Introduction
With an aging global population and changes in
lifestyle choice, chronic kidney disease (CKD) has become a major
threat to public health worldwide (1,2). Renal
interstitial fibrosis is a common pathological outcome of CKD
(3,4), which is associated with the
destruction and disappearance of the renal tissue structure,
including the glomeruli, tubules and interstitium, accompanied by
excessive accumulation of extracellular matrix (ECM) (5,6).
Although kidney damage caused by early-stage renal interstitial
fibrosis is reversible, fibrosis during the scar-formation period
is irreversible (7). Therefore, it
is of great significance to explore the roles of relevant
biological indicators that prevent and delay the progression of
fibrosis, and to formulate therapeutic strategies and prognostic
risk assessment measures for patients with renal interstitial
fibrosis.
Hypoxia affects the function of kidney cells
(8,9). Acute hypoxia can lead to tubular
damage, causing changes in kidney function and subsequent apoptosis
(10). In addition, chronic hypoxia
also stimulates tubular cells, as well as the fibrosis of
interstitial fibroblasts and renal microvascular endothelial cells
(11). Hypoxia-inducible factor 1α
(HIF-1α) is a key factor in the regulation of intracellular oxygen
metabolism. Under normal conditions, HIF-1α is expressed at a low
level in all tissues, whereas under hypoxic conditions, HIF-1α
expression is a correlated with the increase in duration and degree
of hypoxia (12,13). HIF-1α is also closely associated
with tissue and organ fibrosis. A previous study revealed that
surgical hepatic artery ligation in rats induces liver hypoxia and
upregulates HIF-1α, causing fatty lesions and aggravating liver
fibrosis (14). Also, renal hypoxia
can directly induce the upregulation of HIF-1α expression and
promote renal interstitial fibrosis; conversely, inhibiting HIF-1α
can slow the progression of renal interstitial fibrosis (15).
In recent years, microRNAs (miRNAs/miRs) have been
reported to play an important role in the field of gene expression
regulation. A large number of studies have demonstrated that miRNAs
are involved in a number of cellular processes, including
differentiation, proliferation, apoptosis, metabolism,
hematopoiesis, cardiogenesis, body morphogenesis and insulin
secretion (16,17). Moreover, miRNAs also play an
important role in the feedback loop of signal transduction pathways
(18). Numerous studies have
revealed that miRNAs may be closely associated with kidney
physiology, tumors and transplantation, as well as renal
interstitial fibrosis (19,20), suggesting that these molecules serve
key roles in kidney pathophysiology.
Although the relationship between various miRNAs and
renal interstitial fibrosis has been reported (21,22),
the role of miR-212 in renal fibrosis remains unclear. The aim of
the present study was to investigate the association between
miR-212 expression and renal interstitial fibrosis, as well as its
effect on the expression of pro-fibrotic factors. The present study
also sought to analyze the role of miR-212 in renal interstitial
fibrosis by targeting hypoxia-inducible factor 1-α inhibitor
(HIF1AN), so as to provide a novel research direction for the
diagnosis and treatment of renal fibrosis.
Materials and methods
Peripheral blood collection
A total of 73 patients (31 males, 42 females; age
range, 32–64, mean age, 47.18±11.63 years) with renal interstitial
fibrosis who underwent treatment at the Second Affiliated Hospital
of Fujian Medical University (Quanzhou, China) were included in the
present study, along with an additional 30 healthy volunteers (17
males, 13 females, age range, 30–64, mean age 45.74±10.92 years).
The recruitment criteria for renal interstitial fibrosis were as
follows: i) Evidence from renal pathology in accordance with World
Health Organization criteria (23).
The primary diagnostic features were intimal thickening, medial
hypertrophy, reduplication of the internal elastic lamina and
glomerular sclerosis; ii) participants were between 18 and 60 years
of age; and iii) individuals had no other glomerular diseases or
systemic disorders, such as glomerulonephritis, hypertension
nephropathy or diabetic nephropathy, gout or urinary stones. The
healthy control samples were taken from individuals with normal
blood pressure and renal function. All subjects donated ~3 ml
venous peripheral blood during early morning fasting. The blood
samples were placed in anticoagulation tubes containing EDTA and
immediately mixed. The present study was approved by the Ethics
Committee of the Second Affiliated Hospital of Fujian Medical
University (Quanzhou, China) and written informed consent was
provided by patients or family members of patients and the
volunteers. All surgical procedures adhered to the ethical norms of
clinical experiments.
