Introduction
The placenta is a vital organ that mediates
maternal-fetal exchange of nutrients and waste (1). Placental development is accompanied
by extensive angiogenesis, particularly in the labyrinth, the key
site of fetomaternal interaction that contains both fetal and
maternal blood vessels (2). In
mice, the labyrinthine vasculature elongates and expands to meet
the needs of the growing fetus from embryonic day (E)14.5 until the
end of gestation (3,4). The initial stages of angiogenesis
result in leaky, poorly perfused and easily ruptured vessels
(5). The developing network is
repeatedly pruned and remodeled via the fusion and regression of
existing vessels, until an extensive and highly-branched vascular
tree consisting of blood vessels of different diameters and
functions is established (6,7).
Additionally, capillary extension occurs, cell junctions are
tightened and vascular perfusion becomes regulated (8,9).
Progranulin (PGRN) is a multifunctional growth
factor predominantly expressed by endothelial cells (10). It serves a critical role in
inflammatory processes (11,12)
and angiogenesis (13). High
levels of PGRN have been observed at the sites of wounds and the
placenta, where angiogenesis occurs (10,14,15).
In human breast cancer cells, PGRN is associated with the promotion
of cell proliferation and invasion (16). Furthermore, it is involved in and
mediates angiogenesis by directly stimulating vascular endothelial
growth factor expression (17). An
elevated expression of the PGRN gene has been detected in
uterine glandular epithelial cells (18). It has also been detected in the
original mesenchyme on the proliferating layer around the glands
(18). The extensive expression of
PGRN is detected in villous trophoblast cells, especially
syncytiotrophoblast cells (19).
PGRN can stimulate the cell proliferation of BeWo cells, indicating
a growth-stimulating effect during the development of the placenta
(19). Furthermore, PGRN is a key
factor in blastocyst hatching, adhesion and outgrowth (20). The process of mouse blastocyst
formation is delayed when PGRN expression is inhibited (21). Previous studies have suggested that
dysregulated PGRN expression in human placentas is associated with
pregnancy-related diseases, such as pre-eclampsia (PE) and fetal
growth restriction (FGR) (22,23).
Insufficient trophoblast invasion, abnormal uterine spiral artery
remodeling and placental angiogenesis disorders are identified in
the pathophysiology of PE (24,25).
Proteomics studies have revealed an increasing trend of PGRN
expression levels in maternal plasma from the first to the third
trimester during pregnancy, but after delivery, it decreases to
pre-pregnancy levels (26). This
fluctuation may be associated with placental secretion (27). However, although PGRN has been
linked to blastocyst formation in reproductive development and PE
pathology in several studies (17,20–23),
its specific role in the development of placental blood vessels,
particularly in the placental labyrinth, remains to be
elucidated.
In the present study, PGRN gene knockout mice
(PGRN−/−) were used to examine the role of PGRN
in placental blood vessel development. The observed indices
included placental size, structure, vascularization biomarkers,
such as the vascular smooth muscle cell marker α smooth muscle
actin (SMA) (28), the vascular
endothelial cell marker platelet endothelial cell adhesion
molecule, also known as CD31 (29), and endothelial nitric oxide
synthase (eNOS) (30), as well as
pup bodyweights.
Materials and methods
Mouse models
The animal experiments were approved by the
Biomedical Ethics Committee of Chongqing Medical University and the
Animal Care Committee of Chongqing Medical University (approval no.
2016-41; Chongqing, China). A total of 64 mice were used (ratio of
male to female, ~0.23; weight, 19–24 g). The
PGRN−/− mice (C57BL/6 background) were purchased
from the Jackson Laboratory for Genomic Medicine by Dr Ju Cao at
Chongqing Medical University (12,31).
Sex- and age-matched C57BL/6 mice (C57) were purchased from the
Laboratory Animal Center of Chongqing Medical University
(Chongqing, China) and used as controls. Mice were kept in specific
pathogen-free facilities in a 12-h light/dark cycle with free
access to sterilized food and water. The indoor temperature was
20–26°C (daily temperature difference ≤4°C) and the relative
humidity was 40–70%. The present study was conducted when the mice
were 8–12 weeks old. After 4 days of acclimation, female mice in
estrous were mated with males of the same strain (1–2 females:1
male; PGRN−/− mated with
PGRN−/−; C57 mated with C57). The females were
inspected the following morning. The day a copulatory plug was
found was designated as E0.5. The female mice were sacrificed
humanely under anesthesia on E6.5, E14.5 and E17.5. The placentas
and embryos were removed, weighed and stored at −80°C or embedded
in wax, according to the protocols described subsequently. It was
ensured that animals were treated with kindness and with as little
pain as possible. After the materials were collected, the mice were
sacrificed by anesthesia with 1% pentobarbital sodium (30–45 mg/kg)
and asphyxiation with 20–30% cage volume/min CO2 until
the concentration of CO2 reached 99%. Euthanasia was
confirmed by the observations that the mouse had no heartbeat, had
stopped breathing and the pupil of the animal was dilated.
Histology and H&E staining
Placentas were dissected, fixed with 4%
paraformaldehyde overnight at 4°C and then washed with PBS. For the
paraffin block preparation, washed tissues were serially dehydrated
with 75, 85, 95, 100 and 100% ethanol, soaked in xylene and then
embedded in preheated soft wax at 60°C and preheated paraffin at
80°C, respectively. Paraffin sections (5-µm thick) were de-waxed in
xylene, rehydrated with 100, 100, 95, 85 and 75% ethanol series,
and stained with H&E at room temperature. The sections were
first stained with undiluted hematoxylin for 3–8 min, washed with
tap water and then differentiated with 1% hydrochloric acid alcohol
for 5–10 sec, washed with tap water and then returned the nucleus
from red or pink to blue with 0.6% ammonia water and then rinsed
with running water. Following nuclear staining, the sections were
placed in eosin staining solution for 1–3 min for cytoplasmic
staining. The central sections of the placentas were analyzed for
all experiments. A total of 3 wild-type (WT) and 3
PGRN−/− placentas were examined at each of the
collection points (E6.5, E14.5 and E17.5, respectively). Sections
were observed using a Olympus IX81 upright light microscope, and
images were catured with Olympus CellSens Standard Software (both
Olympus Corporation) and analyzed via ImageJ software v 1.8.0
(National Institutes of Health).
