Introduction
Dilated cardiomyopathy (DCM) is characterized by
left ventricular dilation, which is associated with systolic and
diastolic dysfunction (1).
Schultheiss et al (2)
reported that the prevalence of DCM in Minnesota, USA, is estimated
at 1 case per 250 people. In 2010, the estimated mortality rate
associated with cardiomyopathy was 5.9 deaths per 100,000 global
population (2). The mechanism of
DCM is complex, and there is evidence to indicate that inflammatory
reactions and cardiac remodeling play a key role in compensatory
repair following heart failure (3,4).
Furthermore, cardiomyocyte apoptosis occurs during the inflammatory
process in early heart failure and compensatory ventricular
remodeling (5–9). Doxorubicin (Dox) is an anthracycline
derivative that is an effective treatment for a number of soft and
solid types of human malignancy. However, a number of studies have
used doxorubicin to induce DCM in mice (10,11).
Sox5, a member of the Sox family of transcription
factors, has a key function in the regulation of embryonic
development and determination of cell fate (12). Previous studies have demonstrated
that Sox5 can promote cell proliferation in gastric and lung
cancer, as well as glioma and breast tumors (13–16).
In addition, Sox5 has also been demonstrated to regulate cartilage
formation (17). Previous studies
have demonstrated that Sox5 is associated with the
electrocardiographic PR interval, a higher resting heart rate,
atrial fibrillation and left ventricular mass (18–21).
These results indicate that Sox5 may play a key role in cardiac
function (22). However, to the
best of our knowledge, the role of Sox5 in DCM has not previously
been identified.
In the present study, wild-type mice were
intraperitoneally injected with Dox to induce DCM, and heart
specimens from human patients with DCM were used to investigate the
role of Sox5 in DCM. Furthermore, these results indicate that Sox5
is upregulated in DCM and may be involved in the progression of DCM
by modulating wnt-1/β-catenin signaling. To our best knowledge, the
present study provides the first evidence for an association
between Sox5 expression levels and DCM. This may provide a new
target for interventions in patients with DCM.
Materials and methods
Human tissue
The present study was performed at the Department of
Thoracic and Cardiovascular Surgery, Nanjing First Hospital,
Nanjing Medical University (Nanjing, China). Left ventricular
tissues of heart transplant recipients with DCM were collected, and
mismatched left ventricular tissues were collected from donors as
the normal control group from the Nanjing first hospital from 2013
to 2019 (normal: Male: Female, 2:1, Age, 51±2 years; DCM: Male:
Female, 1:2, age, 60±5 years). Patients with rheumatic heart
disease, infectious endocarditis, inflammatory disease, underlying
genetic syndromes and other causes of DCM were excluded. The left
ventricular tissues were cut into two pieces (section thickness, 4
mm): One was fixed in 4% formalin at room temperature for 12 h and
embedded in paraffin, and the other was frozen in liquid nitrogen
at −196°C. The protocols of all human studies were approved by the
Ethics Committee of Nanjing First Hospital and were performed in
accordance with the relevant guidelines and regulations. All
patients provided informed written consent. The study was performed
according to the Declaration of Helsinki (2000).
Animals and experimental
protocols
C57BL/6 mice (male; 8 weeks old; 22–27 g; n=12) were
obtained from the Institutional Animal Care and Use Committee of
Nanjing Medical University (Nanjing, China). Animals received
humane care and the mice experiments in the present study were
approved by the Institutional Animal Care and Use Committee of
Nanjing Medical University (approval no. SYXK2016-0006). The mice
were randomly assigned to two groups (n=6): Sham or DCM group and
kept in pathogen-free conditions with a 12/12 h light/dark cycle,
25 °C, with ad libitum access to food and water. In the DCM
group, each mouse was injected with a cumulative dose of 25 mg/kg
doxorubicin (Sigma-Aldrich; Merck KGaA) via five intraperitoneal
(i.p.) injections (5 mg/kg i.p.) over 30 days at regular intervals.
The sham group received the same volume of sterile isotonic saline
at the same time points. All mice were sacrificed under anesthesia,
and the hearts were immediately harvested.
Echocardiographic evaluation
Mice were anesthetized using 1.5–2.0% isoflurane by
inhalation, and echocardiography was performed using a Vevo2100
(VisualSonics, Inc.) ultrasound with a 30 MHz linear array
ultrasound transducer. The left ventricular internal diameter in
diastole (LVIDd), left ventricular internal diameter in systole
(LVIDs), left ventricular ejection fraction (EF) and fractional
shortening (FS) were measured from M-mode tracings with a sweep
speed of 50 mm/s at the mid-papillary muscle level. The systole and
diastole phases corresponded to the smallest and largest LV
diameters, respectively. Echocardiographic measurements were taken
in triplicate in M-mode from >3 individual mice per group.