Cell culture
NRK49F cells (Shanghai Cell Bank of the Chinese
Academy of Sciences) were cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum, 100 µg/ml
penicillin and 100 µg/ml streptomycin (all Thermo Fisher
Scientific, Inc.) and maintained at 37°C (5% CO2) in a
humidified incubator.
Transfection
The NRK49F cells were seeded into a 6-well plate at
a density of ~5×105 cells/well. Cells were sub-cultured
to 60% confluence, and then transfected with 40 nM miR-negative
control (NC), miR-212 mimics, NC-inhibitor or miR-212 inhibitor,
HIF1AN small interfering (si)RNA, HIF1AN overexpression plasmid, or
co-transfected with miR-212 mimics and HIF1AN overexpression
plasmid, or the miR-212 inhibitor and HIF1AN siRNA (all from Santa
Cruz Biotechnology, Inc.). Transfection was performed using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. After transfection,
cells were cultured for 24 h in serum-free low-glucose medium
(Gibco; Thermo Fisher Scientific, Inc.), and then cultured in DMEM
containing Angiotensin (Ang) II (1×10−5 mol/l) for 72 h.
The sequences used were: miR-212 mimics:
5′-ACCUUG-GCUCUAGACUGCUUACUtt-3′, miR-212 inhibitor:
5′-AGUAAGCAGUCUAGAGCCAAGGUtt-3′, NC control:
5′-CUACGGCCAUUGACUUGUC-UACUtt-3′.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. cDNA was synthesized using the
RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.), qPCR was performed using the cDNA by SYBR RT-PCR
kit (Takara Bio, Inc.) according to the manufacturer's protocol.
and reaction conditions were 95°C for 30 sec, 40 cycles of 95°C for
5 sec and 60°C for 30 sec. Relative expression was calculated using
the 2−ΔΔCq method (24).
miR-212 expression was assessed relative to that of the internal
reference, U6; HIF1AN, α smooth muscle actin (α-SMA), connective
tissue growth factor (CTGF), collagen α-1(I) chain (COL1A1) and
collagen α-1(III) chain (COL3A1) expression was quantified using
GAPDH as the internal reference. The sequences of the qPCR primers
were as follows: miR-212 forward, 5′-GCCTCCTGACTCCAGGTCC-3′ and
reverse, 5′-GCGCAAAGTGACTGGATGAA-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′; HIF1AN forward,
5′-TTCCCGACTAGGCCCATTC-3′ and reverse,
5′-CAGGTATTCAAGGTCCCATTTCA-3′; α-SMA forward,
5′-CATCACGAACTGGGATGACATG-3′ and reverse,
5′-CATCTTCTCCCTGTTGGCTTTAG-3′; CTG F forward,
5′-TCCTTTCTGAGCAATTCACCAAG-3′ and reverse,
5′-GCACACTCCGTCTTTTTCCTC-3′; COL1A1 forward,
5′-GAGGGCCAAGACGAAGACATC-3′ and reverse,
5′-CAGATCACGTCATCGCACAAC-3′; COL3A1 forward,
5′-GGAGCTGGCTACTTCTCGC-3′ and reverse, 5′-GGGAACATCCTCCTTCAACAG-3′;
and GAPDH forward, 5′-TGTGGGCATCAATGGATTTGG-3′ and reverse,
5′-ACACCATGTATTCCGGGTCAAT-3′.
Western blotting and
immunohistochemistry (IHC) analysis
Total protein was extracted using RIPA lysis buffer
and the protein concentration was determined using a bicinchoninic
acid protein assay (both Thermo Fisher Scientific, Inc.). The
proteins (50 µg/well) were separated via SDS-PAGE on 12% gels, and
then subsequently transferred to polyvinylidene fluoride membranes.