Immunohistochemistry and
immunofluorescence
Paraffin sections (5-µm thick) were de-waxed with
xylene and rehydrated with 100, 100, 95, 85 and 75% ethanol series,
then microwaved in 92–98°C for 15 min for antigen retrieval.
Following cooling, the sections were washed with PBS and blocked
with 10% H2O2 for 10 min at room temperature,
followed by 5% goat serum (cat. no. SP-9001/SP-9002; SPlink
Detection kits, Biotin-Streptavidin HRP Detection Systems; OriGene
Technologies, Inc.) for blocking at 37°C for 30 min. The sections
were then incubated with PGRN (cat. no. ab187070; dilution, 1:200),
CD31 (cat. no. ab9498; dilution, 1:500), eNOS (cat. no. ab76198;
dilution, 1:500; all Abcam) antibodies, in 1% BSA (cat. no. ST023;
Beyotime Institute of Biotechnology) overnight at 4°C.
Subsequently, the sections were kept at room temperature for 30
min, washed with PBS and incubated with biotin-conjugated
homologous secondary antibodies, namely non-diluted biotinylated
goat anti-rabbit or anti-mouse IgG polymer working solution (cat.
no. SP-9001/SP-9002; SPlink Detection kits, Biotin-Streptavidin HRP
Detection Systems; OriGene Technologies, Inc.) at 37°C for 30 min.
Following washing with PBS three times, the sections were incubated
with horseradish peroxidase-conjugated streptavidin for 30 min at
37°C. Finally, the sections were washed three times with PBS and
subjected to diaminobenzidine staining for the immunohistochemical
tests of PGRN, CD31 and eNOS at room temperature. Images were
captured using an Olympus IX81 upright light microscope, and
analyzed via Olympus CellSens Standard Software (both Olympus
Corporation).
In the SMA immunofluorescence assay, the steps
before incubation with the primary antibody were the same as for
immunohistochemistry and the subsequent immunofluorescence tests
were performed in the dark. The sections were first washed three
times with PBS following overnight primary antibody incubation in
4°C by anti-α smooth muscle actin (SMA; 1:200; cat. no. ab7817;
Abcam) and were then incubated with the FITC-labeled goat
anti-mouse secondary antibody (1:200; cat. no. IF-0051; Beijing
Dingguo Changsheng Biotechnology Co., Ltd.) for 1 h at 37°C. The
sections were then washed three times with PBS, followed by a 2-min
incubation with DAPI (1 µg/µl in 1% BSA in PBS) at room
temperature. Coverslips were mounted with the washed sections and
anti-fluorescence quenching sealant was applied. Images were
acquired using an Olympus IX81 upright fluorescence microscope and
analyzed via Olympus CellSens Standard Software (Olympus
Corporation).
Western blot analysis
A total of three mice from each group were used in
this experiment. Mice placentas were homogenized and sonicated in
99% RIPA lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) and 1% phenylmethanesulfonyl fluoride (cat. no. PMSF
ST506; Beyotime Institute of Biotechnology) on ice and then
centrifuged at 15,777 × g for 15 min at 4°C. Samples remained on
ice and the protein concentrations were determined using a BCA
protein assay kit (cat. no. P0010S; Beyotime Institute of
Biotechnology). Next, 2 µg/µl tissue sample, loading buffer (cat.
no. 1610747; Bio-Rad Laboratories, Inc.) and lysis buffer (99% RIPA
lysis buffer + 1% PMSF) were mixed in proportion and boiled for 10
min, followed by the addition of 10 µl dithiothreitol. Proteins
were loaded (20 µg per lane) separated using 10 and 7% SDS-PAGE and
transferred onto a nitrocellulose membrane. The membrane was
blocked with 5% skimmed milk/PBS for 1 h at room temperature,
followed by incubation with the corresponding primary antibodies
overnight at 4°C. Following three washes with PBS, the membranes
were incubated with a specific horseradish peroxidase
(HRP)-conjugated goat anti-rabbit/mouse IgG secondary antibody
(1:5,000; cat. no. SH-0031/0011; Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) for 1 h at room temperature, washed with
PBS three times and then incubated with Immobilon Western
Horseradish Peroxidase (Merck KGaA). Western blots/arrays were
visualized at room temperature using enhanced chemiluminescence
western blotting reagents (cat. no. RPN2109; Cytiva) and a Bio-Rad
ChemiDoc MP Imaging system. Images were analyzed via Image Lab v4.0
(Bio-Rad Laboratories, Inc.). The primary antibodies used were:
PGRN (cat. no. ab187070; dilution, 1:200), CD31 (1:500; cat. no.
ab9498), SMA (cat. no. ab7817; dilution, 1:200) and eNOS (cat. no.
ab76198; dilution, 1:1,000; all from Abcam). Mouse monoclonal
β-actin (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) was
used as an internal control.
Histomorphometric measurements
For histomorphometric measurements, three sections
per placenta taken at 100-µm intervals from the central region near
the site of umbilical cord attachment were analyzed. The areas and
diameters were measured using ImageJ software v1.8.0 (National
Institutes of Health).
Statistical analysis
Data are presented as the mean of ≥6 independent
repeats. All data are presented as mean ± SEM. Statistical
significance was calculated using an unpaired Student's t-test or
Welch's t-test. Weight data were analyzed using a Mann-Whitney U
test. GraphPad Prism v5.0 (GraphPad Software, Inc.) was used for
all statistical analysis including the Kaplan-Meier plot of
survival analysis using the log-rank (Mantel-Cox) and
Gehan-Breslow-Wilcoxon tests. *P<0.05 was considered to indicate
a statistically significant difference.
Results
Lower PGRN expression in
PGRN−/− placentas
Immunoh istochemical analysis of mouse embryos at
E6.5 (after implantation was completed) revealed that PGRN was
present around the embryos (Fig. 1Ab
and d) in WT mice. However, its expression was almost
undetectable in PGRN−/− mice (Fig. 1Aa and c). Immunohistochemistry
revealed high levels of PGRN on E14.5 in the WT mice compared with
in the PGRN−/− mice, particularly in the maternal
decidua and junctional zone, and partly in the labyrinthine layer,
where the process of decidual transformation and fetal
neovascularization is accompanied by extensive angiogenesis
(32–35) (Fig.
1B). On E17.5, PGRN was most easily observed in the WT maternal
decidua, followed by the labyrinthine layer (Fig. 1Cb, d and f), while it was less
expressed in the PGRN−/− maternal decidua and
labyrinthine layer (Fig. 1Ca, c and
e). Additionally, western blot analysis demonstrated that PGRN
expression was significantly lower in PGRN−/−
placentas on E14.5 and E17.5 (Fig.
