Introduction
Atherosclerosis (AS) is a gradual process of
thickening and sclerosis of the vascular wall caused by lipid
accumulation, fibrosis and calcium deposition in the intima of the
affected artery, characterized by subintimal fibrous plaques and/or
atheromas (1). Subsequently, AS
can cause ischemic and hypoxic changes in the corresponding tissues
and organs, thus leading to a series of complications, including
coronary artery disease, hypertension, cerebral infarction and
peripheral arterial disease (2).
The specific pathogenesis of AS is complex and involves multiple
pathophysiological processes, such as inflammation, endothelial
cell injury, immunoreaction, autophagy and apoptosis (3,4).
Vascular endothelial cell injury or endothelial
dysfunction is involved in the initiation and progression of AS
(2). Vascular endothelial cells
are predominantly composed of flat epithelial cells on the inner
surfaces of the heart, blood vessels and lymphatic vessels
(5). Besides acting as a barrier
between blood and blood vessel walls, vascular endothelial cells
can synthesize and release a variety of endothelial-derived
vasoactive factors, therefore exerting various physiological
functions, such as reducing vascular permeability, inhibiting cell
migration and chemotaxis, regulating vasoconstriction and
relaxation, preventing platelet aggregation and anti-adhesion
(5). Upon the stimulation of
various inflammatory factors, the functions and structure of
endothelial cells are damaged, triggering a series of inflammatory
reactions that involved a large number of inflammatory mediators
(6). Damaged vascular endothelial
cells express adhesion molecules, such as intercellular adhesion
molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1),
which can bind to monocytes and lymphocytes in the blood and cause
adhesion reactions (7).
Subsequently, large amounts of chemical chemokines are produced and
released, which promotes the accumulation, migration and deposition
of monocytes and lymphocytes under the vascular endothelium.
Monocyte chemoattractant protein 1 (MCP-1) and monocyte/ macrophage
colony stimulating factor promotes the transformation of monocytes
into macrophages, which can form foam cells by uptake of oxidized
low density lipoprotein (8). A
large number of inflammatory factors, such as interferon (IFN)-γ,
tumor necrosis factor (TNF)-α, and interleukin (IL)-1 are then
released, which in turn further stimulates and exacerbates the
inflammatory response of lymphocytes, macrophages, foam cells and
endothelial cells (9). In the
present study, TNF-α was used to induce vascular inflammation that
could contribute to AS in vitro.
Wnts are a family of highly conserved, secreted
glycoproteins involved in numerous biological processes, such as
proliferation, differentiation, polarity, cell survival and
adhesion (10). Wnts can activate
at least two distinct signaling pathways; the canonical
Wnt/β-catenin and noncanonical pathways, where the latter can be
further classified into the Wnt/Ca2+ and the planar cell
polarity (PCP) pathways (10).
Over the past decades, the Wnt pathway was reported to play a key
role in the occurrence and development of inflammatory diseases,
including AS (11). During the
development of AS, pro-inflammatory factors, such as TNF-α, IFN-γ
and nitric oxide, can activate the Wnt pathway; the signal
transduction of which can be maintained and enhanced by IL-6 and
TNF-α (12). In addition, the Wnt
pathway in turn has a pro-inflammatory effect, which can promote
the release of inflammatory factors and concurrently contribute to
endothelial dysfunction, resulting in plaque formation (12). Wnt family member 5a (Wnt5a), a
member of the Wnt family, was first identified in the early 1990s,
and was shown to play an important role in AS. Christman et
al (13), demonstrated Wnt5a
expression in human and murine atherosclerotic lesions for the
first time. Since then, numerous studies have reported the elevated
expression of Wnt5a in human atherosclerotic lesions and the serum
of patients with AS (14,15).
The receptor tyrosine kinase like orphan receptor 2
(Ror2) is a member of the Ror family that is essential for cell
migration, skeletal and nervous system development and oncogenesis
(16). Ror2 acts as a receptor or
co-receptor for Wnt5a that is critical for activation of the PCP
signaling pathway by Wnt5a (17).
The Wnt5a-Ror2 signaling pathway plays an important role in
regulating tumor cell growth, directional migration, invasion and
cell polarity during organogenesis (18). The Wnt5a-Ror2 axis has been
demonstrated to be involved in inflammation (19,20).
Ror2 and Wnt5a expression levels have been found to be elevated in
atherosclerotic lesions and participate in the formation of
atherosclerotic foam cells (21).
However, the functions of Ror2 in vascular endothelial cell injury
of AS remain to be clarified.
Taken together, the present study aimed to
investigate whether Ror2 could protect vascular endothelial cells
against TNF-α-induced inflammation and apoptosis. Additionally, the
modulatory effect of Wnt5a on Ror2 was explored.
Materials and methods
Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs) were
purchased from American Type Culture Collection and maintained in
RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with
10% heat-inactivated FBS (Thermo Fisher Scientific, Inc.) and 100
U/ml penicillin G. Cells were incubated at 37°C in a humidified
atmosphere containing 5% CO2 and 95% air.
To induce inflammatory response in the HUVECs, cells
were treated with different concentrations of TNF-α (0, 2.5, 5, or
10 ng/ml; PMC3016; Thermo Fisher Scientific, Inc.) at 37°C for 24 h
(22).