The membranes were blocked with 5% non-fat dry milk powder for 1 h
at 37°C, and then incubated overnight at 4°C with the following
primary antibodies (all from Abcam): Anti-HIF1AN (1:500; cat. no.
ab237544), anti-HIF-1α (1:500; cat. no. ab216842), anti-α-SMA
(1:500; cat. no. ab5694), anti-CTGF (1:500; cat. no. ab6992),
anti-COL1A1 (1:500; cat. no. ab34710), anti-COL3A1 (1:500; cat. no.
ab7778) and anti-GAPDH (1:1,000; cat. no. ab9485). A horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (1:1,000;
cat. no. ab150077; Abcam) was then added and the membranes were
incubated at room temperature for a 1 h. The protein bands were
visualized with an ECL Substrate kit (Abcam) and analyzed using
ImageJ (version 1.45; National Institutes of Health).
HIF1AN detection was also assessed by IHC. In brief,
the frozen sections (−20°C) (5-µm thick) were exposed to fresh 3%
hydrogen peroxide for 20 min, and then washed with PBS. The
sections were incubated for 60 min at 37°C in 5% normal blocking
serum (Sigma-Aldrich; Merck KGaA), and then incubated with
anti-HIF1AN (1:500; cat. no. ab237544; Abcam) overnight at 4°C. The
slides were then incubated with a secondary antibody (1:1,000; cat.
no. ab150077; Abcam) for 60 min at room temperature, and with
3,3′-diaminobenzidine as a substrate. Images were captured using a
light microscope (5 fields of one specimen were randomly selected;
magnification, ×500; Nikon Corporation).
Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org) was used to predict the
presence of complementary binding sites between miR-212 and the 3′
untranslated region (UTR) of HIF1AN. A wild-type (WT) 3′-UTR
luciferase reporter plasmid (pMIR-HIF1AN-wt) and mutant (Mut)
reporter plasmid (pMIR-HIF1AN-Mut) were subsequently constructed.
miR-NC, miR-212 mimics, NC-inhibitor or miR-212 inhibitor, and
pMIR-HIF1AN-wt or pMIR-HIF1AN-Mut were co-transfected into NRK49F
cells (6×104 cells/well) at 37°C using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h. The reporter plasmids were obtained
from Promega Corporation. After transfection for 24 h, lysis buffer
was added and the cells were agitated at room temperature for 15
min. The lysates were collected and transferred to an Eppendorf
tube containing 100 µl LAR substrate and 20 µl passive lysis
buffer. Luciferase activity was measured using a Dual-luciferase
assay kit (Promega Corporation). The fluorescence intensity of each
sample was calculated as the ratio of firefly to Renilla
luciferase fluorescence.
Unilateral ureteral obstruction (UUO)
mouse model
A total of 24 male C57 BL/6J mice (age, 6–8 weeks;
weight, 18–22 g) were housed in a temperature-controlled
environment (18–22°C), with 50±5% humidity under a 12 h light/dark
cycle and were provided with free access to food and water. The
mice were randomly assigned to the Sham, UUO, UUO+miR-212 agomir
and UUO+miR-212 antagomir groups (n=6 each). Mice in the UUO group
were anesthetized by intraperitoneal injection of pentobarbital (50
mg/kg), and abdominal wall skin, muscle and peritoneal tissues were
incised at the middle of the abdomen. Blunt forceps were used to
separate the periureteral tissues and to expose the left ureter;
the ureter was then ligated with 4-0 thread, and the peritoneum,
muscle layer and skin were sutured layer by layer. The left ureters
of the Sham group were separated without ligation, and the
remaining methods were conducted in the same manner as those of the
UUO group. Mice in the UUO+miR-212 agomir and UUO+miR-212 antagomir
groups received an intravenous injection of 40 mg/kg miR-212 agomir
or miR-212 antagomir, respectively, every 3 days after modeling; 14
days post-surgery, the mice were all sacrificed by dislocation of
cervical vertebra, and the left kidneys were immediately
removed.
Masson staining
The kidney tissues were fixed in 4% paraformaldehyde
solution for 6–8 h at room temperature, fully automatically
dehydrated, paraffin-embedded and sectioned at 5 µm. Masson lichun
red acidic compound solution was then applied for 10 min, before
rinsing with running water and shaking until dry. Then, the samples
were differentiated for 5 min with 1% phosphomolybdate aqueous
solution. After the phosphomolybdate was removed, the samples were
directly stained with aniline blue for 5 min and rinsed with water.
Then, 95% alcohol, anhydrous alcohol and transparent xylene were
added in sequence, and neutral gum was used to seal each specimen.