1D) when compared with the WT placentas.
 | Figure 1.PGRN in developing embryos on E6.5
and placentas on E14.5 and E17.5. Immunohistochemistry was
performed on sections of embryos on (A) E6.5, and placental tissues
on (B) E14.5 and (C) E17.5. (A) Boxed areas in (a) and (b) are
magnified in (c) and (d), respectively. (B and C) Boxed areas in
(a) are magnified in (c) and (e). Boxed areas in (b) are magnified
in (d) and (f). (D) Western blot analysis of placental tissue on
E14.5 and E17.5. Scale bar, 500 µm; magnified scale bar, 50 µm.
**P<0.01. PGRN, progranulin; E, embryonic day; -/-,
PGRN−/− mice; +/+, WT mice; WT, wild-type; EM, embryo;
MD, maternal decidua; JZ, junctional zone; LB, labyrinthine layer;
ACTB, β-actin. |
Smaller placentas in
PGRN−/− mice
The embryos of PGRN−/− and WT C57
mice were visible, as were the decidual cells around them (Fig. 2A). PGRN−/−
placentas exhibited normal structure of the three compartments
(maternal decidua, junctional zone and labyrinthine layer).
However, the total area of the PGRN−/− placentas
was significantly smaller compared with that of the WT placentas,
and the area of the labyrinth zone was reduced on E14.5 (Fig. 2Ba and b, and 2C) and E17.5
(Fig. 2Be and f, and 2D). The
labyrinth appeared to be more compact, with more clusters of
densely packed trophoblast cells in the PGRN−/−
placenta on E14.5 and E17.5 (Fig. 2Bc
and g), compared with the WT placenta (Fig. 2Bd and h). The fetal blood vessel
spaces were decreased in the PGRN−/− mice
compared with the WT placenta, and the embryonic erythrocytes,
which were less frequently detected, appeared to be squeezed into
the narrowed vessels (Fig. 2Bc and
g). Furthermore, the weight of the PGRN−/−
placentas was significantly lower compared with that of the WT
placentas on E14.5 and E17.5 (Fig. 2E
and F).
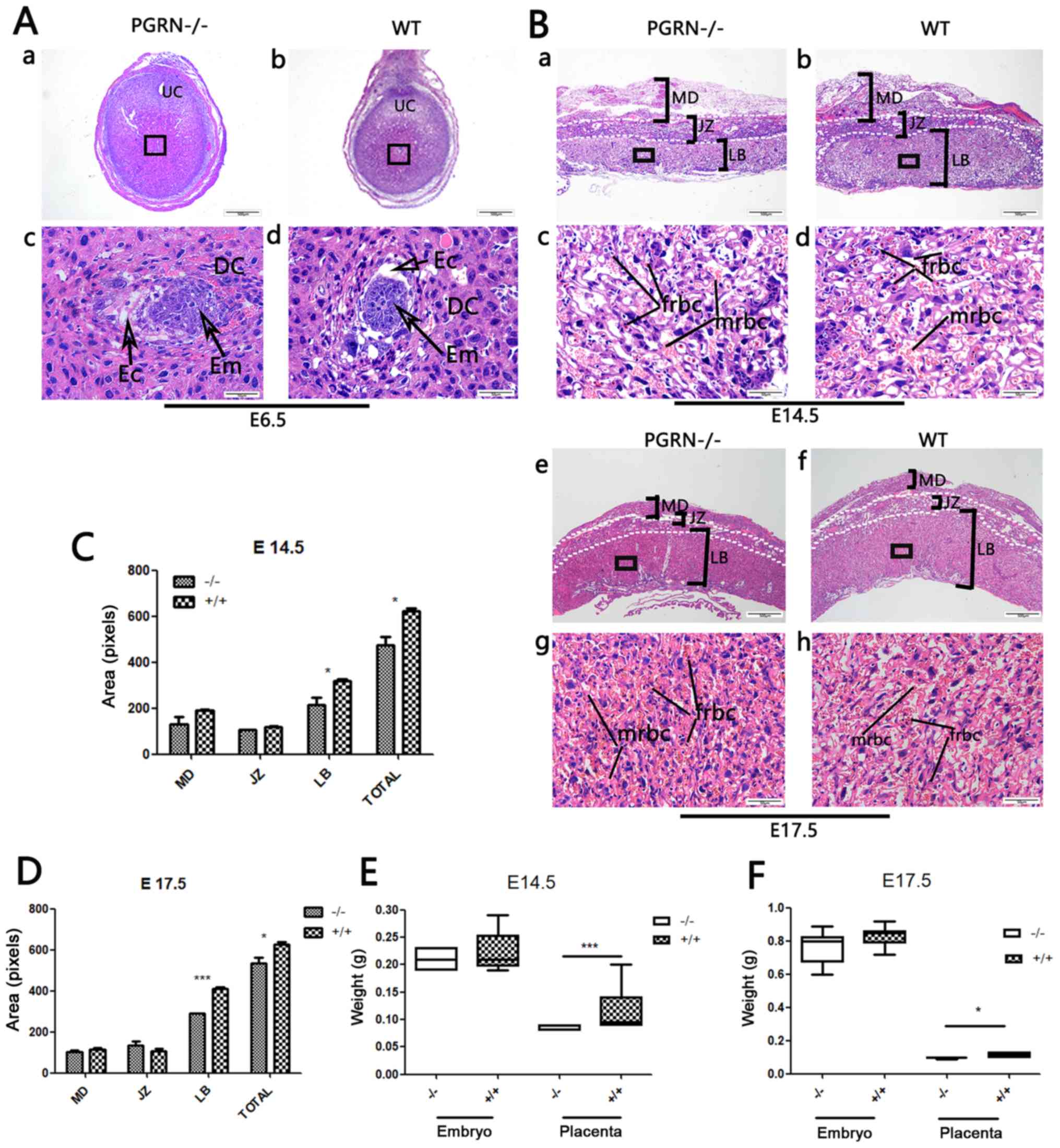 | Figure 2.Changes in PGRN−/− mouse
placentas and embryos. (A) H&E staining of (a and c)
PGRN−/− sections and (b and d) WT (C57) embryos on E6.5.