Plasmids and transfection
Short hairpin RNA (shRNA) sequences targeting Ror2
(−1:
5′-CCGGCCGCTACCAT-CAGTGCTATAACTCGAGTTATAGCACTGATGGTAGCGGTTTTT-3′;
−2: 5′-GCCCGATTCCAACTCTGAAAG-3′) and Wnt5a (−1:
5′-C-CGGCCTGTTCAGATGTCAGAAGTACTCGAGTACTTCTGACATCTGAACAGGTTTTTG-3′;
−2:
5′-TGGTGCTGCTATGTCAAATGCAAGATTCAAGAGATCTTGCATTTGACATAGCAGCACCTTTTTTC-3′)
were designed and constructed, then cloned into plasmids (Santa
Cruz Biotechnology, Inc.). A total 2 µg/ml of Ror2 and Wnt5a shRNA
plasmids or scrambled shRNAs (5′-GCCCAGCCAAGACATGGAAAT-3′) were
transfected into HUVECs using Lipofectamine™ 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were then
transferred to normal RPMI-1640 culture medium containing 10% FBS
and cultured for 48 h. Ror2 and Wnt5a expression levels were
measured by reverse transcription-quantitative PCR (RT-qPCR)
assays.
Western blotting and
immunoprecipitation (IP) assays
Total proteins of HUVECs were extracted using RIPA
lysis buffer (Thermo Fisher Scientific, Inc.) with Complete EDTA
Free Protease and Phosphatase Inhibitors (Roche Diagnostics).
Individual protein concentrations were determined using a
bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). A
total of 10 µg samples were then separated by 12% gradient sodium
dodecyl sulfate polyacrylamide gel electrophoresis, transferred to
polyvinylidene fluoride membranes (Thermo Fisher Scientific, Inc.),
blocked with 5% non-fat milk at room temperature for 2 h, and
incubated overnight with specific primary antibodies. The primary
antibodies (all purchased from Santa Cruz Biotechnology, Inc.) used
were as follows: Ror2 (cat. no. sc-374174; 1:500), ICAM-1 (cat. no.
sc-8439; 1:500), VCAM-1 (cat. no. sc-13160; 1:500), phosphorylated
(p)-IκBα (cat. no. sc-52900; 1:200), p65 (cat. no. sc-8008; 1:500),
IκBα (cat. no. sc-1643; 1:400), lamin B (cat. no. sc-374015;
1:200), Bcl-2 (cat. no. sc-7382; 1:1,000), Bax (cat. no. sc-7480;
1:1,000), cleaved-caspase 3 (cat. no. sc-271759; 1:200),
cleaved-caspase 7 (cat. no. sc-56067; 1:200), caspase 3 (cat. no.
sc-7272; 1:500), caspase 7 (cat. no. sc-56063, 1:500), Wnt5a (cat.
no. sc-365370; 1:500) and GAPDH (cat. no. sc-47724; 1:1,000). GAPDH
was used as the loading control. Following overnight incubation at
4°C, membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (goat anti-rabbit, cat.
no. sc-2004, 1:10,000 and goat anti-mouse, cat. no. sc-2005,
1:10,000; Santa Cruz Biotechnology, Inc.). Finally, membranes were
visualized using the ECL system and ImageJ software (v1.46r;
National Institutes of Health) was used to quantify the intensity
of each protein band.
The interaction between Ror2 and Wnt5a proteins in
HUVECs was validated using IP. For the IP assay, soluble protein
samples were pre-incubated with protein G/A-agarose (Cell Signaling
Biotechnology, Inc) at 4°C overnight and then incubated with 100 µl
protein G/A-agarose pre-coupled to antibody against primary
antibodies for ≥3 h at room temperature. The mixtures were then
washed with PBS, boiled and subjected to western blotting.
RT-qPCR
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed to cDNA using the PrimeScript RT reagent kit with gDNA
Eraser (Takara Bio, Inc.), according to the manufacturer's
instructions. A total of 50 ng cDNA was subsequently used for qPCR
using TB Green® Fast qPCR Mix (Takara Biotechnology Co.,
Ltd.). The following primers were used: Ror2 forward,
5′-TTACAGAGGAACGGCAAGCA-3′ and reverse, 5′-CTGCTGTCTCGGGGACGTTT-3′;
Wnt5a forward, 5′-ATTCTGGCTCCACTTGTTGCT-3′ and reverse,
5′-TTCATACCTAGCGACCACCA-3′; ICAM-1 forward,
5′-ATGGCAACGACTCCTTCTCG-3′ and reverse, 5′-GCCGGAAAGCTGTAGATGGT-3′;
and VCAM-1 forward, 5′-TGGATAATGTTTGCAGCTTCTCA-3′ and reverse,
5′-CGTCACCTTCCCATTCAGTG-3′. Human GAPDH was used as the control
with the following sequences: Forward, 5′-AATGGGCAGCCGTTAGGAAA-3′
and reverse, 5′-AATGGGCAGCCGTTAGGAAA-3′. The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95°C for 30 sec; and 40 cycles of 95°C for 5 sec
and 60°C for 15 sec, followed by default of melt curve (Applied
Biosystems 7500; Thermo Fisher Scientific, Inc.). Differential
expression of mRNA was calculated using the 2−ΔΔCq
method (23).