All images were captured using a light microscope (magnification,
×500; Nikon Corporation) and analyzed using ImageJ (version 1.45;
National Institutes of Health).
Sirius red staining
The paraffin-embedded kidney sections were dewaxed
with xylene, rehydrated in a descending alcohol series and stained
with Sirius red staining solution for 10 min at room temperature.
The sections were lightly rinsed and then stained with Mayer's
hematoxylin staining solution for 8 min at room temperature, rinsed
again with running water for 10 min, and then sealed with neutral
gum. All images were captured using a light microscope
(magnification, ×500; Nikon Corporation) and analyzed using ImageJ
(version 1.45; National Institutes of Health).
Statistical analysis
The data are presented as the mean ± standard
deviation. The Student's t-test was used to compare the mean values
between two groups. One-way analysis of variance and Bonferroni's
post hoc test were used to evaluate the differences among groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Association between the expression of
miR-212 and renal interstitial fibrosis
The expression of miR-212 was detected in the
peripheral blood of healthy subjects and patients with renal
interstitial fibrosis using RT-qPCR. The results showed that
compared with the healthy subjects, miR-212 expression was
significantly increased in patients with renal interstitial
fibrosis (Fig. 1A). Following
construction of a UUO mouse model, Masson's staining was performed
on the resulting kidney tissue sections. The results revealed that
the glomeruli and renal tubules were intact in the Sham group, and
the renal tubular epithelial cells were regularly arranged.
However, the UUO mice exhibited obvious atrophic tubular
dilatation, disordered tubule arrangement and a large amount of
densely interwoven blue collagen fibers deposited in the renal
interstitium (Fig. 1B), indicating
that the UUO mouse model had been successfully constructed. The
RT-qPCR results indicated that the expression of miR-212 in the
kidneys of the UUO mice was significantly higher than in those of
the Sham group (Fig. 1C). These
results indicated a potential association between aberrant miR-212
expression and renal interstitial fibrosis.
miR-212 induces the expression of
α-SMA
RT-qPCR was used to determine the expression levels
of miR-212 in NRK49F cells following Ang II stimulation for 72 h.
The results showed that the expression of miR-212 was significantly
increased in Ang II-treated NRK49F cells compared with untreated
cells (Fig. 2A). miR-212 mimics or
inhibitor were transfected into NRK49F cells to up- or downregulate
miR-212, and compared with that of the control group, miR-212
expression was significantly increased in cells transfected with
miR-212 mimics (Fig. 2G), but
significantly decreased following transfection with miR-212
inhibitors (Fig. 2H). These
findings confirmed that transfection was successful. Cells
transfected with miR-NC or miR-212 mimics were then stimulated with
Ang II. Compared with the untreated controls, the western blotting
results revealed that Ang II induced the expression of α-SMA, which
was further upregulated by miR-212 overexpression (Fig. 2B). On the other hand, NRK49F cells
stimulated with TGF-β1 for 72 h, or cultured under hypoxic
conditions, also expressed significantly increased levels of
miR-212 and α-SMA, and overexpression of miR-212 further
upregulated α-SMA protein expression (Fig. 2C-F). These results suggested that
following NRK49F cell activation, miR-212 expression is
significantly increased, which subsequently promotes the expression
of α-SMA.
miR-212 targets and negatively
regulates HIF1AN
TargetScan was used to predict potential target
genes of miR-212, and the results showed that the 3′-UTR region of
HIF1AN contains a complementary binding site for miR-212 (Fig. 3A). NRK49F cells were subsequently
co-transfected with pMIR-HIF1AN-wt or pMIR-HIF1AN-Mut, together
with miR-NC, miR-212 mimics, an NC-inhibitor or miR-212 inhibitor.
The results of the Dual-luciferase reporter assay showed that
miR-212 mimics significantly inhibited the luciferase activity of
the pMIR-HIF1AN-wt plasmid, whereas transfection with an miR-212
inhibitor exerted the opposite result; however, neither had a
significant effect on the luciferase activity of the
pMIR-HIF1AN-Mut plasmid (Fig. 3B).