(c and d) Higher magnifications of the boxed areas in (a) and (b)
show the embryos. (B) H&E staining of PGRN−/−
sections and WT (C57) placentas on (a-d) E14.5 and (e-h) E17.5.
Higher magnifications of the boxed areas in (a), (b), (e) and (f)
show the structure of the labyrinth and the red blood cells of
PGRN−/− and WT placentas on the maternal and fetal sides
on (c and d) E14.5 and (g and h) E17.5. (C) Morphometrical analysis
of sections in (Ba and b) E14.5. (D) Morphometrical analysis of
sections in (Be and f) E17.5. (E) Weights of placentas and embryos
from WT and PGRN−/− mice on E14.5 were analyzed. (F)
Weights of placentas and embryos from WT and PGRN−/−
mice on E17.5 were analyzed. Scale bar, 500 µm; magnified scale
bar, 50 µm. *P<0.05, ***P<0.001. PGRN, progranulin; H&E,
hematoxylin and eosin; -/-, PGRN−/− mice; +/+, WT mice;
WT, wild-type; E, embryonic day; Em, embryo; MD, maternal decidua;
JZ, junctional zone; LB, labyrinthine layer; frbc, fetal red blood
cells; mrbc, maternal red blood cells. |
Abnormal placental vascularization in
PGRN−/− mice
To further investigate the altered placental
vascularization, western blot analysis and immunohistochemistry of
CD31 expression were conducted. CD31 staining was observed in the
vascular endothelial cells of WT mice, most of which resided in the
labyrinth zone, while the rest was expressed in the maternal
decidua on E14.5 (Fig. 3Ab) and
E17.5 (Fig. 3Bb). A markedly lower
amount of CD31 was observed in the labyrinthine layer of the
PGRN−/− placentas on E14.5 (Fig. 3Aa and c). In addition, the blood
vessels in the WT placental labyrinth aligned in a polar manner
from the maternal to the fetal side, while there were fewer vessels
in the PGRN−/− placental labyrinth, which were
arrayed in a disorderly manner (Fig.
3Ac and d, and 3Bc and d). On
E14.5, the labyrinthine layer of the PGRN−/−
placentas exhibited a higher density and a considerably more
disordered trophoblast cell distribution (Fig. 3Ac). Additionally, a
higher-magnification examination of the maternal decidua revealed
that the vascular endothelium was more compacted and blood vessel
expansion appeared to be restricted, which led to a smaller vessel
lumen in the PGRN−/− placenta (Fig. 3Ae) compared with the WT placenta
(Fig. 3Af). Smaller terminal
sinusoids were observed in the PGRN−/− placental
labyrinth, compared with the WT placental labyrinth, accompanied by
inordinate trophoblast cells on E14.5 (Fig. 3Ac and d). During the development of
the normal placenta, as the chorioallantoic interface underwent
more extensive branching, the trophoblast-lined sinusoid spaces
became progressively smaller (Fig.
3Bd). However, the sinusoid spaces of the labyrinth in the
PGRN−/− placentas on E17.5 were much larger
compared with those in the WT placentas (Fig. 3Bc). CD31 was expressed at higher
levels on E17.5 than on E14.5, in the PGRN−/−
(Fig. 3Ba and c) and WT groups
(Fig. 3Bb and d), especially in
the labyrinthine layer. The difference in the staining density of
CD31 between the two groups was more apparent on E17.5 than on
E14.5. Western blot analysis revealed that CD31 expression in WT
placentas was significantly higher compared with that in the
PGRN−/− placentas on E14.5 and E17.5 (Fig. 3C).
 | Figure 3.CD31 expression in developing
placentas from PGRN−/− and WT mice on E14.5 and E17.5.
Immunohistochemical detection of CD31 in the serial placental
sections on (A) E14.5 and (B) E17.5. (A and B) Boxed areas in (a)
are magnified in panels (c) and (e). Boxed areas in (b) are
magnified in panels (d) and (f). (C) Western blot analysis and
quantification of the results in placentas on E14.5 and E17.5.
Scale bar, 500 µm; magnified scale bar, 50 µm. *P<0.05. CD31,
cluster of differentiation 31; PGRN, progranulin; -/-,
PGRN−/− mice; WT, wild-type; E, embryonic day; MD,
maternal decidua; JZ, junctional zone; LB, labyrinthine layer; MBS,
maternal blood sinus; FBV, fetal blood vessel; VEC, vascular
endothelial cells; mrbc, maternal red blood cells; ACTB,
β-actin. |
To gain a more detailed understanding, placental SMA
was stained using immunofluorescence to examine the smooth muscles
of the blood vessels. The SMA staining results demonstrated that
the number of fetal red blood cells was lower in the
PGRN−/− placenta labyrinthine layer (Fig. 4A and C) compared with the WT
placentas (Fig. 4B and D). A
higher-magnification examination on E14.5 revealed a smaller
vascular diameter, as well as less vascular distribution and fetal
red blood cells in the PGRN−/− placentas
(Fig. 4A). In WT placentas, the
fetal blood vessels were elongated and radiated uniformly from the
chorionic plate to the labyrinthine layer (Fig. 4B). On E17.5, extensive branching of
the fetal capillaries was observed in the WT placentas. In
accordance with the observations on E14.5, the vascular smooth
muscles of the WT placentas appeared elongated in long, straight
segments and ran parallel to each other toward the decidua
(Fig. 4D). By contrast, the
vascular smooth muscles in the PGRN−/− placentas
were randomly aligned, abnormally branched and irregular in
diameter (Fig. 4C). Furthermore,
it was observed that the fetal blood vessels were dilated, the
smooth muscle cells were separated from the trophoblast and
branching of the fetal capillaries was defective in the
PGRN−/− placentas (Fig. 4C). Western blot analysis
demonstrated that the WT placentas exhibited significantly higher
protein expression levels of SMA compared with the
PGRN−/− placentas on both E14.5 and E17.5
(Fig. 4E and F).
 | Figure 4.SMA expression in developing
placentas of PGRN−/− and WT mice on E14.5 and E17.5.