Hematoxylin-eosin (HE) and Masson's
staining
Mouse hearts were removed, immediately immersed in
4% neutral phosphate-buffered paraformaldehyde for 12 h at room
temperature, embedded in paraffin, and sectioned (4 µm-thick).
Sections were stained with HE or Masson's trichrome at the room
temperature for 5 min and were observed to identify morphological
changes and fibrosis in the myocardium under a light
microscope.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from mouse heart tissues
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.). Equal quantities of RNA (1 µg) were transcribed into cDNA
using the PrimeScript™ RT Reagent kit with gDNA Eraser (Takara).
Quantitative TaqMan PCR assays were performed with SYBR Premix Ex
TaqTM II (Takara) using the Applied Biosystems 7500 Real Time PCR
System. The thermocycling conditions were as follows: 95°C for 15
min, followed by 40 cycles (94°C for 15 sec; 60°C for 32 sec; 72°C
for 60 sec). All data were normalized to the level of GAPDH and are
expressed as the fold increase relative to expression levels in a
sham control mouse. Primers used for quantitative real-time PCR
were as follows: Atriopeptin (ANP): Forward,
5′-AAGAACCTGCTAGACCACCTGGAG-3′ and reverse,
5′-TGCTTCCTCAGTCTGCTCACTCAG-3′; brain natriuretic peptide (BNP):
Forward, 5′-GGAAGTCCTAGCCAGTCTCCAGAG-3′ and reverse,
5′-GCCTTGGTCCTTCAAGAGCTGTC-3′; collagen 1: Forward,
5′-TGGTCCTGCTGGTCCTGCTG-3′ and reverse,
5′-CTGTCACCTTGTTCGCCTGTCTC-3′; Collagen 3: Forward,
5′-TCTCCTGGTGCTGCTGGTCAC-3′ and reverse,
5′-TCCATGTGGTCCAACTGGTCCTC-3′; TNF-α: Forward,
5′-ACGGCATGGATCTCAAAGAC-3′ and reverse, 5′-AGATAGCAAATCGGCTGACG-3′;
TGF-β1: Forward, 5′-GCAACAATTCCTGGCGTTACCTTG-3′ and reverse,
5′-CAGCCACTGCCGTACAACTCC-3′; IL-6: Forward,
5′-ACAACGATGATGCACTTGCAGA-3′ and reverse,
5′-GATGAATTGGATGGTCTTGGTC-3′; and IL-10: Forward,
5′-GCTCTTACTGACTGGCATGAG-3′ and reverse,
5′-CGCAGCTCTAGGAGCATGTG-3′.
Immunohistochemical (IHC)
staining
Human and mouse heart tissues collected for
morphological analysis were prepared as 4-µm-thick serial
paraffin-embedded sections and rehydrated in graded alcohol for 1 h
at room temperature. The sections were treated with 3% hydrogen
peroxide for 15 min at 37°C to block endogenous peroxidase activity
and incubated in goat serum at room temperature for 1.5 h (OriGene
Technologies, Inc.) to prevent non-specific binding of antibodies.
The sections were then incubated separately for 14 h at 4°C with
antibodies against Sox5 (anti-human,1:100; cat. no. 26041; Abcam;
anti-mouse,1:100; cat. no. 13216-1-AP; Proteintech) and wnt-1
(1:100; cat. no. 15251; Abcam) and then with goat anti-rabbit IgG
(cat. no. KIT-5004; MXB) for 1 h at 37°C in a humidified box. The
signal of each antibody was developed using diaminobenzidine (DAB;
OriGene Technologies, Inc.) substrate. The sections were
counterstained with hematoxylin at room temperature for 6 min, and
images were captured using a ZEISS microscope with an A1 camera.
The IHC results were evaluated based on the Fromowitz
semi-quantitative analysis score, which evaluated the brown
chromogen intensity (range, 0–7). The average score of each slice,
as determined by two independent observers, was used for later
comparisons.
TUNEL staining
Frozen mice ventricular tissues embedded in optical
cutting temperature compound were cut into 4 µm-thick sections and
fixed in 4% paraformaldehyde at room temperature for 16 min. The
TUNEL assay was performed according to the instructions of the
in situ apoptosis detection kit (Roche Diagnostics
(Shanghai) Co., Ltd.) and examined using a fluorescence microscope.