The effects of miR-212 on the expression of HIF1AN were detected by
RT-qPCR and western blotting. The results suggested that the
expression of HIF1AN mRNA and protein was significantly decreased
in NRK49F cells following miR-212 upregulation (Fig. 3C and E). By contrast, HIF1AN mRNA
and protein expression were significantly increased following
miR-212 inhibition (Fig. 3D and F).
These results indicated that miR-212 may negatively regulate the
expression of the target gene, HIF1AN.
miR-212 regulates the expression of
pro-fibrotic factors
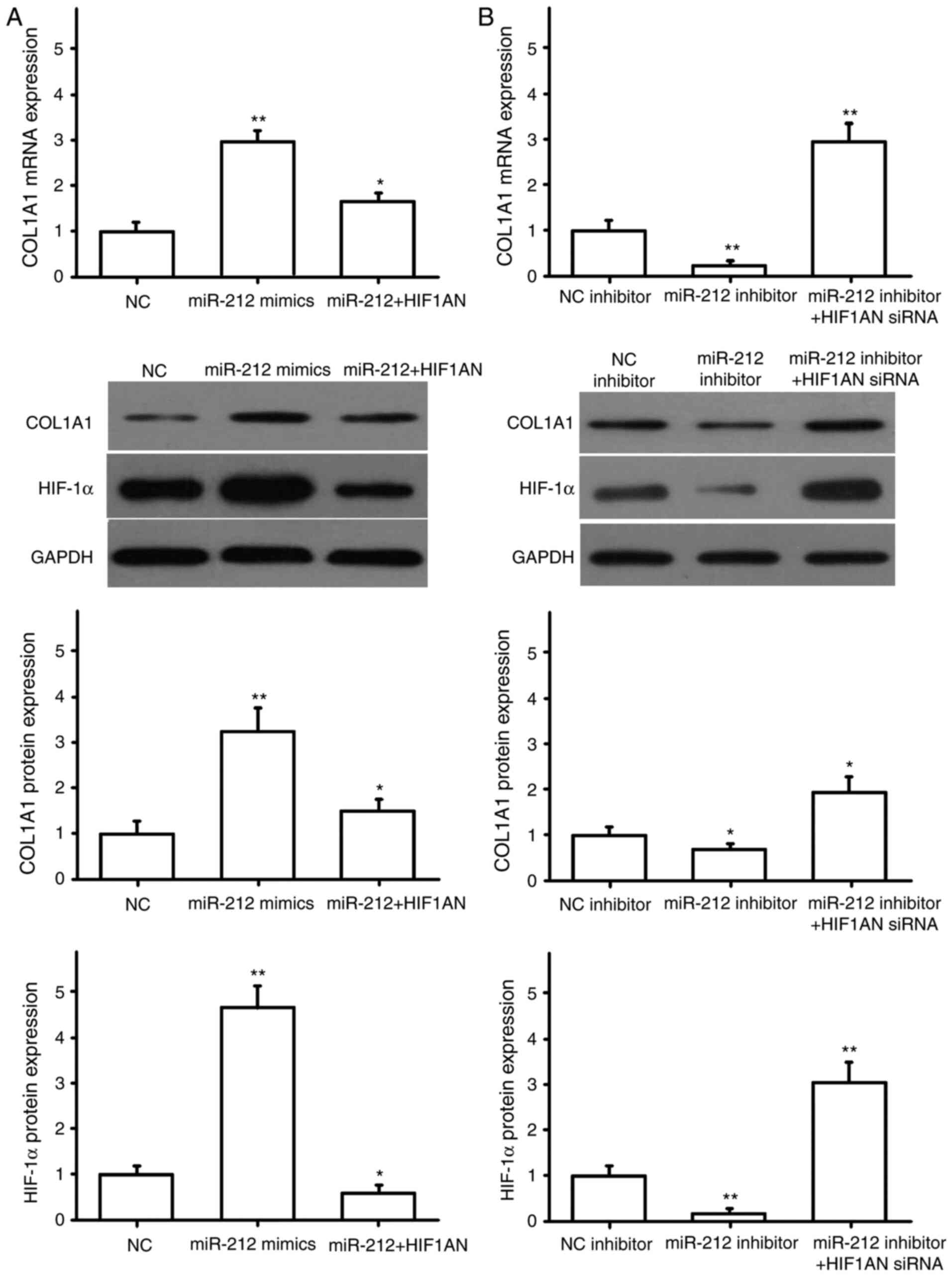
NRK49F cells were transfected with miR-NC or miR-212
mimics, HIF1AN siRNA, HIF1AN overexpression plasmid, and
co-transfected with miR-212 mimics and HIF1AN. The transfection
efficiency for HIF1AN siRNA and the overexpression plasmid were
detected by RT-qPCR. The results showed the HIF1AN siRNA and HIF1AN
overexpression plasmid were successfully transfected (Fig. 4A). The expression of α-SMA, CTGF,
COL1A1 and COL3A1 were then detected by RT-qPCR and western
blotting. The results indicated that miR-212 overexpression
promoted the expression of α-SMA (Fig.