Immunofluorescence evaluation of SMA in (A) the placentas of
PGRN−/− mice on E14.5, (B) the placentas of WT mice on
E14.5, (C) the placentas of PGRN−/− mice on E17.5 and
(D) the placentas of WT mice on E17.5. Western blot analysis was
performed on placental tissue on (E) E14.5 and (F) E17.5. Scale
bar, 50 µm. *P<0.05. SMA, smooth muscle actin; -/-,
PGRN−/− mice; PGRN, progranulin; WT, wild-type; E,
embryonic day; TGC, trophoblast giant cells; fbv, fetal blood
vessels; mrbc, maternal red blood cells; frbc, fetal red blood
cells; ACTB, β-actin. |
Decreased expression of vasodilation
factors in PGRN−/− placentas
eNOS was detected in the labyrinthine layer of
PGRN−/− and WT mice on both E14.5 and E17.5
(Fig. 5Aa and b, and Ba and b).
However, it was only observed in the junctional zone on E14.5, but
not on E17.5 (Fig. 5Aa and b, and Ba
and b). The expression levels of eNOS in the labyrinthine layer
were much higher in WT mice compared with in
PGRN−/− mice on E14.5 and E17.5 (Fig. 5Ac and d, and Bc and d). Western blot analysis
demonstrated that eNOS expression in the WT group was higher than
that in the PGRN−/− group on E14.5 and E17.5
(Fig. 5C).
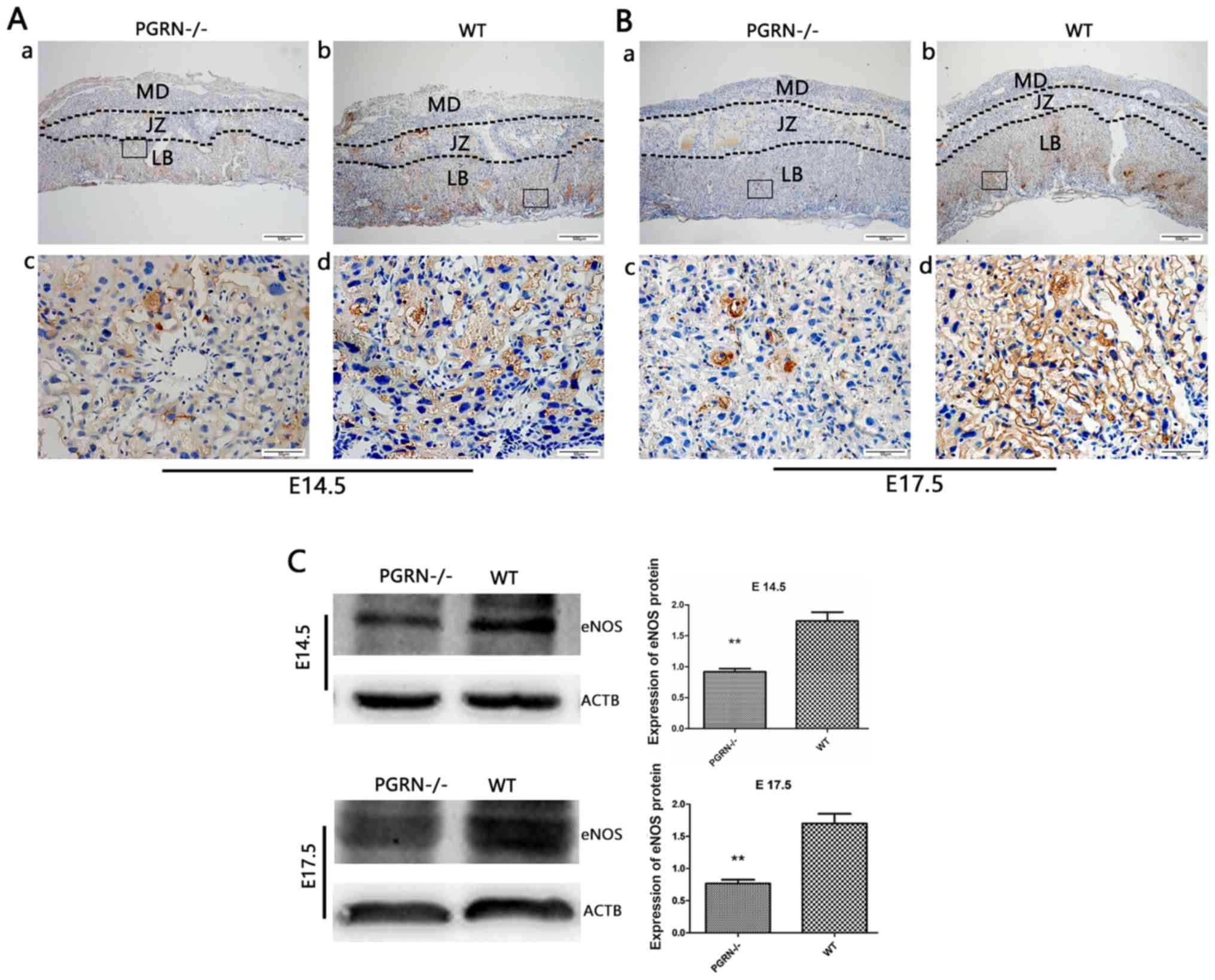 | Figure 5.eNOS expression in the developing
placenta is lower in PGRN−/− than in WT placentas on
both E14.5 and E17.5, particularly in the labyrinthine layer of the
placenta. Immunohistochemistry was performed on serial placental
sections from (A) E14.5 and (B) E17.5 to determine eNOS expression.
(A and B) Boxed areas in (a) are magnified in (c). Boxed areas in
(b) are magnified in (d). (C) Western blotting and relative gray
analysis was performed on placentas on E14.5 and E17.5. Scale bar,
500 µm; magnified scale bar, 50 µm. **P<0.01. eNOS, endothelial
nitric oxide synthase; -/-, PGRN−/− mice; PGRN,
progranulin; WT, wild-type; E, embryonic day; MD, maternal decidua;
JZ, junctional zone; LB, labyrinthine layer; ACTB, β-actin. |
Growth retardation in newborn
PGRN−/− pups
PGRN−/− mice were viable and
fertile (Fig. 6A). The number of
pups produced was not affected by the parental genotype (Fig. 6A). No significant difference was
observed between the weights of the embryos of the two groups on
E14.5 and E17.5 (Fig. 2E and F).
However, the body weights of the newborn pups of the
PGRN−/- mice were significantly lower compared
with the WT pups (Fig. 6B).
Furthermore, survival analysis demonstrated that the mortality of
PGRN−/− pups was significantly higher than that
of WT controls (Fig. 6C).
Discussion
Previous studies have demonstrated that PGRN serves
a role in vascular development (36,37).