Images were captured using Olympus BX-51 light microscope
(magnification, ×200). Only nuclei that were clearly located in
cardiac myocytes were considered. The apoptotic index was
calculated as the percentage of the number of TUNEL-positively
stained nuclei to the number of DAPI- stained nuclei from 8 random
fields.
Western blotting analysis
Total protein samples (30 µg) were extracted from
left ventricular tissues and separated by 5% SDS-PAGE. The proteins
were transferred to PVDF membranes (EMD Millipore), which were
washed twice in TBS with Tween-20 (1:1,000; Promega Corporation)
for 10 min each and blocked with TBST containing 5% BSA
(Sigma-Aldrich; Merck KGaA) for 1 h at 4°C. The membranes were
incubated with the following primary antibodies in TBST with Tween
plus 5% BSA overnight at 4°C: Anti-sox5 (anti-human, 1:100; cat.
no. 26041; Abcam; anti-mouse, 1:100; cat. no. 13216-1-AP;
ProteinTech Group, Inc.)), anti-wnt-1 (1:1,000; cat. no. 15251;
Abcam), anti-β-catenin [1:1,000; cat. no. 8480s; Cell Signaling
Technology, Inc. (CST)], anti-phosphorylated β-catenin (1:1,000;
cat. no. 4176s; CST), anti-bax (1:1,000; cat. no. 2772s; CST),
anti-cleaved-caspase3 (1:1,000; cat. no. 9661s; CST), anti-caspase
9 (1:1,000; cat. no. 9508T, CST) and horseradish peroxidase
(HRP)-conjugated monoclonal mouse anti-GAPDH (1:5,000; cat. no.
KC-5G5; Kangchen BioTech Co., Ltd.).. The PVDF membranes were
washed with TBST (Tween-20, 1:1,000; Promega Corporation) three
times for 10 min each. Subsequently, the PVDF membranes were
incubated with goat anti-mouse IgG/HRP (1:5,000, cat. no.
bs-0296G-HRP; BIOSS), goat anti-rabbit IgG or an HRP-conjugated
antibody (1:5,000; cat. no. 7074P2; Cell Signaling Technology,
Inc.) at room temperature for 1 h. Specific proteins were detected
using Immobilon Western Chemiluminescent HRP Substrate (EMD
Millipore) and images were captured using a Chemi Scope system
(3300 Mini; Clinx Science Instruments Co., Ltd.). The results were
analyzed using Chemi Analysis Software (Clinx Science Instruments
Co., Ltd.) by semi-quantifying the mean gray value of each blot.
All results are representative of at least three independent
experiments.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Differences among groups were assessed using analysis of
variance followed by Tukey's post-hoc test. Comparisons between two
groups were performed using paired Student's t test. All
statistical analyses were performed using SPSS software (version
17.0; SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Result
Expression levels of Sox5 and activation of the
wnt/β-catenin pathway in the hearts of patients with DCM.
Myocardial tissue samples were selected from five patients with
DCM. The mismatched left ventricular tissues of donors were
collected as the normal control group. Western blotting analysis
demonstrated that the expression level of Sox5 was increased in DCM
heart tissue compared with the normal control heart tissue
(Fig. 1A). Images of IHC staining
demonstrated that the expression level (Fig. 1C) and localization of Sox5 was the
same in cardiomyocytes in samples from both groups. The present
study also demonstrated that the expression levels of wnt-1 and
β-catenin were increased in DCM hearts, while the expression level
of p-β-catenin was decreased, as determined by western blotting
(Fig. 1A). IHC demonstrated that
the expression levels of wnt-1 were the same in both groups
(Fig. 1C). The results
demonstrated that Sox5 and wnt/β-catenin pathway-associated
proteins are highly expressed in DCM.
Dox-induced cardiac dilatation and left ventricular
dysfunction in mice. In order to further investigate
the role of Sox5 in DCM, a mouse model of Dox-induced DCM was used.
Gross anatomical analysis demonstrated that the hearts of mice
treated with Dox were larger than those of mice treated with saline
(Fig. 2A). Furthermore, the
heart-to-body weight ratio of the sham group was lower than that of
the DCM group (Fig. 2B). The EF
and FS were notably decreased in the DCM group compared with the
sham group; in contrast, the LVIDd and LVIDs were notably increased
in the DCM group (Fig. 2C and D).
HE staining of the hearts demonstrated that the sham group
exhibited an increased myocardial thickness and decreased cardiac
chamber diameter compared with those of the DCM group. Masson's
trichrome staining demonstrated that the collagen content was
notably elevated in the DCM group (Fig. 2E). These results demonstrated that
mice treated with Dox exhibited pathological changes associated
with DCM.
