Introduction
Sepsis is defined as serious organ dysfunction
induced by a dysregulated host response to infection (1). Despite progress in therapeutic
strategies, sepsis remains the leading cause of mortality of
patients in intensive care units (2).
High mortality from sepsis is correlated with immune
impairment, which may be caused by T lymphocyte dysfunction
(3–5). Septic events trigger high levels of
immune cell apoptosis, including apoptosis of T lymphocytes,
resulting in suppressed immune functions, and often inducing
increased susceptibility to secondary infections (6–8).
T cell apoptosis caused by sepsis is now considered
to be associated with the programmed death ligand-1
(PD-L1)-programmed death-1 (PD-1) signaling axis, and the increase
in PD-L1 expression during sepsis is an important indicator of
immunosuppression (9).
Immunotherapy targeting the PD-1/PD-L1 signaling axis is a hot
topic in current research, and the tumor microenvironment is an
important factor affecting the effect of this immunotherapy for
numerous tumors, such as lung tumor, glioblastoma multiforme and
colon cancer (10–12). Atezolizumab is an anti-PD-L1
antibody that inhibits binding of PD-L1 to its receptor PD-1,
thereby restoring the suppression of T lymphocyte activity
(13,14), and has shown efficacy in numerous
types of cancer including locally advanced and metastatic
urothelial carcinoma, non-small-cell lung cancer and metastatic
renal cell carcinoma (15–17). For non-small-cell lung cancer,
Fehrenbacher et al (16)
conducted a multicenter, open-label, randomized controlled trial to
evaluate the effectiveness of atezolizumab and found that the
overall survival of patients was significantly longer in the
atezolizumab group than that in the docetaxel group (median overall
survival, 12.6 vs. 9.7 months). Since immunosuppression of T
lymphocytes also occurs in septic microenvironments (9,18), it
was hypothesized that atezolizumab may alleviate T lymphocyte
immunosuppression and improve prognosis during sepsis. In the
present study, an animal sepsis model in vivo and a T
lymphocyte cell line in vitro were used to evaluate the
effects of atezolizumab during sepsis.
Materials and methods
Mouse model of sepsis and drug
intervention
A total of 129 male C57BL/6 mice (age, 8–10-weeks
old; weight, 20–25 g) were purchased from the Experimental Animal
Center of Guangxi Medical University (Guangxi Zhuang Autonomous
Region, China). All mice were housed in specific pathogen-free
facilities at 25°C and 55% humidity, under a 12-h light/dark cycle
for 1 week before surgery. All mice had free access to water and
food. The sepsis model was generated using the cecal ligation and
puncture (CLP) procedure, according to a reported method (19). Briefly, mice were anesthetized by
intraperitoneal injection of 40 mg/kg pentobarbital sodium
(Sigma-Aldrich; Merck KGaA). Then, an incision was made in the
lower abdomen. The cecum was ligated in the middle, and the distal
cecum was punctured right through using a 21-gauge needle. A small
amount of stool was squeezed into the abdominal cavity, and the
abdominal incision was closed layer by layer. Mice in the control
group underwent the same procedure as the mice in the sepsis group,
with the exception of the CLP. All procedures were conducted under
sterile conditions.
Mice were randomly assigned to three groups: Sham,
CLP and atezolizumab groups. In the atezolizumab group, septic mice
were treated with intraperitoneal injection of atezolizumab (100 µg
on days 1 and 4; Shanghai TheraMabs Biotechnology Co., Ltd.). Mice
in the other groups were treated with intraperitoneal injection of
an isotype control antibody (100 µg on days 1 and 4; Shanghai
TheraMabs Biotechnology Co., Ltd.).
The present study was approved by the Animal Care
Committee of Guangxi Medical University (approval no. 201901006),
and all experiments were conducted in accordance with the
Laboratory Animal Guideline for Ethical Review of Animal Welfare
issued by the National Standard GB/T35892-2018 of the People's
Republic of China. Blood was collected by cardiac puncture from
mice anesthetized by pentobarbital sodium intraperitoneal injection
in this study. Mice were euthanized following blood collection, or
when they met criteria for humane endpoints, including loss of
crawling ability and loss of eating ability after surgery. Mice
were euthanized with CO2 followed by cervical
dislocation, and a flow rate of 30% displacement of chamber volume
per minute of CO2 was used for euthanasia. The
confirmation criteria for mouse death was the arrest of breathing
and heartbeat.
Detection of PD-L1 in blood and bone
marrow neutrophils by flow cytometry
Blood and bone marrow were harvested from the hearts
and thighs of mice at 24, 48, 72 and 96 h after surgery. Mice were
euthanized before collection of bone marrow. Red blood cells in
whole blood cells were lysed using Red Blood Cell Lysis Buffer
(Beijing Solarbio Science & Technology Co., Ltd.). Cells were
stained with allophycocyanin (APC)-anti-Ly6G and PE-anti-PD-L1
antibodies (BD Pharmingen; BD Biosciences), and then quantified by
flow cytometry on a FACSCalibur instrument (BD Biosciences),
according to the manufacturer's instructions. PD-L1+
neutrophils were defined as the Ly6G+PD-L1+
population, and data was analyzed using FlowJo software (version
10; FlowJo, LLC).
Detection of PD-1 expression in T
lymphocytes in blood by flow cytometry
Blood samples were harvested from the hearts of mice
at 72 h post-surgery, ~1 ml blood was collected from each mouse.