Furthermore, studies have suggested that PGRN expression in the
human placenta is closely associated with certain pregnancy-related
diseases, such as PE and fetal FGR (22,23).
The pathogenesis of PE and FGR is complex and cannot be explained
by a single change, including the lack of trophoblast invasion and
abnormal placental angiogenesis, as well as for several other
reasons such as persistent placental hypoxia and the release of
numerous mediators into the maternal circulation (24,25).
The significant increase in serum PGRN levels in women with PE
(22) suggests that the incidence
of PE is uncertain, due to insufficient PGRN, which may be due to
other reasons that lead to insufficient placental angiogenesis in
PE and FGR. In order to promote angiogenesis, PGRN in the body is
elevated but the angiogenesis outcome of PE and FGR cannot be
completely rectified. The specific association and underlying
mechanism require further research. In the present study, a
PGRN knockout mouse model was used to determine whether PGRN
served a role in the development of placental vasculature,
particularly in the labyrinth.
Abnormal PGRN expression in endothelial cells
influences angiogenesis in vivo (38). Studies have reported high levels of
PGRN expression in the placenta of minks and mice (14,39).
In adults, rapidly renewed epithelial cells, such as corneal or
intestinal epithelial cells in deep crypts, express PGRN
genes at high levels, while most epithelial cells in the resting
state of mitosis, such as the epithelial cells of the lungs or
renal tubules, have relatively low expression levels (18). Additionally, the findings of the
present study demonstrated PGRN expression in the placentas of WT
mice, which reflected the expression and distribution of PGRN in
mouse placentas under normal conditions. It has been reported that
PGRN can increase the capillary size and number when added to
rodent dermal wounds (10).
Another study reported that PGRN influences vasculogenesis by
regulating vascular permeability in the brain (40). The data of the present study
revealed that the knockout of the PGRN gene in a mouse model
resulted in placental blood vessels of a smaller diameter and
reduced distribution. A previous study demonstrated that PGRN
affects angiogenic behavior in vitro and in vivo
(41). Other studies examined the
effect of PGRN in isolated superior rat mesenteric artery rings and
revealed that it is involved in the regulation of vascular tone by
regulating eNOS and smooth muscle (36,42).
Another study suggested that eNOS contributes to changes in uterine
placental blood vessels and increases in uterine artery blood flow
during pregnancy (30).
Furthermore, PGRN reportedly upregulates the Akt/eNOS
phosphorylation level in human umbilical vein endothelial cells
(37,43). The present study demonstrated that
the expression of eNOS and SMA in PGRN−/−
placentas was lower compared with that in WT placentas. In the
experimental results of the present study, it could be observed
under a high magnification that PGRN−/− genotype
maternal decidual vascular endothelial cells were denser, blood
vessel walls were thicker and the placental vascular lumen diameter
was reduced compared with WT placental cells. These phenomena
suggested that, when PGRN is knocked out, the defective
expression of eNOS in the mouse placenta may cause vascular
remodeling disorders. Therefore, the present findings, combined
with those of previous studies, demonstrated that PGRN regulates
placental angiogenesis.
The maternal-fetal interface is mainly made up of
the maternal decidua, the junctional zone and the labyrinthine
layer, which undergo extensive angiogenesis during pregnancy
(35). The labyrinthine layer is a
widely vascularized tissue and abnormal vascular formation can lead
to aberrant placental development (32,35).
Previous studies have demonstrated that follicle stimulating
hormone receptor, TEK receptor tyrosine kinase, vascular cell
adhesion molecule-1, ESX homeobox 1 (Esx1), connexin 45,
platelet-derived growth factor B/β-receptor and aryl hydrocarbon
receptor nuclear translocator (ARNT) are important genes during
vascular development, whose knockout results in a defective
labyrinth (44–49). Additional studies have reported
genes, such as the translocon-associated protein subunit γ, Esx1,
ARNT and retinoid X receptors, to be associated with normal
labyrinth development, and knocking out any one of them can lead to
a narrowed labyrinth, abnormal placental development, fetal growth
retardation or even fetal death (39,46,49,50).
Additionally, a narrowed labyrinth and distributed dysplastic fetal
capillaries in PGRN−/− mice were observed in the
present study. These findings suggested that insufficient PGRN
leads to abnormal blood vessel development and can result in a
defective labyrinth and insufficient placental development. When
comparing the placenta weights of PGRN−/− and WT
mice on E14.5 and E17.5, the placenta weight of
PGRN−/− mice was significantly lower compared
with that of WT normal mouse placentas. This result demonstrated
that the absence of PGRN expression in mice can delay placental
development. The difference in chorionic artery branching between
the normal and FGR placentas is caused by the different placenta
sizes (51). FGR placentas show
microvascular degeneration and extreme vascular deficiencies in the
surrounding area, and these features are likely to limit the
placental ability to meet fetal nutritional needs in late pregnancy
(51). The data from the present
study revealed that the weight of the PGRN−/−
placentas and newborn pups, and the survival rate of
PGRN−/− pups, were significantly lower compared
with those of mice in the WT group. Therefore, insufficient PGRN
may affect pup weight and survival rate as a result of the
disordered labyrinth and abnormal placental function.
In the present study, it was revealed that knocking
out PGRN led to a smaller diameter and less distribution of
the blood vessels in the placenta and affected the body weight of
the pups following their birth. This result suggested that the
bodyweight of the pups was not directly affected by the
underdeveloped placenta. However, the PGRN−/− pup
body weight increased at a significantly slower rate compared with
WT pups, which might be due to two possible reasons. First, that
the underdeveloped placenta hindered the normal development of the
pups (39,46,49,50)
and second, that the PGRN−/- gene prevented the
pups from normal postnatal growth due to problems associated with
angiogenesis in key organs (40,52,53).
Therefore, to what extent the underdeveloped placenta and the
PGRN−/− gene that the pups carry affect the
growth of the pups requires further investigation.
In conclusion, the present study demonstrated that
the absence of PGRN may lead to aberrant angiogenesis in the
placental labyrinth, further disrupting the structure of the
nutrient-waste exchange system, which may give rise to adverse
pregnancy outcomes. Further analysis of PGRN knockout mice
should be conducted to provide insights into the molecular
mechanisms regulating the vasculature of the placenta. The results
of the present study expanded the list of known growth factors
regulating placental angiogenesis, by demonstrating that placental
PGRN is essential for appropriate angiogenesis of the placenta,
particularly in the labyrinth.
