesicular bodies. Mol Biol Cell. 12:3451–3464. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yasumoto K, Yokoyama K, Takahashi K,
Tomita Y and Shibahara S: Functional analysis of
microphthalmia-associated transcription factor in pigment
cell-specific transcription of the human tyrosinase family genes. J
Biol Chem. 272:503–509. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarkar D, Boukerche H, Su ZZ and Fisher
PB: mda-9/syntenin: Recent insights into a novel cell signaling and
metastasis-associated gene. Pharmacol Ther. 104:101–115. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin J, Jiang H and Fisher P:
Characterization of a novel melanoma differentiation associated
gene, mda-9, that is down regulated during terminal cell
differentiation. Mol Cell Differ. 4:317–333. 1996.
|
|
10
|
Kegelman TP, Das SK, Emdad L, Hu B,
Menezes ME, Bhoopathi P, Wang XY, Pellecchia M, Sarkar D and Fisher
PB: Targeting tumor invasion: The roles of MDA-9/Syntenin. Expert
Opin Ther Targets. 19:97–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao D, Ohlendorf J, Chen Y, Taylor DD,
Rai SN, Waigel S, Zacharias W, Hao H and McMasters KM: Identifying
mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One.
7:e468742012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang P, Liu W, Zhu C, Yuan X, Li D, Gu W,
Ma H, Xie X and Gao T: Silencing of GPNMB by siRNA inhibits the
formation of melanosomes in melanocytes in a MITF-independent
fashion. PLoS One. 7:e429552012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosoi J, Abe E, Suda T and Kuroki T:
Regulation of melanin synthesis of B16 mouse melanoma cells by 1
alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res.
45:1474–1478. 1985.PubMed/NCBI
|
|
14
|
Martínez-Esparza M, Jiménez-Cervantes C,
Solano F, Lozano JA and García-Borrón JC: Mechanisms of
melanogenesis inhibition by tumor necrosis factor-alpha in B16/F10
mouse melanoma cells. Eur J Biochem. 255:139–146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Shang J, Ping F and Zhao G:
Alcohol extract from Vernonia anthelmintica (L.) willd seed
enhances melanin synthesis through activation of the p38 MAPK
signaling pathway in B16F10 cells and primary melanocytes. J
Ethnopharmacol. 143:639–647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basrur V, Yang F, Kushimoto T, Higashimoto
Y, Yasumoto K, Valencia J, Muller J, Vieira WD, Watabe H,
Shabanowitz J, et al: Proteomic analysis of early melanosomes:
Identification of novel melanosomal proteins. J Proteome Res.
2:69–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung E, Kim JH, Kim MO, Jang S, Kang M, Oh
SW, Nho YH, Kang SH, Kim MH, Park SH and Lee J: Afzelin positively
regulates melanogenesis through the p38 MAPK pathway. Chem Biol
Interact. 254:167–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Makpol S, Jam FA, Rahim NA, Khor SC,
Ismail Z, Yusof YA and Wan Ngah WZ: Comparable down-regulation of
TYR, TYRP1 and TYRP2 genes and inhibition of melanogenesis by
tyrostat, tocotrienol-rich fraction and tocopherol in human skin
melanocytes improves skin pigmentation. Clin Ter. 165:39–45.
2014.
|
|
19
|
Watt B, van Niel G, Raposo G and Marks MS:
PMEL: A pigment cell-specific model for functional amyloid
formation. Pigment Cell Melanoma Res. 26:300–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vachtenheim J and Borovanský J:
‘Transcription physiology’ of pigment formation in melanocytes:
Central role of MITF. Exp Dermatol. 19:617–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan L, Ma X, Wen B, Su Z, Zheng X, Liu Y,
Li H, Chen Y, Wang J, Lu F, et al: Microphthalmia-associated
transcription factor/T-box factor-2 axis acts through Cyclin D1 to
regulate melanocyte proliferation. Cell Prolif. 48:631–642. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khaled M, Larribere L, Bille K, Aberdam E,
Ortonne JP, Ballotti R and Bertolotto C: Glycogen synthase kinase
3beta is activated by cAMP and plays an active role in the
regulation of melanogenesis. J Biol Chem. 277:33690–33697. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiménez-Cervantes C, Martínez-Esparza M,
Pérez C, Daum N, Solano F and García-Borrón JC: Inhibition of
melanogenesis in response to oxidative stress: Transient
downregulation of melanocyte differentiation markers and possible
involvement of microphthalmia transcription factor. J Cell Sci.
114:2335–2344. 2001.PubMed/NCBI
|
|
24
|
D'Mello SA, Finlay GJ, Baguley BC and
Askarian-Amiri ME: Signaling pathways in melanogenesis. Int J Mol
Sci. 17(pii): E11442016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim DS, Jeong YM, Park IK, Hahn HG, Lee
HK, Kwon SB, Jeong JH, Yang SJ, Sohn UD and Park KC: A new
2-imino-1,3-thiazoline derivative, KHG22394, inhibits melanin
synthesis in mouse B16 melanoma cells. Biol Pharm Bull. 30:180–183.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bu J, Ma PC, Chen ZQ, Zhou WQ, Fu YJ, Li
LJ and Li CR: Inhibition of MITF and tyrosinase by
paeonol-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am
J Chin Med. 36:245–263. 2008. View Article : Google Scholar : PubMed/NCBI
|