Enzyme-linked immunosorbent assay
(ELISA)
The generation of inflammatory factors TNF-α (cat.
no. ab181421), IL-1β (cat. no. ab100562), IL-6 (cat. no. ab178013)
and MCP-1 (cat. no. ab179886) was determined using ELISA kits
(Abcam) according to the manufacturer's instructions. Briefly, cell
supernatants were added to 96-well plates, and then incubated with
biotin-conjugated antibodies (included in the kits) targeting
TNF-α, IL-1β, IL-6 and MCP-1 at 37°C for 1 h. Following incubation
with working solution at 37°C for 30 min and TMB solution for 15
min in darkness, the absorbance at a wavelength of 450 nm was
detected using a microplate reader (Thermo Fisher Scientific,
Inc.).
Flow cytometry
Cell apoptosis was assessed by flow cytometry using
propidium iodide (PI) staining. In brief, cells were gently washed
twice with PBS, digested with 0.25% trypsin and centrifuged at 200
× g for 5 min at 4°C. Following resuspension of the cell pellet
with 1 ml NaCl/Pi, cells were incubated with PI for 15 min in a
dark room at room temperature and immediately analyzed using a flow
cytometer (Becton, Dickinson and Company). Data were analyzed by
flow cytometry software (iSort Automated Cell Sorter, vA.0; Thermo
Fisher Scientific, Inc).
Statistical analysis
All experiments were repeated at least three times
and data are expressed as the mean ± standard deviation. Student's
t-test was used for comparison between two groups, one-way ANOVA
was used for comparison among multiple groups and Tukey's post hoc
test was used for pairwise comparison. P<0.05 was considered to
indicate a statistically significant difference (24).
Results
Ror2 is upregulated by TNF-α stimuli
in HUVECs
Firstly, the protein expression of Ror2 in HUVECs
treated with different concentrations of TNF-α was detected by
western blotting. As presented in Fig.
1A, Ror2 expression was enhanced by TNF-α in a
concentration-dependent manner, suggesting the potential modulatory
effect of Ror2 on TNF-α dose-dependent increase in vascular
endothelial cell injury. This result was in accordance with a
previous study, in which Ror2 expression was reported to be
significantly expressed at a higher level in advanced human
atherosclerotic lesions compared with less advanced lesions
(21). Furthermore, the expression
level of Ror2 was the highest following treatment with 10 ng/ml
TNF-α. Therefore, 10 ng/ml was selected as the concentration of
TNF-α to be used in the following experiments based on the present
and previous results (22,25,26).
To further investigate the effects of Ror2 on TNF-α-induced
vascular endothelial cell injury, two shRNA expression vectors,
shRNA-Ror2-1 and shRNA-Ror2-2, were used to knock down the
expression of Ror2 in HUVECs, and cells transfected with empty
plasmids were used as a negative control. The results revealed a
more efficient knockdown effect of shRNA-Ror2-1 compared with
shRNA-Ror2-2. shRNA-Ror2-1 produced a nearly 50% reduction in the
expression level of Ror2, which is significant and enough to affect
downstream inflammatory response (27), therefore it was selected for
subsequent experiments (Fig.
1B).
Knockdown of Ror2 inhibits