Red blood cells in whole blood cells were lysed using Red Blood
Cell Lysis Buffer (Beijing Solarbio Science & Technology Co.,
Ltd.). Cells were stained with APC-anti-CD3 (eBioscience; Thermo
Fisher Scientific, Inc.) and PE-anti-PD-1 (eBioscience; Thermo
Fisher Scientific, Inc.) antibodies, and then quantified by flow
cytometry on a FACSCalibur instrument (BD Biosciences) according to
the manufacturer's instructions. Data was analyzed as
aforementioned.
Detection of T lymphocyte early/late
apoptosis in vivo by flow cytometry
Blood was harvested from the hearts of mice at 72 h
after surgery. Red blood cells were lysed using Red Blood Cell
Lysis Buffer (Beijing Solarbio Science & Technology Co., Ltd.).
Cells were stained with APC-anti-CD3 (eBioscience; Thermo Fisher
Scientific, Inc.) antibody, FITC-Annexin V and PI (MultiSciences
Biotechnology Co., Ltd.). T lymphocyte apoptosis was detected by
flow cytometry as aforementioned, according to the manufacturer's
instructions. CD3+ Annexin V+ cells were
defined as apoptotic T lymphocytes in blood. Data was analyzed as
aforementioned.
Detection of blood endotoxin and
intestinal permeability
Serum was collected from mice in the sham, CLP and
atezolizumab groups at 24, 48 and 72 h after surgery using
pyrogen-free tubes. Endotoxin levels were measured using an
End-point Chromogenic Tachypleus amebocyte Lysate kit
(Xiamen Bioendo Technology Co., Ltd.) according to the
manufacturer's instructions, and calculated from a standard curve
by reading the absorbance at 405 nm using an ELISA instrument
(BioTek Instruments, Inc.). All experiments were performed in
triplicate.
Intestinal permeability was measured at 24, 48 and
72 h after surgery using FITC-dextran (FD40S; Sigma-Aldrich; Merck
KGaA), according to a reported method (20). FD40S (0.5 ml, 22 mg/ml) was
administered to mice by gavage, 5 h before sacrifice. Blood was
harvested from the mice hearts at the time of sacrifice. The
extracted plasma was diluted using the same volume of PBS, and then
the concentration of FD40S was measured using a fluorescence
spectrophotometer (Shimadzu Corporation), with excitation and
emission wavelengths of 470 and 515 nm, respectively. A standard
curve was drawn using measurements from serial samples at different
concentrations. All experiments were performed in triplicate.
Histological examination of the ileum
by hematoxylin and eosin (H&E) staining
At 72 h after modeling, distal ileum specimens (~1
cm) were collected, fixed in 4% paraformaldehyde (Wuhan Boster
Biological Technology, Ltd.) for 24 h at room temperature and
embedded in paraffin. Then, 5-µm thick sections were cut and
stained with H&E. The sections were stained with hematoxylin
for 5 min and then dehydrated with 70 and 90% ethanol, followed by
staining with eosin for 3 min; Images were obtained using a
microscope (Olympus BX53; Olympus Corporation). Histopathology was
assessed according to the Chiu grading system, and
histopathological scores (0-5 scale) were calculated following
observation by light microscopy at ×200 magnification. The Chiu
scoring standard of the ileal mucosa is divided into six levels
according to the severity of injury: i) 0, the intestinal mucosal
epithelium is normal without injury; ii) 1, the intestinal mucosal
subepithelial space is widened and the capillaries have hyperemia;
iii) 2, the intestinal mucosal subepithelial space is further
extended and elevated, and separation of the epithelium and lamina
propria occurs; iv) 3, part of the villous epithelium of the
intestinal mucosa falls off; v) 4, the intestinal mucosal villous
epithelium completely falls off, and capillary hemorrhages can be
observed; and vi) 5, the intestinal mucosal lamina propria
disintegrates, and mucosal bleeding ulcer occurs (21,22).
Expression of tight junction protein
in the ileum is detected by immunohistochemistry
At 72 h after modeling, a section of the ileum was
cut under anesthesia with intraperitoneal injection of 40 mg/kg
pentobarbital sodium (Sigma-Aldrich; Merck KGaA). The procedure for
obtaining pathological slices was the same as that described above.
Next, the slices were blocked with 5% goat serum (Beijing Solarbio
Science & Technology Co., Ltd.) for 15 min at room temperature,
then incubated overnight at 4°C with anti-claudin-1 (1:100; cat.
no. Ab15098; Abcam), anti-occludin (1:100; cat. no. 168986; Abcam)
and anti-zonula occludens-1 (ZO-1; 1:100; cat. no. 21773-1-AP;
ProteinTech Group, Inc.) antibodies. After a wash with PBS, tissue
was incubated with horseradish peroxidase-labeled goat anti-rabbit
IgG secondary antibody (1:100; cat. no. abs20002ss; Absin, Inc.) at
37°C for 30 min, according to the manufacturer's instructions.
Images were obtained using a microscope (Olympus BX53; Olympus
Corporation; magnification, ×200). The expression of these proteins
was calculated using ImageJ software (version 1.8.0; National
Institutes of Health).
Purification of neutrophils and
co-culture with a T lymphocyte cell line
Neutrophils were purified from blood samples from
the CLP or sham groups 72 h after surgery using a Neutrophil
Extraction kit (Beijing Solarbio Science & Technology Co.,
Ltd.), according to the manufacturer's instructions.