Acknowledgements
The authors would like to thank Dr Ju Cao at
Chongqing Medical University, China, for kindly donating
PGRN−/− mice (C57BL/6 background).
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81971406, 81771607,
81871185, 81961128004 and 81701477), The 111 Project [grant no.
Yuwaizhuan (2016)32], The National Key Research and Development
Program of Reproductive Health & Major Birth Defects Control
and Prevention (grant no. 2016YFC1000407), Chongqing Health
Commission (grant nos. 2017ZDXM008 and 2018ZDXM024) and Chongqing
Science & Technology Commission (grant nos. cstc2017jcyjBX0060
and cstc2018jcyjAX0359).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BX and XC designed and performed the experiments,
conducted the statistical analysis of the data and drafted the
manuscript. TL, HZ, YD and CC participated in the conception and
experimental design of the project, provided the materials needed
for the experiment and helped write the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Experimental Animal Ethics Committee of Chongqing Medical
University (approval no. 2016-41).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sibley CP, Brownbill P, Glazier JD and
Greenwood SL: Knowledge needed about the exchange physiology of the
placenta. Placenta. 64 (Suppl 1):3482–S15. 2018. View Article : Google Scholar
|
|
2
|
Muntener M and Hsu YC: Development of
trophoblast and placenta of the mouse. A reinvestigation with
regard to the in vitro culture of mouse trophoblast and placenta.
Acta Anat (Basel). 98:241–252. 1977.PubMed/NCBI
|
|
3
|
Adamson SL, Lu Y, Whiteley KJ, Holmyard D,
Hemberger M, Pfarrer C and Cross JC: Interactions between
trophoblast cells and the maternal and fetal circulation in the
mouse placenta. Dev Biol. 250:358–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malassine A, Frendo JL and Evain-Brion D:
A comparison of placental development and endocrine functions
between the human and mouse model. Hum Reprod Update. 9:531–539.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carmeliet P and Collen D: Role of vascular
endothelial growth factor and vascular endothelial growth factor
receptors in vascular development. Curr Top Microbiol Immunol.
237:133–158. 1999.PubMed/NCBI
|
|
7
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carmeliet P and Conway EM: Growing better
blood vessels. Nat Biotechnol. 19:1019–1020. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leach L, Lammiman MJ, Babawale MO, Hobson
SA, Bromilou B, Lovat S and Simmonds MJ: Molecular organization of
tight and adherens junctions in the human placental vascular tree.
Placenta. 21:547–557. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Z, Ong CH, Halper J and Bateman A:
Progranulin is a mediator of the wound response. Nat Med.
9:225–229. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jian J, Konopka J and Liu C: Insights into
the role of progranulin in immunity, infection, and inflammation. J
Leukoc Biol. 93:199–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin F, Banerjee R, Thomas B, Zhou P, Qian
L, Jia T, Ma X, Ma Y, Iadecola C, Beal MF, et al: Exaggerated
inflammation, impaired host defense, and neuropathology in
progranulin-deficient mice. J Exp Med. 207:117–128. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eguchi R, Nakano T and Wakabayashi I:
Progranulin and granulin-like protein as novel VEGF-independent
angiogenic factors derived from human mesothelioma cells. Oncogene.
36:714–722. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Desmarais JA, Cao M, Bateman A and Murphy
BD: Spatiotemporal expression pattern of progranulin in embryo
implantation and placenta formation suggests a role in cell
proliferation, remodeling, and angiogenesis. Reproduction.
136:247–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Winther H, Leiser R, Pfarrer C and Dantzer
V: Localization of micro- and intermediate filaments in
non-pregnant uterus and placenta of the mink suggests involvement
of maternal endothelial cells and periendothelial cells in blood
flow regulation. Anat Embryol (Berl). 200:253–263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tangkeangsirisin W and Serrero G: PC
cell-derived growth factor (PCDGF/GP88, progranulin) stimulates
migration, invasiveness and VEGF expression in breast cancer cells.
Carcinogenesis. 25:1587–1592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tangkeangsirisin W, Hayashi J and Serrero
G: PC cell-derived growth factor mediates tamoxifen resistance and
promotes tumor growth of human breast cancer cells. Cancer Res.
64:1737–1743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daniel R, He Z, Carmichael KP, Halper J
and Bateman A: Cellular localization of gene expression for
progranulin. J Histochem Cytochem. 48:999–1009. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stubert J, Richter DU, Gerber B and Briese
V: Expression pattern of progranulin in the human placenta and its
effect on cell proliferation in the choriocarcinoma cell line BeWo.
J Reprod Dev. 57:229–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin J, Diaz-Cueto L, Schwarze JE,
Takahashi Y, Imai M, Isuzugawa K, Yamamoto S, Chang KT, Gerton GL
and Imakawa K: Effects of progranulin on blastocyst hatching and
subsequent adhesion and outgrowth in the mouse. Biol Reprod.
73:434–442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Díaz-Cueto L, Stein P, Jacobs A, Schultz
RM and Gerton GL: Modulation of mouse preimplantation embryo
development by acrogranin (epithelin/granulin precursor). Dev Biol.
217:406–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Açıkgöz AS, Tüten A, Öncül M, Eskalen S,
Dinçgez BC, Şimşek A, Yüksel MA and Guralp O: Evaluation of
maternal serum progranulin levels in normotensive pregnancies, and
pregnancies with early- and late-onset preeclampsia. J Matern Fetal
Neonatal Med. 29:2658–2664. 2016.PubMed/NCBI
|
|
23
|
Stubert J, Schattenberg F, Richter DU,
Dieterich M and Briese V: Trophoblastic progranulin expression is
upregulated in cases of fetal growth restriction and preeclampsia.
J Perinat Med. 40:475–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kadyrov M, Kingdom JC and Huppertz B:
Divergent trophoblast invasion and apoptosis in placental bed
spiral arteries from pregnancies complicated by maternal anemia and
early-onset preeclampsia/intrauterine growth restriction. Am J
Obstet Gynecol. 194:557–563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tal R: The role of hypoxia and
hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol
Reprod. 87:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aghaeepour N, Lehallier B, Baca Q, Ganio
EA, Wong RJ, Ghaemi MS, Culos A, El-Sayed YY, Blumenfeld YJ, Druzin
ML, et al: A proteomic clock of human pregnancy. Am J Obstet
Gynecol. 218:347.e1–347.e14. 2018. View Article : Google Scholar
|
|
27
|
Stubert J, Szewczyk M, Spitschak A, Knoll
S, Richter DU and Pützer BM: Adenoviral mediated expression of
anti-inflammatory progranulin by placental explants modulates
endothelial cell activation by decrease of ICAM-1 expression.