TNF-α-induced inflammation in HUVECs
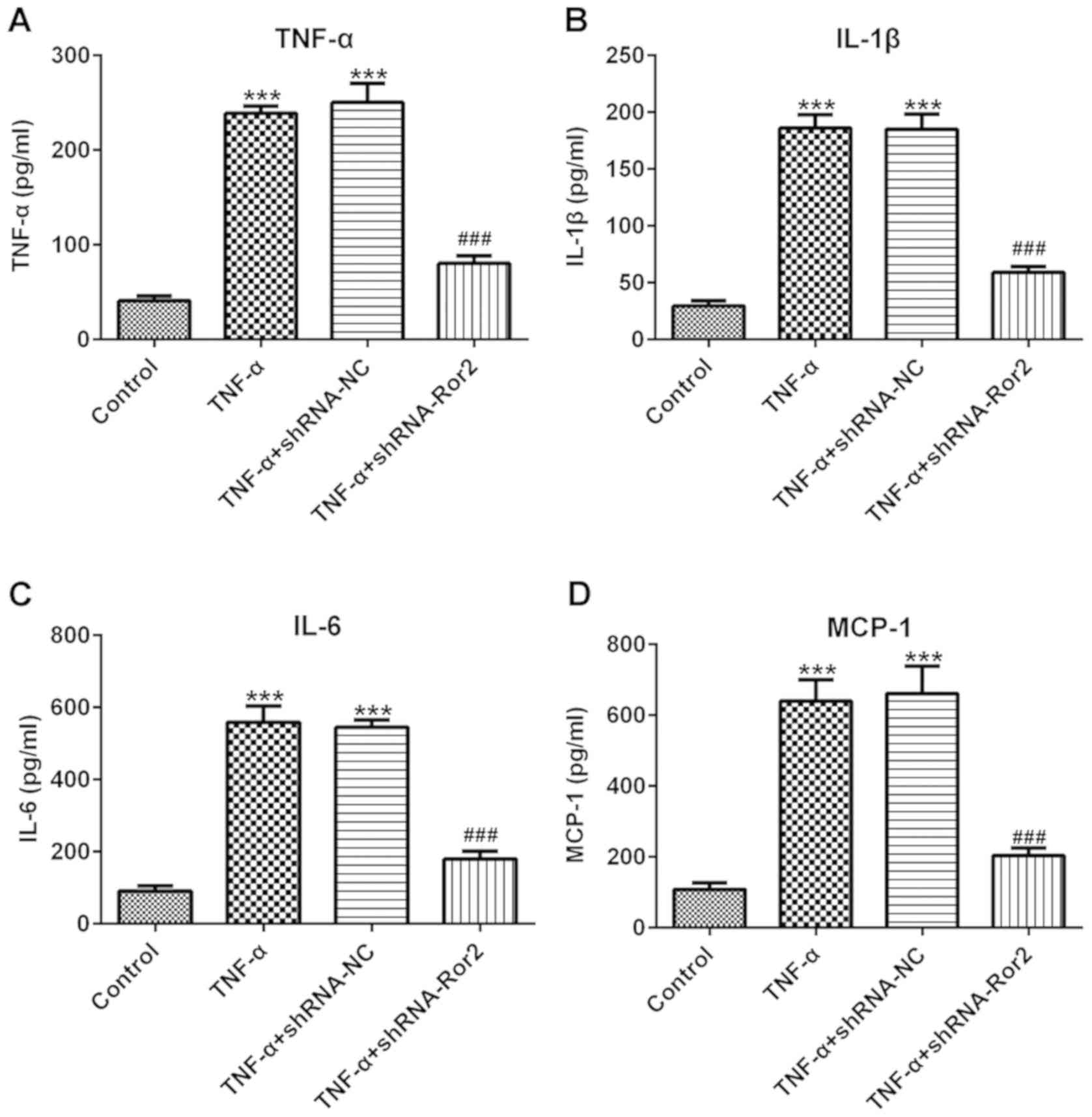
It was then investigated whether Ror2 silencing
could protect HUVECs against TNF-α-induced injury. As presented in
Fig. 2, TNF-α treatment resulted
in a significant increase in the release of inflammatory cytokines,
including TNF-α, IL-1β, IL-6 and MCP-1 compared with controls.
Meanwhile, cells with Ror2-knockdown released significantly fewer
inflammatory cytokines compared with the TNF-α groups. Results from
RT-qPCR and western blotting revealed that compared with controls,
TNF-α resulted in a significant increase in the mRNA and protein
expression levels of adhesion molecules ICAM-1 and VCAM-1, whereas
Ror2-silencing significantly decreased the mRNA and protein
expression of ICAM-1 and VCAM-1 (Fig.
3), indicating the inhibitory effect of Ror2-knockdown on
TNF-α-induced inflammation in endothelial cells.
Knockdown of Ror2 obstructs the
TNF-α-induced activation of NF-κB
NF-κB is a protein complex consisting of p65 and p50
(28). Following binding to TNF-α
receptor, TNF-α can activate IκBα kinase, which plays a role in
phosphorylating IκBα (28).
Following phosphorylation, IκBα can be recognized and degraded by
the proteasomes (28). As a
result, NF-κB is activated by release from the cytoplasmic
NF-κB/IκBα complex and translocation into the nucleus, thereby
activating the expression of target genes, such as TNF-α and IL-1
(28). As presented in Fig. 4, upon treatment with TNF-α, the
levels of p-IκBα and nuclear p65 significantly increased compared
with controls, while p65 levels significantly decreased in the
cytoplasm. However, Ror2-knockdown significantly inhibited the
TNF-α-induced increase of p-IκBα and recovered p65 expression. In
the canonical NF-κB pathway, the release of the p65/p50 complex
represents the transcription activation of NF-κB pathway (29). These results revealed that
knockdown of Ror2 could prevent activation of NF-κB.
 | Figure 4.Ror2-knockdown inhibits the
activation of NF-κB induced by TNF-α. (A) Representative immunoblot
analysis for p-IκBα, nuclear p65, cytoplasmic p65 and lamin B in
HUVECs. (B) Relative protein expression of p-IκBα, nuclear p65,
cytoplasmic p65 and lamin B in different groups. n=3. ***P<0.001
vs. control; ##P<0.05 and ###P<0.001
vs. TNF-α. TNF-α, tumor necrosis factor-α; shRNA, short hairpin
RNA; NC, negative control; p-, phosphorylated; Ror2, receptor
tyrosine kinase-like orphan receptors 2; HUVEC, human umbilical
vein endothelial cells. |
Knockdown of Ror2 reduces
TNF-α-induced HUVEC apoptosis
To observe the effect of Ror2-knockdown on cell
apoptosis induced by TNF-α, flow cytometry and western blotting
were performed. As presented in Fig.
5, TNF-α resulted in a significant increase in the apoptosis
rate of HUVECs compared with controls, while cells transfected with
shRNA-Ror2 exhibited a lower apoptosis rate compared with the TNF-α
group. Compared with controls, following TNF-α stimulation, the
protein expression of Bcl-2 significantly decreased, while Bax,
cleaved caspase-3 and cleaved caspase-7 expression significantly
increased, indicating the enhancement of apoptotic activity.