The CTLL-2 mouse T lymphocyte cell line (Fenghbio
Co., Ltd.) was used for in vitro experiments. T lymphocytes
were cultured in RPMI-1640 supplemented with 10% fetal bovine
serum, 100 U/ml penicillin, 100 ng/ml streptomycin and 100 U/ml
recombinant human IL-2 (all Beijing Solarbio Science &
Technology Co., Ltd.) at 37°C under a 5% CO2
atmosphere.
Purified neutrophils and CTLL-2 cells were
co-cultured in 6-well culture plates at a 1:1 ratio
(1×106 cells/ml) under the culture conditions described
above. Samples from the atezolizumab group were treated with 10
µg/ml atezolizumab overnight at 37°C, while those in the other two
groups were treated with 10 µg/ml isotype control antibody
overnight at 37°C as a control.
Detection of T lymphocyte early/late
apoptosis by flow cytometry after co-culture
After co-culture of T lymphocyte CTLL-2 cells with
neutrophils isolated from septic mice for 24 h, cells were
collected for staining with APC-anti-CD3 antibody, FITC-Annexin V
and PI (MultiSciences Biotech Co., Ltd.). T lymphocyte apoptosis
was detected by flow cytometry as aforementioned and the apoptosis
rate was calculated by FlowJo software, according to the
manufacturer's instructions. Cells that stained positive for
Annexin V were considered apoptotic.
Detection of PD-1 expression in T
lymphocyte cell lines by flow cytometry after co-culture
To confirm that CTLL-2 cells expressed PD-1, the
positive cells were detected by flow cytometry. After co-culture of
CTLL-2 cells with neutrophils isolated from septic mice for 24 h,
cells were collected for staining with PE-anti-PD-1 antibody
(eBioscience; Thermo Fisher Scientific, Inc.). PD-1 expression in
CTLL-2 cells was detected by flow cytometry and analyzed as
aforementioned, according to the manufacturer's instructions.
Calculation of survival time of mice
after operation
After operation, the survival times of mice in the
CLP and atezolizumab groups were recorded. Mouse death was
determined by physical examination, and death was confirmed when
there was no respiration and heartbeat. Survival rates were
monitored continuously for 144 h, and 12 mice were studied in the
CLP and atezolizumab groups. Mice were monitored once an hour.
After 144 h of monitoring, the living mice were sacrificed under
anesthesia by intraperitoneal injection of sodium
pentobarbital.
Statistical analysis
Continuous, normally distributed variables are
presented as the mean ± standard deviation, while non-normally
distributed data are presented as the mean (minimum, maximum). A
Student's t-test was applied for comparisons between two groups. If
the data were not normally distributed between two groups, a
Mann-Whitney U test was used. Comparisons among three groups were
conducted using ANOVA followed by an LSD test. Mouse survival times
were evaluated by Kaplan-Meier analysis, and statistical
significance was determined by the log-rank test using SPSS version
17.0 (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Sepsis mouse model is successfully
established by the CLP method
After recovery from the CLP procedure, mice with
sepsis showed mental depression, erection of whole-body hair,
trembling and slow crawling. In addition, bloody or purulent
ascites, and intestinal tube swelling and expansion could be
observed in their abdominal cavity, and their distal cecum was
black and necrotic with various degrees of adhesion around it
(Fig. 1A-C).
Expression of PD-L1 in neutrophils is
increased in blood and bone marrow during sepsis
PD-L1 expression in blood and bone marrow
neutrophils was measured at various time points by flow cytometry.
In blood, compared with that of the sham group, the proportion of
PD-L1+ neutrophils in the CLP group was 4.433±0.77% (vs.
2.647±1.117%, P=0.085), 9.583±2.222% (vs. 3.867±1.22%, P=0.017),
16.3±3.716% (vs. 7.003±0.512%, P=0.013) and 12.933±1.877% (vs.
4.387±2.531%, P=0.009) at 24, 48, 72 and 96 h, respectively
(Table SI). Hence, the proportion
of PD-L1+ neutrophils was significantly higher in the
CLP group compared with that in the sham group at 48, 72 and 96 h
(P<0.05; Fig. 2A). In the bone
marrow, compared with that of the sham group, the proportion of
PD-L1+ neutrophils in the CLP group was 10.867±0.493%
(vs. 6.197±1.127%, P=0.003), 14.7±1.353% (vs. 5.2±2.05%, P=0.003),
25.367±6.037% (vs. 9.8±1.928%, P=0.013) and 17.433±2.616% (vs.
9.417±2.029%, P=0.014) at 24, 48, 72 and 96 h, respectively
(Table SII). These data
demonstrated that the proportion of PD-L1+ neutrophils
was significantly higher in the CLP group than in the sham group at
all the time points tested (P<0.05; Fig. 2B).
Atezolizumab treatment reduces
endotoxin levels and intestinal mucosal permeability in septic
mice
The blood endotoxin levels measured at 24, 48 and 72
h after surgery were 0.224±0.008, 0.241±0.128 and 0.23±0.064 EU/ml,
respectively, in the sham group; 0.496±0.102, 0.742±0.153 and
0.956±0.162 EU/ml, respectively, in the CLP group; and 0.477±0.069,
0.488±0.145 and 0.564±0.128 EU/ml, respectively, in the
atezolizumab group (Table SIII).
Compared with those in the sham group, the endotoxin levels were
significantly increased at 24, 48 and 72 h in the CLP group
(P<0.01; Fig. 3A). Furthermore,
relative to those in the CLP group, the endotoxin levels were
significantly decreased at 48 and 72 h in the atezolizumab group
(P<0.05; Fig. 3A).