Inflammatory reactions, fibrosis and apoptosis were
increased in the hearts of Dox-treated mice. DCM is associated with
acute myocardial inflammation and heart failure (1). RT-qPCR analysis of heart tissues
demonstrated a notable increase in heart failure markers (ANP and
BNP), pro-inflammatory factors (IL-6 and TNF-α) and
anti-inflammatory factors (TGF-β and IL-10) in the DCM group
compared with the sham group. The present study also found that the
mRNA levels of fibrosis markers (collagen 1 and 3) were increased
in the DCM group (Fig. 3A), which
was consistent with the results of Masson's trichrome staining. In
addition, the present study tested the expression levels of
apoptosis-associated proteins in mouse heart tissues. The western
blot results showed that the expression levels of pro-apoptotic
genes, including Bax, cleaved-caspase3 and caspase 9, were
increased in the DCM group. Furthermore, the number of
TUNEL-positive nuclei was notably increased in the DCM group
(Fig. 3B-D).
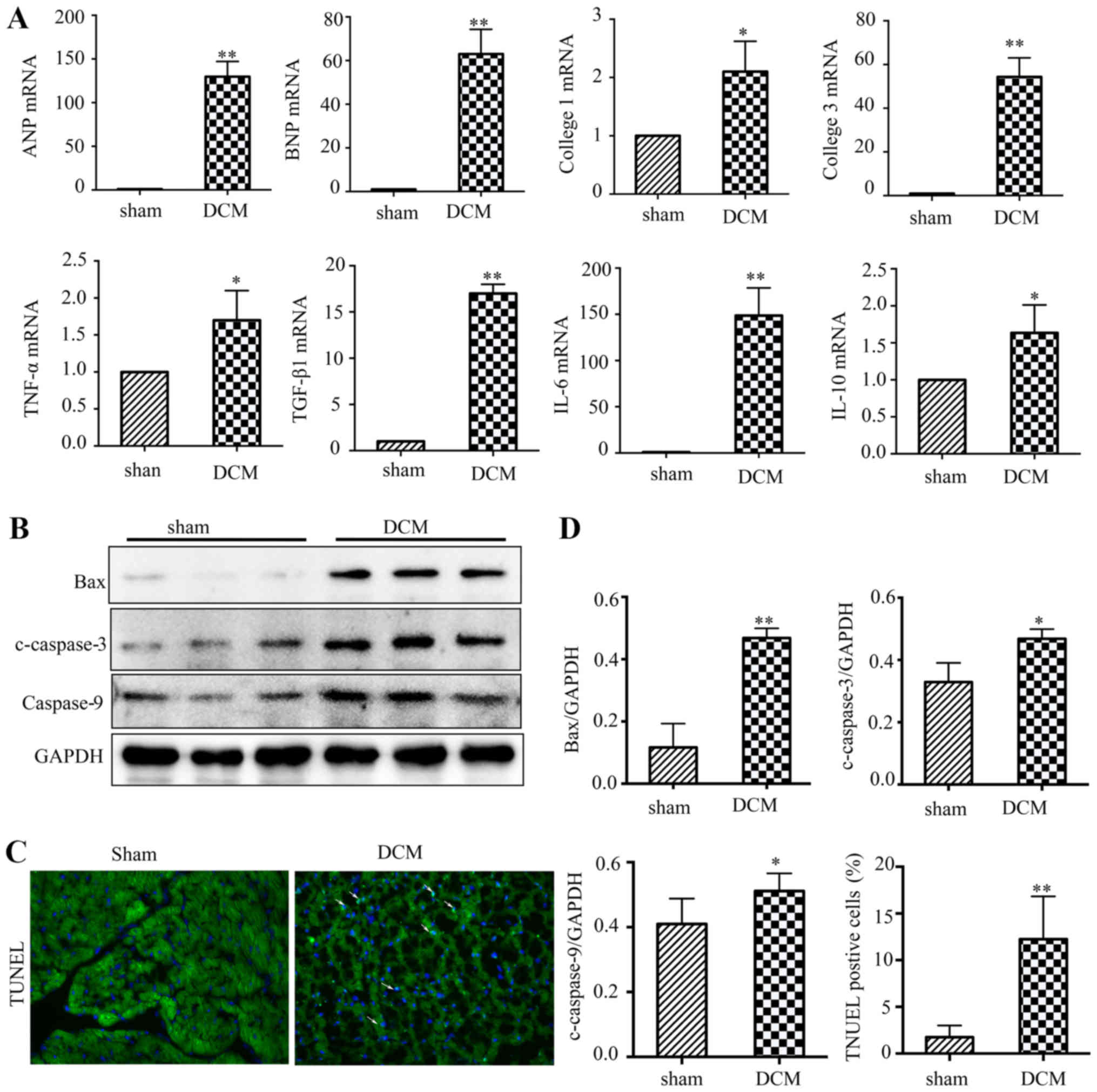 | Figure 3.Inflammatory reactions, fibrosis and
apoptosis are activated in the hearts of Dox-treated mice. (A) The
relative mRNA levels of heart failure markers (ANP and BNP),
fibrosis markers (collagen 1 and collagen 3), pro-inflammatory
factors (IL-6 and TNF-α) and anti-inflammatory factors (TGF-β and
IL-10). (B) Representative western blotting of Bax, c-caspase3 and
caspase9 expression levels in heart tissues from WT mice following
drug injection. (C) Representative images of TUNEL staining
(magnification, ×200) of heart tissues from WT mice following drug
injection. (D) Quantitative analyses of western blots and TUNEL
staining. (n=6). *P<0.05; **P<0.01 vs. sham group. Dox,
doxorubicin; ANP, atriopeptin; BNP, brain natriuretic peptide; WT,
wild type; DCM, dilated cardiomyopathy. |
Increased expression levels of Sox5 and activation
of the wnt/β-catenin pathway in the hearts of Dox-treated
mice. High expression levels of Sox5 and activation of the
wnt/β-catenin pathway have been reported in the hearts of human
patients with DCM (Fig. 1).
Western blotting and IHC analysis demonstrated increased expression
levels of Sox5 and wnt-1 in the DCM group compared with the sham
group (Fig. 4A and C). In
addition, increased expression levels of β-catenin and decreased
expression levels of p-β-catenin were exhibited in the DCM group
(Fig. 4A). In conclusion, Sox5 is
upregulated in DCM and may be involved in the development of DCM
via modulation of Wnt-1/β-catenin signaling.
Discussion
DCM is a type of heart failure characterized by
ventricular dilatation. In the absence of hypertension, coronary
artery or valvular disease, DCM is the most significant and third
most common cause of heart failure and treatment requires heart
transplantation (23). However,
the pathogenesis of DCM is not clear. Sox5 can downregulate the
cyclin D1/cyclin-dependent kinase 4 complex to regulate cell
proliferation and apoptosis (24,25).