Although caspase-7 was considered to be redundant compared with
caspase 3, studies have confirmed the crucial role of caspase 7 in
apoptosis and inflammation (30,31).
As a downstream effector of caspase 8/9/10/1, caspase 7 needs to be
evaluated during apoptosis. Furthermore, Ror2-knockdown also
rescued the protein expression of these apoptosis-related proteins
(Fig. 5B), suggesting the
inhibitory effect of Ror2-knockdown on TNF-α-induced HUVEC
apoptosis.
Ror2 interacts with Wnt5a and can be
regulated by Wnt5a
Finally, to investigate whether the protective
effect of Ror2 knockdown on TNF-α-induced vascular endothelial cell
injury was mediated by its upstream ligand Wnt5a, the alteration of
Wnt5a protein levels upon TNF-α stimulation was investigated. The
results demonstrated that, consistent with previous findings
(14,32), Wnt5a expression was enhanced by
TNF-α in a concentration-dependent manner (Fig. 6A), which indicated the potential
modulatory effect of Wnt5a on TNF-α-induced HUVECs. Subsequently,
IP assay confirmed the interaction of Ror2 and Wnt5a in HUVECs
under TNF-α stimulation (Fig. 6B).
To further investigate the effect of Wnt5a on Ror2 expression in
TNF-α-treated HUVEC, Wnt5a was knocked down by shRNA-Wnt5a
(Fig. 6C). ShRNA-Wnt5a-1 exerted a
superior knockdown effect and significantly reduced the protein
expression of Ror2 in HUVECs exposed to TNF-α compared with control
and shRNA-negative control groups (Fig. 6C and D). These results suggested
that Ror2 was likely to exert its functions on TNF-α-induced HUVEC
injury via binding to Wnt5a.
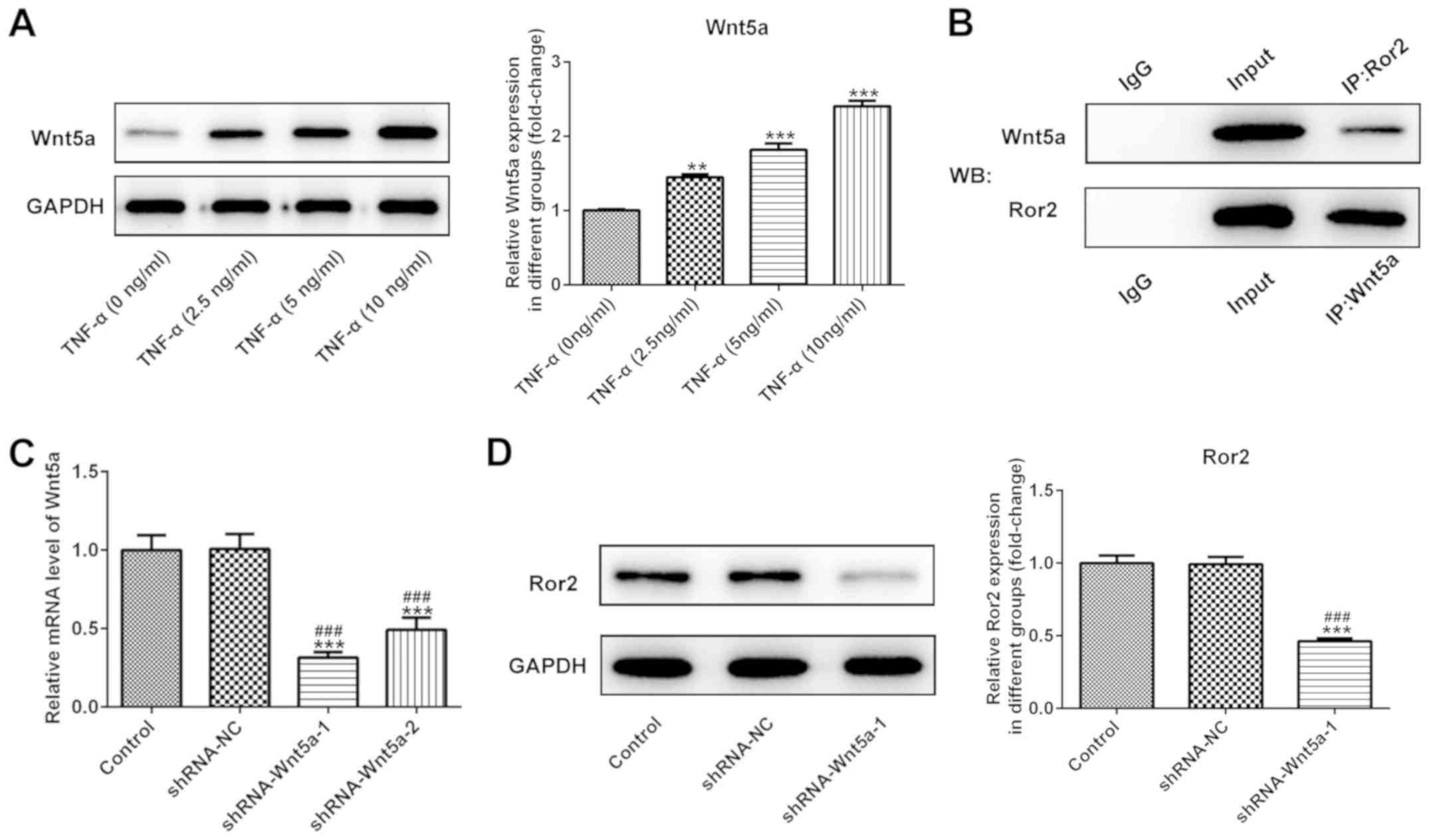 | Figure 6.Interaction of Ror2 with Wnt5a. (A)
Representative immunoblot analysis and relative protein expression
of Wnt5a following stimulation of HUVECs with different
concentrations of TNF-α. n=3. **P<0.01 and ***P<0.001 vs.