The degree of intestinal permeability was indicated
by the concentration of FD40S in the blood. The concentrations of
FD40S at 24, 48 and 72 h after surgery were 1.754±0.575,
2.758±0.636 and 3.473±1.434 µg/ml, respectively, in the sham group;
8.919±2.681, 25.024±3.68 and 32.283±3.456 µg/ml, respectively, in
the CLP group; and 8.519±2.335, 13.1±4.098 and 17.981±3.211 µg/ml,
respectively, in the atezolizumab group (Table SIV). Compared with that in the sham
group, FD40S was significantly increased in the CLP group at 24, 48
and 72 h (P<0.01; Fig. 3B).
Furthermore, relative to that of the CLP group, the FD40S
concentration was significantly decreased in the atezolizumab group
at 48 and 72 h (P<0.01; Fig.
3B).
Atezolizumab treatment decreases ileum
histological scores in septic mice
The ileal mucosa of the CLP group showed increased
villous epithelial shedding, bleeding and ulcers. In the
atezolizumab group, the ileal mucosa exhibited varying degrees of
widening of the subcutaneous space and shedding of villi
epithelium, but ulcers of the mucosa were rare. Evaluation of
histopathological scores by H&E-stained distal ileum samples
indicated that the mean score in the CLP group was significantly
higher than that in the sham group (3.5±1.069 vs. 0.875±0.835,
P<0.01), while the mean score in the atezolizumab group was
significantly lower than that in the CLP group (2.25±1.035 vs.
3.5±1.069, P<0.05) (Fig. 4;
Table SV).
Atezolizumab treatment increases the
expression of tight junction proteins in the ileum during
sepsis
The expression of three tight junction proteins in
the ileum was assessed by immunohistochemistry (Fig. 5A-C; Table SVI). The expression of claudin-1
protein in the sham, CLP and atezolizumab groups was 1.03±0.061,
0.652±0.17 and 0.846±0.127, respectively. The expression of
claudin-1 protein in the CLP group was significantly lower than
that in the sham group (P<0.05), while the expression of
claudin-1 protein in the atezolizumab group was higher than that in
the CLP group (P<0.05). The expression levels of occludin
protein in the sham, CLP and atezolizumab groups were 1.02±0.125,
0.68±0.194 and 0.888±0.122, respectively. Compared with that in the
sham group, the expression of occludin protein in the CLP group was
significantly lower (P<0.05), while the expression of occludin
protein in the atezolizumab group was significantly higher than
that in the CLP group (P<0.05). The expression levels of ZO-1
protein in the sham, CLP and atezolizumab groups were 1.01±0.137,
0.794±0.124 and 0.896±0.092, respectively. The ZO-1 protein
expression in the CLP group was significantly lower than that in
the sham group (P<0.05). The ZO-1 protein expression in the
atezolizumab group was higher than that in the CLP group, but the
difference between the two groups was not statistically significant
(P>0.05).
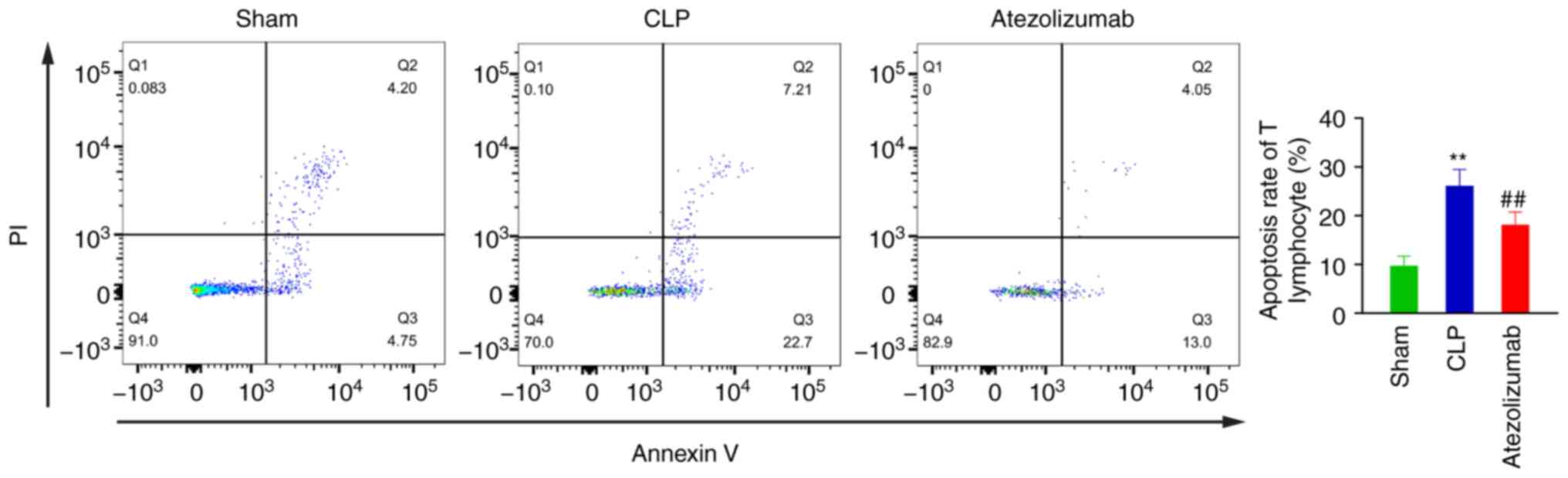
Atezolizumab treatment decreases T
lymphocyte apoptosis during sepsis in vivo and in vitro
In vivo, 72 h after surgery, the apoptosis
rates of T lymphocytes in the sham, CLP and atezolizumab groups
were 9.735±1.966, 26.158±3.342 and 18.014±7.415%, respectively.