It can also upregulate the protein expression levels of N-cadherin,
vimentin and fibronectin to promote fibrosis (17). Sox5 is expressed in numerous human
tissues, including the testis, liver, lung, fetal brain and heart
(26). Liu et al (27) demonstrated that overexpression of
Sox5 can promote inflammatory responses and reverse
microRNA-193a-3p mimic-mediated decrease in chondrocyte apoptosis
in human osteoarthritis. However, studies have demonstrated that
inhibiting Sox5 can promote apoptosis in rheumatoid arthritis and
lung cancer (24,25). Axelsson et al (28) demonstrated that Sox5 protein is
present both in the nucleus and in the cytosol of pancreatic
sections. Stolt et al (29)
demonstrated that the transcription factor Sox5 modulates Sox10
function during melanocyte development and that Sox5 is expressed
in the nuclei of murine melanocytes. In the present study, Sox5 was
highly expressed in DCM myocardial tissue compared with normal
myocardial tissue, and histological manifestations, such as severe
inflammatory responses, collagen deposition and apoptosis, were
increased in DCM. Therefore, it was hypothesized that Sox5 may be a
protective factor in DCM, and its specific role will be further
studied in Sox5-knockout mice. Previous studies have indicated that
Sox5 plays a key role in heart function (18–21).
In the present study, western blotting analysis demonstrated a
notable increase in the expression level of Sox5 in the hearts of
patients with DCM; this was confirmed via IHC. To the best of our
knowledge, these results are the first to demonstrate that Sox5
expression level is associated with DCM.
Dox is associated with dose-dependent
cardiotoxicity, which can progress to heart failure. Administration
of Dox at doses >1 mg/kg results in a decreased survival rate
and classic signs of DCM (30). In
order to study the effect of Sox5 on dilated cardiomyopathy in
vivo, the present study constructed a mouse model of DCM via
i.p. injection of Dox into wild-type mice. In the present study,
the hearts of mice treated with Dox were larger, and the
heart-to-body weight ratio of the DCM group was higher than that of
the sham group. The body weight of mice treated with Dox was
significantly decreased compared with that of mice treated with
saline. In addition, following Dox treatment, the mice became thin
and responded poorly. Furthermore, the mRNA levels of heart failure
markers (ANP and BNP) were significantly increased, as determined
by PCR, which indicated that mice treated with Dox experienced
heart failure. Moreover, the Dox-treated mice exhibited
pathological manifestations of DCM. HE and Masson's trichrome
staining demonstrated that Dox-treated mice exhibited dilated
ventricles, disordered arrangement of cardiac cells, collagen
deposition and fibrosis. The echocardiography results indicated
that Dox-treated mice exhibited an increased ventricular inner
diameter, a decreased ejection fraction and heart failure
consistent with DCM.