TNF-a (0 ng/ml). (B) The protein interaction of Ror2 and Wnt5a was
assessed by IP. n=3. (C) HUVECs were transfected with the indicated
vectors, and the mRNA levels of Wnt5a was detected. n=3.
***P<0.001 vs. control; ###P<0.001 vs. shRNA-NC.
(D) Alteration of Ror2 protein expression following Wnt5a
knockdown. n=3. ***P<0.001 vs. control; ###P<0.001
vs. shRNA-NC. TNF-α, tumor necrosis factor-α; shRNA, short hairpin
RNA; NC, negative control; Ror2, receptor tyrosine kinase-like
orphan receptors 2; HUVEC, human umbilical vein endothelial cells;
Wnt5a, Wnt family member 5a; IgG, immunoglobulin G; IP,
immunoprecipitation; WB, western blot. |
Discussion
Specific Wnt/receptor/co-receptor combinations are
particularly important in determining downstream signaling. As a
member of the receptor tyrosine kinase family, Ror2 can act as a
receptor or co-receptor of Wnt5a to participate in the regulation
of non-classical Wnt-Ca2+ and Wnt-PCP signaling
pathways, thus mediating cell proliferation, migration, adhesion
and location (33). To the best of
our knowledge, the present study was the first to demonstrate the
function of Ror2 in vascular endothelial cell injury and revealed
that Ror2-knockdown could suppresses TNF-α-induced inflammation and
apoptosis in vascular endothelial cells.
Ror2 has been identified to be involved in the early
formation of chondrocytes and may be required for cartilage and
growth plate development (34,35).
Following more in-depth research, Ror2 was shown to be highly
expressed in a variety of cancer tissues and correlated with
patient prognosis (36–38). Studies have reported that
Wnt5a-Ror2 signaling is involved in the regulation of inflammatory
diseases, such as atherosclerosis, and Ror2 is highly expressed in
atherosclerotic plaques and atherosclerotic patient sera, similar
to Wnt5a (20,27,39).
These findings suggested that Ror2 may regulate the development of
AS. Vascular endothelial cell injury is the initial step of AS and
directly influences the development of AS (2). In the present study, TNF-α was used
to induce vascular endothelial cell injury to stimulate AS in
vitro. It was identified that Ror2 protein expression was
upregulated by TNF-α in a concentration-dependent manner. Based on
the present and previous findings (21–23),
it has been demonstrated that Ror2 plays an important role in AS.
To verify this, Ror2 was knocked down in the current study to
observe whether the injury of HUVECs could be alleviated. In
addition, since TNF-α has been demonstrated to increase Ror2
expression, the present study aimed to uncover a therapy for
alleviating TNF-α-induced injury, instead of aggravating injury,
therefore, overexpression of Ror2 will not be covered in this
study.
Inflammatory response is a marker of vascular
endothelial cell damage (6). Upon
stimulation of inflammatory factor TNF-α, increased levels of
TNF-α, IL-1β, IL-6 and MCP-1 were released. Additionally, the
expression levels of ICAM-1 and VCAM-1 were significantly enhanced
by TNF-α stimulation. ICAM-1 and VCAM-1 are adhesion molecules that
can bind to monocytes and lymphocytes and cause adhesion reactions
(25). MCP-1 can promote the
transformation of monocytes into macrophages, leading to the
formation of foam cells (40,41).
The present results demonstrated that cells that transfected with
shRNA-Ror2 exhibited lower levels of TNF-α, IL-1β, IL-6, MCP-1,
ICAM-1 and VCAM-1 compared with normal HUVECs upon TNF-α
stimulation, indicating the inhibitory effect on inflammation and
AS progression. However, further immunofluorescence staining
experiments are needed to directly reflect the enhancement of
inflammation.
The NF-κB signaling pathway can be activated by
TNF-α, thereby activating target gene transcription and generating
cellular responses, such as immunity, inflammation and stress
(42). The present results
demonstrated that Ror2 silencing significantly inhibited the
phosphorylation of IκBα and the nuclear translocation of p65 in
TNF-α stimulated cells. Wnt5a has been implicated to regulate NF-κB
signaling (42). Ror2 silencing
may block the phosphorylation of IκBα via preventing the
transcription of downstream genes, thus affecting NF-κB signaling.
In addition, knockdown of Ror2 successfully inhibited the cell
apoptosis induced by TNF-α. These results revealed that
Ror2-knockdown could prevent activation of NF-κB signaling and
inhibit cell apoptosis.