Compared with the that in the sham group, the apoptosis rate of T
lymphocytes in the CLP group was significantly increased
(P<0.01; Fig. 6), while the
apoptosis rate of T lymphocytes in the atezolizumab group was
significantly decreased relative to that in the CLP group
(P<0.01; Fig. 6).
After co-culture with neutrophils from septic mice
for 24 h, the apoptosis rates of T lymphocytes in the sham, CLP and
atezolizumab groups were 8±1.638, 27±2.855 and 12.93±2.15%,
respectively (Table SVII).
Compared with that in the sham group, the apoptosis rate of T
lymphocytes in the CLP group was significantly increased
(P<0.01; Fig. 7), while the
apoptosis rate of T lymphocytes in the atezolizumab group was
significantly decreased relative to that in the CLP group
(P<0.01; Fig. 7).
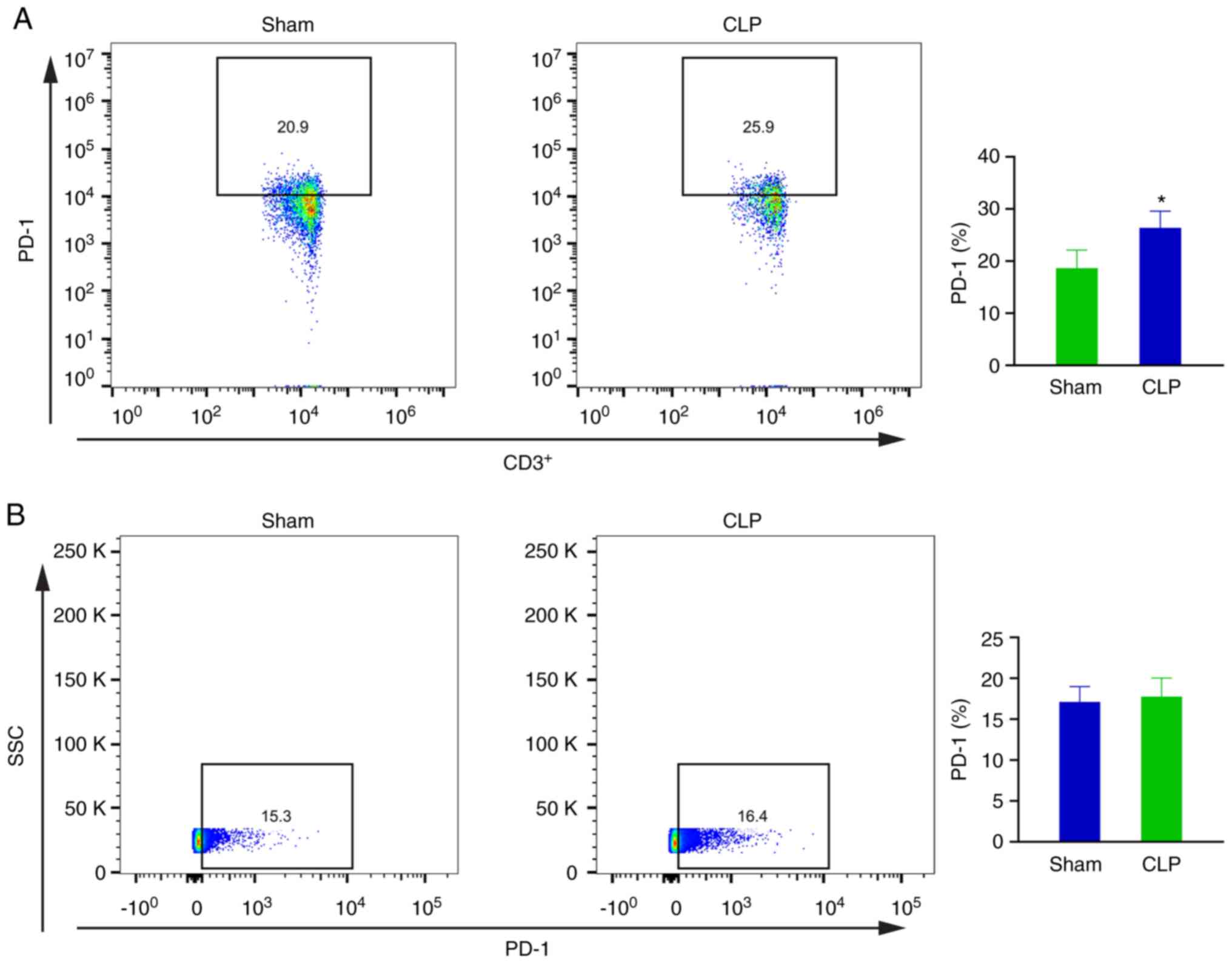
Confirmation of PD-1 expression in T
lymphocytes in vivo and T lymphocytes cell line in vitro
At 72 h after surgery, the PD-1 expression rates in
blood T lymphocytes in the sham and CLP groups were 18.65±3.483 and
26.388±3.2%, respectively. The difference between two groups was
statistically significant (P<0.05; Fig. 8A).