Inflammation is involved in the pathogenesis of DCM.
DCM is associated with inflammation, as documented by increased
levels of inflammatory cytokines, such as IL-6, IL-10 and TNF-α
(31). PCR analysis of the
expression levels of pro-inflammatory factors in the DCM group were
consistent with this. Collagen deposition and ventricular
remodeling, which maintain the function of the heart, are important
during the progression of DCM (32,33).
Consistent with the Masson's trichrome staining results, RT-qPCR
demonstrated that the expression levels of collagen (collagen 1 and
collagen 3) were increased in the DCM group compared with the sham
group. Apoptosis is a form of programmed cell death controlled by
numerous genes that maintains the stability of the internal
environment (34). Previous
studies have demonstrated that cell apoptosis promotes the
progression of Dox-induced DCM and may be the molecular mechanism
underlying DCM (35). The cysteine
aspartate protein (caspase) family participates in the regulation
of cell apoptosis. Caspase-9, which is an initiator of apoptosis,
triggers an executioner caspase (caspase 3 or 7), ensuring
continuity of the process (34).
In the present study, TUNEL staining and analysis of Bax,
cleaved-caspase3 and caspase9 expression levels using western
blotting demonstrated a notable increase in apoptosis in the DCM
group compared with the sham group.
Wnt proteins are a family of secreted cysteine-rich
glycoproteins involved in a number of cellular processes, including
proliferation, differentiation, senescence and apoptosis. The
wnt/β-catenin pathway has been demonstrated to play a role in DCM
(36). In previous studies, Sox5
has been demonstrated to regulate the wnt/β-catenin pathway
(37,38). β-catenin is the key effector of the
wnt/β-catenin pathway and functions as a transcriptional
co-activator that is critical for target gene expression level. In
the absence of wnt ligands, β-catenin is bound by the scaffold
protein Axin, which facilitates its phosphorylation by glycogen
synthase kinase 3-b via a destruction complex. When wnt ligands
bind to the Frizzled and low-density lipoprotein receptor 5/6
complex, the β-catenin destruction complex becomes dysfunctional
(37,38). However, the mechanism of this
process is not fully understood. When the wnt/β-catenin pathway is
activated, the expression level of β-catenin in cytosol increases,
and β-catenin translocates to the nucleus to form a complex with
the transcription factor TCF/LEF, leading to the activation of
target genes (39,40). The present study demonstrated that
the expression levels of wnt/β-catenin pathway-associated proteins
were altered in DCM. In light of the expression level pattern of
Sox5 and the reported association between the wnt/β-catenin pathway
and Sox5, it was hypothesized that sox5 may be associated with the
wnt/β-catenin pathway during the development of DCM.
In conclusion, the present study demonstrated that
Sox5 may be associated with acute inflammation, apoptosis, collagen
deposition and ventricular remodeling during the development of
DCM.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation of Nanjing Provincial Level in 2018
(grant no. BK20181119), the Postgraduate Research & Practice
Innovation Program of Jiangsu Province (grant no. SJCX19_0328) and
the Jiangsu Provincial Special Program of Medical Science (no.
BE2017610).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL designed the experiment and wrote the manuscript.
BJ constructed the mouse model. YC, LY and YX performed mechanism
research and molecular biological detection. WC and ZQ
conceptualized the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nanjing First Hospital (approval no.
KY20190404-03-KS-01) and were performed in accordance with the
relevant guidelines and regulations.
Patient consent for publication
All patients provided informed written consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jefferies JL and Towbin JA: Dilated
cardiomyopathy. Lancet. 375:752–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schultheiss HP, Fairweather D, Caforio
ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A,
Mazzanti A, McMurray J and Priori SG: Dilated cardiomyopathy. Nat
Rev Dis Primers. 5:322019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cutler MJ, Jeyaraj D and Rosenbaum DS:
Cardiac electrical remodeling in health and disease. Trends
Pharmacol Sci. 32:174–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mann DL: Inflammatory mediators and the
failing heart: Past, present, and the foreseeable future. Circ Res.