4B), CTGF (Fig. 5A), COL1A1
(Fig. 6A) and COL3A1 (Fig. 7A), and that HIF1AN upregulation
reversed these effects (Figs.
4–7). After downregulating the
expression of miR-212 in NRK49F cells, the expression levels of
pro-fibrotic factors, including α-SMA (Fig. 4C), CTGF (Fig. 5B), COL1A1 (Fig. 6B) and COL3A1 (Fig. 7B), were also significantly reduced,
and inhibiting HIF1AN expression reversed this effect (Figs. 4–7).
Concurrently, HIF-1α protein expression was also altered with the
expression of miR-212, and HIF-1α was found to be negatively
regulated by HIF1AN (Figs.
4–7). These results suggested
that miR-212 may be involved in the regulation of renal
interstitial fibrosis by inhibiting HIF1AN gene expression.
Role of miR-212 in renal interstitial
fibrosis in UUO mice
The role of miR-212 in renal interstitial fibrosis
was further studied using an in vivo mouse UUO model.
Masson's staining revealed that compared with the UUO mice, those
in the UUO+miR-212 agomir group exhibited increased tubular
dilatation and fibrosis. On the contrary, mice in the UUO+miR-212
antagomir group possessed slightly dilated renal tubules and the
pathological changes to the interstitium were alleviated. Sirius
red staining also indicated that upregulating miR-212 expression
promoted renal interstitial fibrosis in UUO mice, whereas
downregulating miR-212 produced an inhibitory effect. Furthermore,
the IHC results showed that the expression of HIF1AN in the kidney
tissue of UUO mice was notably reduced, and that the overexpression
of miR-212 could further inhibit HIF1AN expression. By contrast,
inhibiting miR-212 expression upregulated HIF1AN expression
(Fig. 8A). In addition, miR-212
agomirs induced the expression of COL1A1 and COL3A1 in the kidney
tissues of the UUO mice, whereas miR-212 antagomirs had the
opposite effect (Fig. 8B and C).
RT-qPCR showed that the miR-212 agomirs and miR-212 antagomirs were
successfully transfected in mice (Fig.
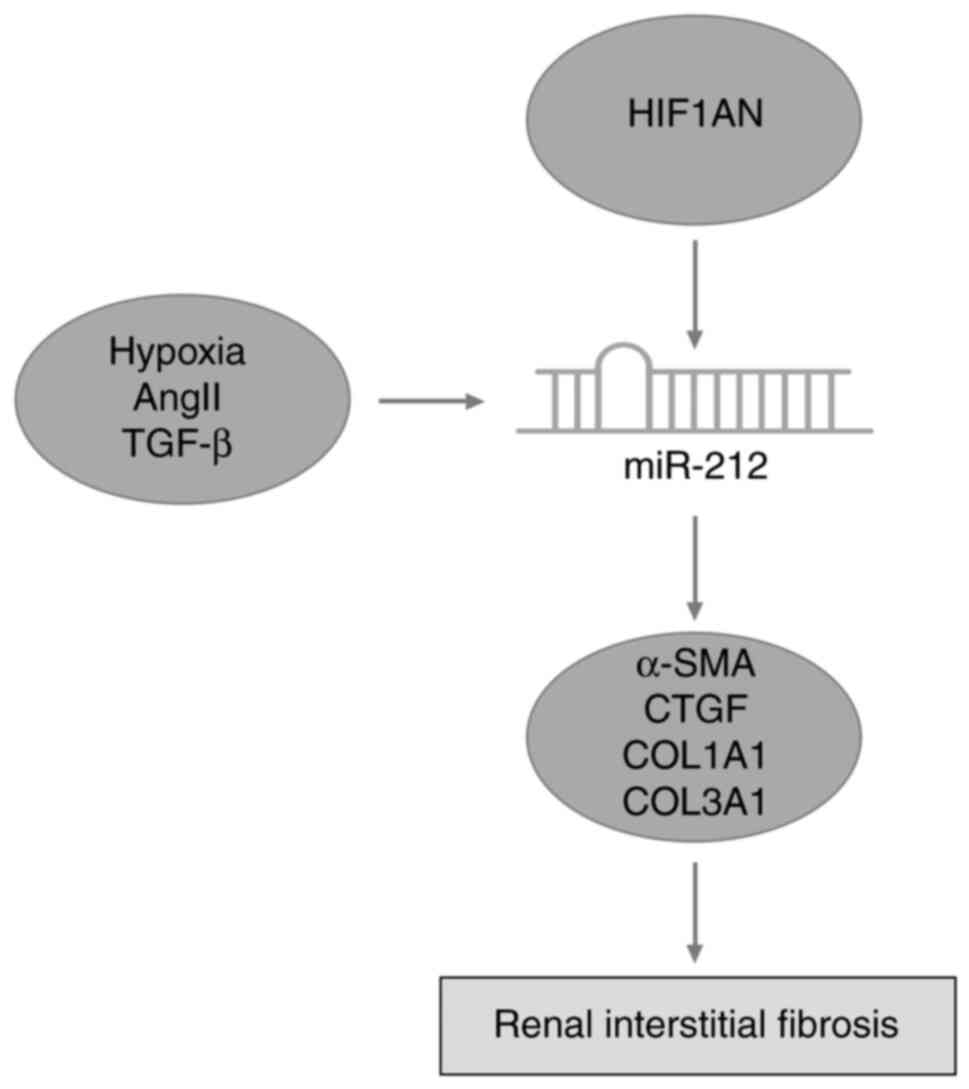
8D). Collectively, the results of the present study indicated
that miR-212 serves a catalytic role in the progression of renal
interstitial fibrosis (Fig. 9).
Discussion
The pathological progression of renal interstitial
fibrosis involves multiple factors, and the primary clinical
manifestations include glomerular sclerosis, tubular atrophy,