Following co-culture with neutrophils from septic
mice for 24 h, PD-1 was found to be expressed in ~17.105±1.878 and
17.763±2.263% of CTLL-2 cells in the sham and CLP groups,
respectively. No significant difference between these two groups
was found (P>0.05; Fig. 8B).
Atezolizumab treatment improves the
survival of septic mice
To investigate the role of atezolizumab in mouse
survival, the death rates of mice in the CLP and atezolizumab
groups were recorded (Table
SVIII). As shown in Fig. 9,
within 90 h, ~50% of mice in the CLP group had died, while the
corresponding rate was 25% for the atezolizumab group. Hence,
septic mice treated with atezolizumab clearly survived for longer
than those treated with isotype control antibody (P<0.05;
Fig. 9).
Discussion
There are currently two main mouse models for
studying sepsis at the animal level. One is induced by
lipopolysaccharide (LPS) and the other is under CLP procedure. CLP
is a method to create an animal model of polymicrobial sepsis. As
the CLP model usually induces a longer-lasting systemic
inflammatory response, it is suitable for experimental observation
of longer-term treatment (23).
Moreover, the CLP model is also superior to the LPS model in
studying physiological functions (24).
The cause of high mortality in sepsis has always
been a concern. Boomer et al (25) conducted autopsies of patients
following sepsis and found that the main cause of death was
immunosuppression rather than the excessive inflammatory response.
Lymphocyte apoptosis is now considered an important step in the
development of immunosuppression in patients with sepsis, in which
T lymphocyte apoptosis is a key factor (8,26,27).
In addition to T lymphocyte apoptosis, PD-1 and PD-L1 are also
indicators of immunosuppression (9,28,29).
The interaction of PD-L1 and PD-1, which are
expressed on the surface of T lymphocytes, results in
immunosuppression. Hence, an antibody specifically targeting PD-L1
is suitable for alleviation of the suppression of effector T
lymphocytes (30). Atezolizumab is
a monoclonal antibody targeting the immunosuppressive receptor
PD-L1, and its effectiveness has been verified in numerous
diseases, such as urothelial and renal cell carcinoma and
non-small-cell lung cancer (15–17).
Immunotherapy with anti-PD-1 or anti-PD-L1
antibodies target the PD-1-PD-L1 pathway. Anti-PD-1 antibodies are
reported to alleviate immunosuppression of T lymphocytes and
improve the survival rate of septic mice (31). Since neutrophils are the most
abundant white blood cells in the blood, the inhibitory effect of
PD-L1+_neutrophils on T lymphocytes via the PD-L1-PD-1
axis may be considerable.
In the present study, it was found that the
population of neutrophils expressing PD-L1 markedly increased
during sepsis. Septic mice treated with atezolizumab had less
endotoxin burden, reduced damage of the intestinal mucosa and
intestinal permeability, higher expression of intestinal tight
junction proteins and survived for a longer period. Finally, both
in vivo and in vitro experiments confirmed that
atezolizumab can protect T lymphocytes against the effects of
PD-L1+ positive neutrophils.
The gut is often considered to be the driver of
multiple serious diseases, including sepsis (20). The intestinal mucosal barrier is
important to guard against the bacterial and endotoxin attacks
experienced in the gut. The morphology of this barrier mainly
comprises intestinal epithelial cells and tight junction proteins
(32,33). Sepsis often leads to intestinal
epithelial cell apoptosis (34) and
tight junction protein destruction (20,35),
resulting in increased permeability (36,37).
Damage to the intestinal mucosal barrier leads to bacterial and
endotoxin translocation into circulation, which exacerbates sepsis
(38), and may even cause multiple
organ failure (39,40). Therefore, the intestinal mucosal
barrier, intestinal permeability and endotoxin levels are important
indicators reflecting the severity of sepsis.
A large number of endotoxins appear in the blood
circulation during the onset of sepsis, and endotoxin is an
important factor that disrupts the intestinal mucosal barrier
(41). The mechanism of
atezolizumab to protect the intestinal mucosal barrier may be that
it can enhance the function of T cells in the body, thereby
reducing blood circulation endotoxin, which demonstrates its
ability to protect the intestinal mucosal barrier. The detailed
underlying mechanism is not yet clear, and further research is
needed to determine it.
To date, no studies on atezolizumab for the
treatment of sepsis have been published. There are few reports of
other anti-PD-L1 antibody reagents used in the treatment of sepsis,
but often performed only in vitro or in vivo
experiments. In the present study, it was verified that treatment
with atezolizumab protected T lymphocytes during sepsis via in
vivo and in vitro experiments, and demonstrated that it
also improves the prognosis of septic mice in vivo. Zhang
et al (42) reported that
PD-L1 blockade decreased lymphocyte apoptosis in vivo, and
PD-L1 expression in blood neutrophils was significantly increased
during sepsis. It was presented in the present study that in
addition to the increase in PD-L1 expressed by blood neutrophils,
PD-L1 expressed by bone marrow neutrophils also increased during
sepsis. This suggested that PD-L1 expression may occur in multiple
organs at the same time during the onset of sepsis.
Apoptosis during sepsis is triggered by numerous
pathways, with multiple cell death stimuli involved. Hence, it is
difficult to prevent T lymphocyte apoptosis by blockade of a single
factor (18). Although multiple
factors contribute to cell death, the PD-1-PD-L1 axis is an
important pathway affecting T lymphocytes (42,43).