91:988–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crow MT, Mani K, Nam YJ and Kitsis RN: The
mitochondrial death pathway and cardiac myocyte apoptosis. Circ
Res. 95:957–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hikoso S, Ikeda Y, Yamaguchi O, Takeda T,
Higuchi Y, Hirotani S, Kashiwase K, Yamada M, Asahi M, Matsumura Y,
et al: Progression of heart failure was suppressed by inhibition of
apoptosis signal-regulating kinase 1 via transcoronary gene
transfer. J Am Coll Cardiol. 50:453–462. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Q, Zhou HJ, Zhang H, Huang Y,
Hinojosa-Kirschenbaum F, Fan P, Yao L, Belardinelli L, Tellides G,
Giordano FJ, et al: Thioredoxin-2 inhibits mitochondrial reactive
oxygen species generation and apoptosis stress kinase-1 activity to
maintain cardiac function. Circulation. 131:1082–1097. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maejima Y, Kyoi S, Zhai P, Liu T, Li H,
Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, et al: Mst1
inhibits autophagy by promoting the interaction between Beclin1 and
Bcl-2. Nat Med. 19:1478–1488. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller EJ, Li J, Leng L, McDonald C,
Atsumi T, Bucala R and Young LH: Macrophage migration inhibitory
factor stimulates AMP-activated protein kinase in the ischaemic
heart. Nature. 451:578–582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong YJ, Kim TK, Hong D, Park CH, Yoo SJ,
Wickum ME, Hur J, Lee HJ, Kim YJ, Suh YJ, et al: Myocardial
characterization using dual-energy CT in doxorubicin-induced DCM:
Comparison with CMR T1-mapping and histology in a rabbit model.
JACC Cardiovasc Imaging. 9:836–845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marechal X, Montaigne D, Marciniak C,
Marchetti P, Hassoun SM, Beauvillain JC, Lancel S and Neviere R:
Doxorubicin-induced cardiac dysfunction is attenuated by
ciclosporin treatment in mice through improvements in mitochondrial
bioenergetics. Clin Sci (Lond). 121:405–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lefebvre V: The SoxD transcription
factors-Sox5, Sox6, and Sox13-are key cell fate modulators. Int J
Biochem Cell Biol. 42:429–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou H, Wang S, Wang S, Wu H, Yu J, Chen Q,
Cui W, Yuan Y, Wen X, He J, et al: SOX5 interacts with YAP1 to
drive malignant potential of non-small cell lung cancer cells. Am J
Cancer Res. 8:866–878. 2018.PubMed/NCBI
|
|
14
|
Sun C, Ban Y, Wang K, Sun Y and Zhao Z:
SOX5 promotes breast cancer proliferation and invasion by
transactivation of EZH2. Oncol Lett. 17:2754–2762. 2019.PubMed/NCBI
|
|
15
|
Ueda R, Yoshida K, Kawase T, Kawakami Y
and Toda M: Preferential expression and frequent IgG responses of a
tumor antigen, SOX5, in glioma patients. Int J Cancer.
120:1704–1711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You J, Zhao Q, Fan X and Wang J: SOX5
promotes cell invasion and metastasis via activation of
Twist-mediated epithelial-mesenchymal transition in gastric cancer.
Onco Targets Ther. 12:2465–2476. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hattori T, Coustry F, Stephens S,
Eberspaecher H, Takigawa M, Yasuda H and de Crombrugghe B:
Transcriptional regulation of chondrogenesis by coactivator Tip60
via chromatin association with Sox9 and Sox5. Nucleic Acids Res.
36:3011–3024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pfeufer A, van Noord C, Marciante KD,
Arking DE, Larson MG, Smith AV, Tarasov KV, Müller M, Sotoodehnia
N, Sinner MF, et al: Genome-wide association study of PR interval.
Nat Genet. 42:153–159. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eijgelsheim M, Newton-Cheh C, Sotoodehnia
N, de Bakker PI, Müller M, Morrison AC, Smith AV, Isaacs A, Sanna
S, Dörr M, et al: Genome-wide association analysis identifies
multiple loci related to resting heart rate. Hum Mol Genet.
19:3885–3894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olesen MS, Holst AG, Jabbari J, Nielsen
JB, Christophersen IE, Sajadieh A, Haunsø S and Svendsen JH:
Genetic loci on chromosomes 4q25, 7p31, and 12p12 are associated
with onset of lone atrial fibrillation before the age of 40 years.