abnormal composition and excessive deposition of ECM and renal
interstitial fibrosis (25,26). As a result of progressively in-depth
research, increasing numbers of miRNAs have been found to be
associated with renal fibrosis (27–29).
miRNAs are a highly conserved class of non-coding single-stranded
RNAs, 19–25 nucleotides in length, that inhibit gene expression at
the post-transcriptional level by associating with the 3′-UTRs of
their target genes. miR-200a has been reported to inhibit the
development of renal interstitial fibrosis by targeting TGF-β2
(30), and miR-29b can inhibit
fibrosis by regulating the expression of collagen genes COL1A1,
COL3A1 and COL4A1 in mice kidney medullary epithelial cells
(31). Other studies have indicated
that miR-21 is highly expressed in the renal tissues of patients
with renal fibrosis (29). Although
the association between specific miRNAs and renal interstitial
fibrosis has already been reported (32,33),
the role and influence of miR-212 in renal interstitial fibrosis
are yet to be elucidated. In the present study, miR-212 was found
to be significantly elevated in the peripheral blood of patients
with renal interstitial fibrosis, compared with that of the healthy
controls. Moreover, the expression of miR-212 in the kidney tissues
of UUO mice was also significantly higher compared with in those of
the sham group, indicating a potential association between
alterations in miR-212 expression and renal interstitial
fibrosis.
Ang II promotes the proliferation of renal
interstitial fibroblasts, induces myofibroblast (MFB) activation,
increases ECM secretion and promotes renal interstitial fibrosis
(26,34). As such, continuous intravenous
infusion of Ang II can induce renal tubular atrophy, renal
interstitial expression of α-SMA and ECM accumulation (35,36).
MFBs are the primary synthesizers of the ECM and TGF-β in the renal
interstitium, and their large numbers are closely associated with
the degree of renal interstitial fibrosis (37). In the present study, the expression
of miR-212 and α-SMA in NRK49F cells was found to increase
significantly following stimulation with Ang II, TGF-β1 or hypoxia,
and transfection with miR-212 mimics further upregulated the
expression of α-SMA protein. These results suggested that the
overexpression of miR-212 may promote the activation of NRK49F
cells.