Immunotherapy targeting this pathway can decrease T lymphocyte
apoptosis, thereby alleviating immunosuppression during sepsis.
This type of immunotherapy is expected to become an important
method for the treatment of sepsis in the future.
However, the present study had some limitations.
First, although T lymphocyte apoptosis is an important factor in
the development of immunosuppression, other factors also contribute
to this process. Whether these factors are also affected by
atezolizumab requires further evaluation. Second, animal models of
sepsis cannot completely replicate sepsis onset in humans; hence,
the effects of atezolizumab on humans may differ from those on
mice. Future studies on atezolizumab administration in patients
with sepsis warrants consideration. In conclusion, treatment with
atezolizumab may be an effective method for the immunosuppression
induced by sepsis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant no. 81860334) and the
Natural Science Foundation of Fujian Province (grant no.
2019J01585).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC drafted the manuscript and performed the
experiments with the assistance of RC. SH and BZ performed the data
analysis. SZ designed the study and corrected the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
Committee of Guangxi Medical University (approval no.
201901006).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CLP
|
cecal ligation and puncture
|
|
PD-L1
|
programmed death ligand-1
|
|
LPS
|
lipopolysaccharide
|
|
PD-1
|
programmed death-1
|
|
FD40S
|
fluorescently labeled dextran 40S
|
|
APC
|
allophycocyanin
|
|
H&E
|
hematoxylin and eosin
|
|
ZO-1
|
zonula occludens-1
|
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Florian B: Mayr, Sachin Yende and Derek C
Angus: Epidemiology of severe sepsis. Virulence. 5:4–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patil NK, Bohannon JK, Luan L, Guo Y,
Fensterheim B, Hernandez A, Wang J and Sherwood ER: Flt3 ligand
treatment attenuates T cell dysfunction and improves survival in a
murine model of burn wound sepsis. Shock. 47:40–51. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramonell KM, Zhang W, Hadley A, Chen CW,
Fay KT, Lyons JD, Klingensmith NJ, McConnell KW, Coopersmith CM and
Ford ML: CXCR4 blockade decreases CD4+ T cell exhaustion
and improves survival in a murine model of polymicrobial sepsis.
PLoS One. 12:e01888822017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boomer JS, To K, Chang KC, Takasu O,
Osborne DF, Walton AH, Bricker TL, Jarman SD II, Kreisel D,
Krupnick AS, et al: Immunosuppression in patients who die of sepsis
and multiple organ failure. Surv Anesthesiol. 56:272–273. 2012.
View Article : Google Scholar
|
|
6
|
Cao C, Chai Y, Shou S, Wang J, Huang Y and
Ma T: Toll-like receptor 4 deficiency increases resistance in
sepsis-induced immune dysfunction. Int Immunopharmacol. 54:169–176.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oami T, Watanabe E, Hatano M, Sunahara S,
Fujimura L, Sakamoto A, Ito C, Toshimori K and Oda S: Suppression
of T cell autophagy results in decreased viability and function of
T cells through accelerated apoptosis in a murine sepsis model.
Crit Care Med. 45:e77–e85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hotchkiss RS, Osmon SB, Chang KC, Wagner
TH, Coopersmith CM and Karl IE: Accelerated lymphocyte death in
sepsis occurs by both the death receptor and mitochondrial
pathways. J Immunol. 174:5110–5118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JF, Li JB, Zhao YJ, Yi WJ, Bian JJ,
Wan XJ, Zhu KM and Deng XM: Up-regulation of programmed cell death
1 ligand 1 on neutrophils may be involved in sepsis-induced
immunosuppression: An animal study and a prospective case-control
study. Anesthesiology. 122:852–863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dougall WC, Aguilera AR and Smyth MJ: Dual
targeting of RANKL and PD-1 with a bispecific antibody improves
anti-tumor immunity. Clin Transl Immunology. 8:e010812019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oliver AJ, Davey AS, Keam SP, Mardiana S,
Chan JD, von Scheidt B, Beavis PA, House IG, Van Audernaerde JR,
Darcy PK, et al: Tissue-specific tumor microenvironments influence
responses to immunotherapies. Clin Transl Immunology. 8:e10942019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walker DG, Shakya R, Morrison B, Neller
MA, Matthews KK, Nicholls J, Smith C and Khanna R: Impact of
pre-therapy glioblastoma multiforme microenvironment on clinical
response to autologous CMV-specific T-cell therapy. Clin Transl
Immunology. 8:e010882019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balar AV, Galsky MD, Rosenberg JE, Powles
T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J,
Perez-Gracia JL, et al IMvigor210 Study Group, : Atezolizumab as
first-line treatment in cisplatin-ineligible patients with locally
advanced and metastatic urothelial carcinoma: A single-arm,
multicentre, phase 2 trial. Lancet. 389:67–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al POPLAR Study Group, :
Atezolizumab versus docetaxel for patients with previously treated
non-small-cell lung cancer (POPLAR): A multicentre, open-label,
phase 2 randomised controlled trial. Lancet. 387:1837–1846. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McDermott DF, Sosman JA, Sznol M, Massard
C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV,
et al: Atezolizumab, an anti-programmed death-ligand 1 antibody, in
metastatic renal cell carcinoma: Long-term safety, clinical
activity, and immune correlates from a phase ia study. J Clin
Oncol. 34:833–842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hotchkiss RS, Monneret G and Payen D:
Sepsis-induced immunosuppression: From cellular dysfunctions to
immunotherapy. Nat Rev Immunol. 13:862–874. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoseph BP, Klingensmith NJ, Liang Z, Breed
ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML and
Coopersmith CM: Mechanisms of intestinal barrier dysfunction in
sepsis. Shock. 46:52–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bouboulis G, Bonatsos VG, Katsarou AI,
Karameris A, Galanos A, Zacharioudaki A, Theodoropoulos G, Zografos
G, Papalois AE and Toutouzas K: Experimental hemorrhagic shock
protocol in swine models: The effects of 21-aminosteroid on the
small intestine. Curr Ther Res Clin Exp. 88:18–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. Exp Surg.