The role of Wnt5a in AS has been extensively
studied, however the present study aimed to investigate whether the
effect of Ror2 on vascular endothelial cell injury could be
mediated by Wnt5a. The interaction between Wnt5a and Ror2 in
TNF-α-treated HUVECs was confirmed by IP assay, and the western
blotting results suggested that the protein expression of Ror2 was
decreased by Wnt5a-knockdown. However, the protein level of Ror2
was not completed abolished by Wnt5a-knockdown, implying other
ligands could mediate Ror2 expression, which needs to be
investigated in future work. In addition, whether Wnt5a-knockdown
could abolish or affect the inhibitory effect of Ror2-knockdown on
vascular endothelial cell injury during the development of AS
remains to be clarified. Our results confirmed that in the presence
of shRNA-Wnt5a, the protein expression of Ror2 in HUVECs exposed to
TNF-α was significantly reduced. In addition, the IP assay further
confirmed their interaction. These results are sufficient to
suggest that Ror2 was likely to exert its functions on
TNF-α-induced HUVEC injury via binding to Wnt5a. A recent study
(27) indicated that knockdown of
Wnt5a suppressed LPS-induced cholesterol accumulation and
inflammatory response in VSMCs. The present study illustrated that
inhibition of Wnt5a receptor-Ror2 could also prevent TNF-α
stimulated inflammation, NF-κB activation and apoptosis and
suggested the participation of other ligands in regulating
Ror2-mediated HUVEC injury. Furthermore, AS may be driven by an
innate immune response through myeloid cells. In future studies,
the decline of protective T regulatory cells in the course of AS
would be covered.
In conclusion, the present study identified
Wnt5a/Ror2 as a novel regulator in TNF-α-induced HUVECs
inflammation and apoptosis. Knockdown of Ror2 may be a potential
therapeutic approach in treating or relieving AS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HZ and XY conceived and designed the study. XY, SZ,
HY and RS acquired the data. WG, ZG and XL analysed and interpreted
the data. HZ and XY drafted the manuscript and revised it for
critically important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schaftenaar F, Frodermann V, Kuiper J and
Lutgens E: Atherosclerosis: The interplay between lipids and immune
cells. Curr Opin Lipidol. 27:2981–215. 2016. View Article : Google Scholar
|
|
2
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gisterå A and Hansson GK: The immunology
of atherosclerosis. Nat Rev Nephrol. 13:368–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolf D and Ley K: Immunity and
Inflammation in Atherosclerosis. Circ Res. 124:315–327. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial Cell Dysfunction and the Pathobiology of
Atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sitia S, Tomasoni L, Atzeni F, Ambrosio G,
Cordiano C, Catapano A, Tramontana S, Perticone F, Naccarato P,
Camici P, et al: From endothelial dysfunction to atherosclerosis.
Autoimmun Rev. 9:830–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Förstermann U, Xia N and Li H: Roles of
vascular oxidative stress and nitric oxide in the pathogenesis of
atherosclerosis. Circ Res. 120:713–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eelen G, de Zeeuw P, Simons M and
Carmeliet P: Endothelial cell metabolism in normal and diseased
vasculature. Circ Res. 116:1231–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cochain C and Zernecke A: Macrophages in
vascular inflammation and atherosclerosis. Pflugers Arch.
469:485–499. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matthijs Blankesteijn W and Hermans KC:
Wnt signaling in atherosclerosis. Eur J Pharmacol. 763((Pt A)):
122–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krishna SM, Seto SW, Jose RJ, Li J, Morton
SK, Biros E, Wang Y, Nsengiyumva V, Lindeman JH, Loots GG, et al:
Wnt signaling pathway inhibitor sclerostin inhibits angiotensin
II-induced aortic aneurysm and atherosclerosis. Arterioscler Thromb
Vasc Biol. 37:553–566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Christman MA II, Goetz DJ, Dickerson E,
McCall KD, Lewis CJ, Benencia F, Silver MJ, Kohn LD and Malgor R:
Wnt5a is expressed in murine and human atherosclerotic lesions. Am
J Physiol Heart Circ Physiol. 294:H2864–H2870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malgor R, Bhatt PM, Connolly BA, Jacoby
DL, Feldmann KJ, Silver MJ, Nakazawa M, McCall KD and Goetz DJ:
Wnt5a, TLR2 and TLR4 are elevated in advanced human atherosclerotic
lesions. Inflamm Res. 63:277–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhatt PM and Malgor R: Wnt5a: A player in
the pathogenesis of atherosclerosis and other inflammatory
disorders. Atherosclerosis. 237:155–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou XL, Zhang CJ, Peng YN, Wang Y, Xu HJ
and Liu CM: ROR2 modulates neuropathic pain via phosphorylation of
NMDA receptor subunit GluN2B in rats. Br J Anaesth. 123:e239–e248.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu J, Chen L, Cui B, Widhopf GF II, Shen
Z, Wu R, Zhang L, Zhang S, Briggs SP and Kipps TJ: Wnt5a induces
ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and
proliferation. J Clin Invest. 126:585–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arabzadeh S, Hossein G, Salehi-Dulabi Z
and Zarnani AH: WNT5A-ROR2 is induced by inflammatory mediators and
is involved in the migration of human ovarian cancer cell line
SKOV-3. Cell Mol Biol Lett. 21:92016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato A, Kayama H, Shojima K, Matsumoto S,
Koyama H, Minami Y, Nojima S, Morii E, Honda H, Takeda K, et al:
The Wnt5a-Ror2 axis promotes the signaling circuit between
interleukin-12 and interferon-γ in colitis. Sci Rep. 5:105362015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ackers I, Szymanski C, Duckett KJ, Consitt
LA, Silver MJ and Malgor R: Blocking Wnt5a signaling decreases CD36
expression and foam cell formation in atherosclerosis. Cardiovasc
Pathol. 34:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan W, Yu H, Huang S and Zhu P:
Resveratrol Protects against TNF-α-Induced Injury in Human
Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced
Repression of NF-KB and p38 MAPK. PLoS One. 11:e01470342016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Sun J, Gu L and Gao X: Protective
effect of CTRP6 on cerebral ischemia/reperfusion injury by
attenuating inflammation, oxidative stress and apoptosis in PC12
cells. Mol Med Rep. 22:344–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim DH, Lee SM, Lee YJ, Yoon JJ, Tan R, Yu
YC, Kang DG and Lee HS: Effect of Paeotang on tumor necrosis factor
α-induced vascular inflammation in human umbilical vein endothelial
cells. Chin J Integr Med. doi.10.1007/s11655-017-2759-3.