Placenta. 90:109–117. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Honma M, Koizumi F, Wakaki K and Ochiai H:
Co-expression of fibroblastic, histiocytic and smooth muscle cell
phenotypes on cultured adherent cells derived from human palatine
tonsils: A morphological and immunocytochemical study. Pathol Int.
45:903–913. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jackson DE, Gully LM, Henshall TL, Mardell
CE and Macardle PJ: Platelet endothelial cell adhesion molecule-1
(PECAM-1/CD31) is associated with a naive B-cell phenotype in human
tonsils. Tissue Antigens. 56:105–116. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kulandavelu S, Whiteley KJ, Qu D, Mu J,
Bainbridge SA and Adamson SL: Endothelial nitric oxide synthase
deficiency reduces uterine blood flow, spiral artery elongation,
and placental oxygenation in pregnant mice. Hypertension.
60:231–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhixin S, Xuemei Z, Liping Z, Xu F, Tao X,
Zhang H, Lin X, Kang L, Xiang Y, Lai X, et al: Progranulin plays a
central role in host defense during sepsis by promoting macrophage
recruitment. Am J Respir Crit Care Med. 194:1219–1232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Portilho NA and Pelajo-Machado M:
Mechanism of hematopoiesis and vasculogenesis in mouse placenta.
Placenta. 69:140–145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daniel R, Daniels E, He Z and Bateman A:
Progranulin (acrogranin/PC cell-derived growth
factor/granulin-epithelin precursor) is expressed in the placenta,
epidermis, microvasculature, and brain during murine development.
Dev Dyn. 227:593–599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Enders AC: A comparative study of the fine
structure of the trophoblast in several hemochorial placentas. Am J
Anat. 116:29–67. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walentin K, Hinze C and Schmidt-Ott KM:
The basal chorionic trophoblast cell layer: An emerging coordinator
of placenta development. Bioessays. 38:254–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beltowski J: Role of progranulin in the
regulation of vascular tone: (patho)physiological implications.
Acta Physiol (Oxf). 219:706–708. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hwang HJ, Jung TW, Hong HC, Choi HY, Seo
JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH and Yoo HJ:
Progranulin protects vascular endothelium against atherosclerotic
inflammatory reaction via Akt/eNOS and nuclear factor-κB pathways.
PLoS One. 8:e766792013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Toh H, Cao M, Daniels E and Bateman A:
Expression of the growth factor progranulin in endothelial cells
influences growth and development of blood vessels: A novel mouse
model. PLoS One. 8:e649892013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamaguchi YL, Tanaka SS, Oshima N,
Kiyonari H, Asashima M and Nishinakamura R: Translocon-associated
protein subunit Trap-γ/Ssr3 is required for vascular network
formation in the mouse placenta. Dev Dyn. 240:394–403. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanazawa M, Kawamura K, Takahashi T, Miura
M, Tanaka Y, Koyama M, Toriyabe M, Igarashi H, Nakada T, Nishihara
M, et al: Multiple therapeutic effects of progranulin on
experimental acute ischaemic stroke. Brain. 138:1932–1948. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toh H, Daniels E and Bateman A: Methods to
investigate the roles of progranulin in angiogenesis using in vitro
strategies and transgenic mouse models. Methods Mol Biol.
1806:329–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kazama K, Hoshino K, Kodama T, Okada M and
Yamawaki H: Adipocytokine, progranulin, augments
acetylcholine-induced nitric oxide-mediated relaxation through the
increases of cGMP production in rat isolated mesenteric artery.
Acta Physiol (Oxf). 219:781–789. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang F, Wang H, Bao S, Zhou H, Zhang Y,
Yan Y, Lai Y, Teng W and Shan Z: Thyrotropin regulates eNOS
expression in the endothelium by PGRN through Akt pathway. Front
Endocrinol (Lausanne). 9:3532018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stilley JAW and Segaloff DL: Deletion of
fetoplacental Fshr inhibits fetal vessel angiogenesis in the mouse
placenta. Mol Cell Endocrinol. 476:79–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kwee L, Baldwin HS, Shen HM, Stewart CL,
Buck C, Buck CA and Labow MA: Defective development of the
embryonic and extraembryonic circulatory systems in vascular cell
adhesion molecule (VCAM-1) deficient mice. Development.
121:489–503. 1995.PubMed/NCBI
|
|
46
|
Li Y and Behringer RR: Esx1 is an
X-chromosome-imprinted regulator of placental development and fetal
growth. Nat Genet. 20:309–311. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Krüger O, Plum A, Kim JS, Winterhager E,
Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH and Willecke
K: Defective vascular development in connexin 45-deficient mice.
Development. 127:4179–4193. 2000.PubMed/NCBI
|
|
48
|
Ohlsson R, Falck P, Hellström M, Lindahl
P, Boström H, Franklin G, Ahrlund-Richter L, Pollard J, Soriano P
and Betsholtz C: PDGFB regulates the development of the
labyrinthine layer of the mouse fetal placenta. Dev Biol.
212:124–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kozak KR, Abbott B and Hankinson O:
ARNT-deficient mice and placental differentiation. Dev Biol.
191:297–305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wendling O, Chambon P and Mark M: Retinoid
X receptors are essential for early mouse development and
placentogenesis. Proc Natl Acad Sci USA. 96:547–551. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Junaid TO, Brownbill P, Chalmers N,
Johnstone ED and Aplin JD: Fetoplacental vascular alterations
associated with fetal growth restriction. Placenta. 35:808–815.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jackman K, Kahles T, Lane D,
Garcia-Bonilla L, Abe T, Capone C, Hochrainer K, Voss H, Zhou P,
Ding A, et al: Progranulin deficiency promotes post-ischemic
blood-brain barrier disruption. J Neurosci. 33:19579–19589. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fu Y, Sun Y, Zhou M, Wang X, Wang Z, Wei
X, Zhang Y, Su Z, Liang K, Tang W and Yi F: Therapeutic potential
of progranulin in hyperhomocysteinemia-induced cardiorenal
dysfunction. Hypertension. 69:259–266. 2017. View Article : Google Scholar : PubMed/NCBI
|




