Can J Cardiol. 28:191–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Della-Morte D, Beecham A, Rundek T, Wang
L, McClendon MS, Slifer S, Blanton SH, Di Tullio MR and Sacco RL: A
follow-up study for left ventricular mass on chromosome 12p11
identifies potential candidate genes. BMC Med Genet. 12:1002011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li A, Ahsen OO, Liu JJ, Du C, McKee ML,
Yang Y, Wasco W, Newton-Cheh CH, O'Donnell CJ, Fujimoto JG, et al:
Silencing of the Drosophila ortholog of SOX5 in heart leads to
cardiac dysfunction as detected by optical coherence tomography.
Hum Mol Genet. 22:3798–3806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halliday BP, Cleland JGF, Goldberger JJ
and Prasad SK: Personalizing risk stratification for sudden death
in dilated cardiomyopathy: The past, present, and future.
Circulation. 136:215–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Zhang XL, Li XF, Tang YC and Zhao
X: miR-212-3p reduced proliferation, and promoted apoptosis of
fibroblast-like synoviocytes via down-regulating SOX5 in rheumatoid
arthritis. Eur Rev Med Pharmacol Sci. 22:461–471. 2018.PubMed/NCBI
|
|
25
|
Li G, Wang K, Wang J, Qin S, Sun X and Ren
H: miR-497-5p inhibits tumor cell growth and invasion by targeting
SOX5 in non-small-cell lung cancer. J Cell Biochem.
120:10587–10595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Veronique MW, Critcher R, Ashworth A and
Goodfellow PN: Cloning and characterization of SOX5, a new member
of the human SOX gene family. Genomics. 36:354–358. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu F, Liu X, Yang Y, Sun Z, Deng S, Jiang
Z, Li W and Wu F: NEAT1/miR-193a-3p/SOX5 axis regulates cartilage
matrix degradation in human osteoarthritis. Cell Biol Int.
44:947–957. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Axelsson AS, Mahdi T, Nenonen HA, Singh T,
Hänzelmann S, Wendt A, Bagge A, Reinbothe TM, Millstein J, Yang X,
et al: Sox5 regulates beta-cell phenotype and is reduced in type 2
diabetes. Nat Commun. 8:156522017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stolt CC, Lommes P, Hillgartner S and
Wegner M: The transcription factor Sox5 modulates Sox10 function
during melanocyte development. Nucleic Acids Res. 36:5427–5440.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayward R and David SH: Doxorubicin
Cardiotoxicity in the rat: An in vivo characterization. J Am Assoc
Lab Anim Sci. 46:20–32. 2007.PubMed/NCBI
|
|
31
|
Sun X, Shan A, Wei Z and Xu B: Intravenous
mesenchymal stem cell-derived exosomes ameliorate myocardial
inflammation in the dilated cardiomyopathy. Biochem Biophys Res
Commun. 503:2611–2618. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iwata Y, Ohtake H, Suzuki O, Matsuda J,
Komamura K and Wakabayashi S: Blockade of sarcolemmal TRPV2
accumulation inhibits progression of dilated cardiomyopathy.
Cardiovasc Res. 99:760–768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Japp AG, Gulati A, Cook SA, Cowie MR and
Prasad SK: The diagnosis and evaluation of dilated cardiomyopathy.
J Am Coll Cardiol. 67:2996–3010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grilo AL and Mantalaris A: Apoptosis: A
mammalian cell bioprocessing perspective. Biotechnol Adv.
37:459–475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kankeu C, Clarke K, Passante E and Huber
HJ: Doxorubicin-induced chronic dilated cardiomyopathy-the
apoptosis hypothesis revisited. J Mol Med (Berl). 95:239–248. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Le Dour C, Macquart C, Sera F, Homma S,
Bonne G, Morrow JP, Worman HJ and Muchir A: Decreased WNT/β-catenin
signalling contributes to the pathogenesis of dilated
cardiomyopathy caused by mutations in the lamin a/C gene. Hum Mol
Genet. 26:333–343. 2017.PubMed/NCBI
|
|
37
|
Martinez-Morales PL, Quiroga AC, Barbas JA
and Morales AV: SOX5 controls cell cycle progression in neural
progenitors by interfering with the WNT-beta-catenin pathway. EMBO
Rep. 11:466–472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li A, Hooli B, Mullin K, Tate RE, Bubnys
A, Kirchner R, Chapman B, Hofmann O, Hide W and Tanzi RE: Silencing
of the Drosophila ortholog of SOX5 leads to abnormal neuronal
development and behavioral impairment. Hum Mol Genet. 26:1472–1482.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|


