|
|
26
|
Choe JY, Park KY, Lee SJ, Park SH and Kim
SK: Rebamipide inhibits tumor necrosis factor-α-induced
interleukin-8 expression by suppressing the NF-κB signal pathway in
human umbilical vein endothelial cells. Inflamm Res. 59:1019–1026.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang CJ, Zhu N, Liu Z, Shi Z, Long J, Zu
XY, Tang ZW, Hu ZY, Liao DF and Qin L: Wnt5a/Ror2 pathway
contributes to the regulation of cholesterol homeostasis and
inflammatory response in atherosclerosis. Biochim Biophys Acta Mol
Cell Biol Lipids. 1865:1585472020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen XJ, Zhang WN, Chen B, Xi WD, Lu Y,
Huang JY, Wang YY, Long J, Wu SF, Zhang YX, et al:
Homoharringtonine deregulates MYC transcriptional expression by
directly binding NF-κB repressing factor. Proc Natl Acad Sci USA.
116:2220–2225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lamkanfi M and Kanneganti TD: Caspase-7: A
protease involved in apoptosis and inflammation. Int J Biochem Cell
Biol. 42:21–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhatt PM, Lewis CJ, House DL, Keller CM,
Kohn LD, Silver MJ, McCall KD, Goetz DJ and Malgor R: Increased
Wnt5a mRNA expression in advanced atherosclerotic lesions, and
oxidized LDL treated human monocyte-derived macrophages. Open Circ
Vasc J. 5:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Flores-Hernández E, Velázquez DM,
Castañeda-Patlán MC, Fuentes-García G, Fonseca-Camarillo G,
Yamamoto-Furusho JK, Romero-Avila MT, García-Sáinz JA and
Robles-Flores M: Canonical and non-canonical Wnt signaling are
simultaneously activated by Wnts in colon cancer cells. Cell
Signal. 72:1096362020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takeuchi S, Takeda K, Oishi I, Nomi M,
Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, et al: Mouse
Ror2 receptor tyrosine kinase is required for the heart development
and limb formation. Genes Cells. 5:71–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Witte F, Chan D, Economides AN, Mundlos S
and Stricker S: Receptor tyrosine kinase-like orphan receptor 2
(ROR2) and Indian hedgehog regulate digit outgrowth mediated by the
phalanx-forming region. Proc Natl Acad Sci USA. 107:14211–14216.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dai B, Yan T and Zhang A: ROR2 receptor
promotes the migration of osteosarcoma cells in response to Wnt5a.
Cancer Cell Int. 17:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Henry CE, Llamosas E, Daniels B, Coopes A,
Tang K and Ford CE: ROR1 and ROR2 play distinct and opposing roles
in endometrial cancer. Gynecol Oncol. 148:576–584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Debebe Z and Rathmell WK: Ror2 as a
therapeutic target in cancer. Pharmacol Ther. 150:143–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takahashi D, Suzuki H, Kakei Y, Yamakoshi
K, Minami Y, Komori T and Nishita M: Expression of Ror2 associated
with fibrosis of the submandibular gland. Cell Struct Funct.
42:159–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Murphy JM, Jeong K, Rodriguez YAR, Kim JH,
Ahn EE and Lim SS: FAK and Pyk2 activity promote TNF-α and
IL-1β-mediated pro-inflammatory gene expression and vascular
inflammation. Sci Rep. 9:76172019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu JB, Jia L, Li BR, Lan LZ, Ge Q, Zhen
HT and Deng HC: Adiponectin suppresses inflammatory responses at
the early phase of atherosclerosis in hyperglycemic rats. Mol Med
Rep. 3:323–328. 2010.PubMed/NCBI
|
|
42
|
Bergenfelz C, Medrek C, Ekström E,
Jirström K, Janols H, Wullt M, Bredberg A and Leandersson K: Wnt5a
induces a tolerogenic phenotype of macrophages in sepsis and breast
cancer patients. J Immunol. 188:5448–5458. 2012. View Article : Google Scholar : PubMed/NCBI
|