101:478–483. 1970.
|
|
23
|
Seemann S, Zohles F and Lupp A:
Comprehensive comparison of three different animal models for
systemic inflammation. J Biomed Sci. 24:602017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skirecki T, Kawiak J, Machaj E, Pojda Z,
Wasilewska D, Czubak J and Hoser G: Early severe impairment of
hematopoietic stem and progenitor cells from the bone marrow caused
by CLP sepsis and endotoxemia in a humanized mice model. Stem Cell
Res Ther. 6:1422015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boomer JS, To K, Chang KC, Takasu O,
Osborne DF, Walton AH, Bricker TL, Jarman SD II, Kreisel D,
Krupnick AS, et al: Immunosuppression in patients who die of sepsis
and multiple organ failure. JAMA. 306:2594–2605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lang JD and Matute-Bello G: Lymphocytes,
apoptosis and sepsis: Making the jump from mice to humans. Crit
Care. 13:1092009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hotchkiss RS, Swanson PE, Freeman BD,
Tinsley KW, Cobb JP, Matuschak GM, Buchman TG and Karl IE:
Apoptotic cell death in patients with sepsis, shock, and multiple
organ dysfunction. Crit Care Med. 27:1230–1251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsukamoto H, Fujieda K, Miyashita A,
Fukushima S, Iked T, Kubo Y, Senju S, Ihn H, Nishimura Y and
Oshiumi H: Combined blockade of IL6 and PD-1/PD-L1 signaling
abrogates mutual regulation of their immunosuppressive effects in
the tumor microenvironment. Cancer Res. 78:5011–5022. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng Y, Li B, Liang Y, Reeves PM, Qu X,
Ran C, Liu Q, Callahan MV, Sluder AE, Gelfand JA, et al: Dual
blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival
of ovarian tumor-bearing mice by prevention of immunosuppression in
the tumor microenvironment. FASEB J. 33:6596–6608. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Passariello M, D'Alise AM, Esposito A,
Vetrei C, Froechlich G, Scarselli E, Nicosia A and De Lorenzo C:
Novel human anti-PD-L1 mAbs inhibit immune-independent tumor cell
growth and PD-L1 associated intracellular signalling. Sci Rep.
9:131252019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang X, Venet F, Wang YL, Lepape A, Yuan
Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, et al: PD-1
expression by macrophages plays a pathologic role in altering
microbial clearance and the innate inflammatory response to sepsis.
Proc Natl Acad Sci USA. 106:6303–6308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bjarnason I, MacPherson A and Hollander D:
Intestinal permeability: An overview. Gastroenterology.
108:1566–1581. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Turner JR: Intestinal mucosal barrier
function in health and disease. Nat Rev Immunol. 9:799–809. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Perrone EE, Jung E, Breed E, Dominguez JA,
Liang Z, Clark AT, Dunne WM, Burd EM and Coopersmith CM: Mechanisms
of methicillin-resistant Staphylococcus aureus pneumonia-induced
intestinal epithelial apoptosis. Shock. 38:68–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Q, Zhang Q, Wang C, Liu X, Li N and Li
J: Disruption of tight junctions during polymicrobial sepsis in
vivo. J Pathol. 218:210–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suzuki T: Regulation of intestinal
epithelial permeability by tight junctions. Cell Mol Life Sci.
70:631–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haussner F, Chakraborty S, Halbgebauer R
and Huber-Lang M: Challenge to the intestinal mucosa during sepsis.
Front Immunol. 10:8912019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De-Souza DA and Greene LJ: Intestinal
permeability and systemic infections in critically ill patients:
Effect of glutamine. Crit Care Med. 33:1125–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schulz K, Sommer O, Jargon D, Utzolino S,
Clement HW, Strate T and von Dobschuetz E: Cytokine and radical
inhibition in septic intestinal barrier failure. J Surg Res.
193:831–840. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Piton G and Capellier G: Biomarkers of gut
barrier failure in the ICU. Curr Opin Crit Care. 22:152–160.
2016.PubMed/NCBI
|
|
41
|
Dial EJ, Romero JJ, Villa X, Mercer DW and
Lichtenberger LM: Lipopolysaccharide-induced gastrointestinal
injury in rats: Role of surface hydrophobicity and bile salts.
Shock. 17:77–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K,
Wan X, Deng X and Cai Z: PD-L1 blockade improves survival in
experimental sepsis by inhibiting lymphocyte apoptosis and
reversing monocyte dysfunction. Crit Care. 14:R2202010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brahmamdam P, Inoue S, Unsinger J, Chang
KC, McDunn JE and Hotchkiss RS: Delayed administration of anti-PD-1
antibody reverses immune dysfunction and improves survival during
sepsis. J Leukoc Biol. 88:233–240. 2010. View Article : Google Scholar : PubMed/NCBI
|