Hypoxia plays an important role in the occurrence
and development of renal interstitial fibrosis (38–40),
and increased expression of HIF-1α is a direct indicator of hypoxia
(41). Numerous studies have
reported that the excessive activation of HIF-1α is a risk factor
for the development of kidney disease (42–44).
The degree of pathological changes to the renal tissue is also
directly proportional to the number of HIF-1α-positive cells, with
more positive cells indicating a higher degree of damage (45). Matoba et al (46) found that HIF-1α is an important
regulator of renal hardening in diabetic nephropathy. A previous
study has also demonstrated that HIF-1α is highly expressed during
kidney damage caused by poisoning, which promotes renal fibrosis
(47). Higgins et al
(48) reported that HIF-1α promoted
the epithelial-to-mesenchymal transition of renal tubular
epithelial cells, as well as renal fibrosis. The cause of chronic
Ang II-induced kidney injury may be the excessive activation of
HIF-1α, of which HIF1AN is an important inhibitor. In the present
study, the HIF1AN gene was predicted to be a potential target of
miR-212. In addition, Dual-luciferase reporter, RT-qPCR and western
blotting confirmed that miR-212 directly targets and negatively
regulates the 3′-UTR of HIF1AN.
Connective tissue growth factor is associated with
the progression of fibrosis in the kidney, skin, lung and liver,
and is expressed at the highest degree in the kidney (49–51).
CTGF is one of the downstream factors of the TGF-β pro-fibrotic
signaling pathway, which stimulates cellular proliferation and ECM
formation and promotes renal interstitial fibrosis (52). The primary components of the ECM
include collagen I and III, which are heavily involved in renal
interstitial fibrosis (53,54). In the present study, the
overexpression of miR-212 was found to regulate the expression of
HIF-1α and pro-fibrotic factors, such as α-SMA, CTGF, COL1A1 and
COL3A1, whereas the upregulation of HIF1AN reversed the promotive
effects of miR-212. Furthermore, downregulating the expression of
miR-212 significantly reduced that of HIF-1α and the aforementioned
pro-fibrotic factors, which was reversed by inhibiting HIF1AN
expression. The present study further found that miR-212 is
involved in the regulation of renal interstitial fibrosis by
inhibiting its target gene, HIF1AN. Therefore, the role of miR-212
in renal interstitial fibrosis was further investigated by
constructing an in vivo UUO model. The results showed that
the degree of tubular dilatation and fibrosis were increased in UUO
mice overexpressing miR-212, in which the expression of HIF1AN was
notably reduced and the levels of COL1A1 and COL3A1 were
significantly increased. On the contrary, low expression levels of
miR-212 inhibited fibrosis and decreased the expression levels of
COL1A1 and COL3A1, but upregulated the expression of HIF1AN in UUO
mice. These findings were consistent with the in vitro
findings, indicating that miR-212 plays a catalytic role in the
progression of renal interstitial fibrosis.
There are some limitations to the present study.
miR-212 expression was not detected in the renal biopsy tissues;
investigating miR-212 in human renal tissues would be more
beneficial and directly confirm the conclusions drawn herein. Also,
miR-212 has other target genes, including recombinant mothers
against decapentaplegic homolog 4 (55) and frizzled family receptor 5
(56), and so it was hypothesized
that HIF1AN is not the only downstream target of miR-212 in the
regulation of renal fibrosis. Systemic analysis of the downstream
genes of miR-212 will be key to further investigations.
In conclusion, high expression levels of miR-212
were closely associated with the occurrence of renal interstitial
fibrosis, and miR-212 induced the expression of fibroblast
growth-promoting factors and promoted fibrosis in UUO mice.
Furthermore, miR-212 was revealed to play an important role in
renal interstitial fibrosis by targeting HIF1AN.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Quanzhou
Science and Technology Bureau (grant no. 2018Z125).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and YTL designed the study and wrote the
manuscript. YZ, GXZ and LSC performed the experiments. YTL and SHS
analyzed the data and YZ drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Affiliated Hospital of Fujian Medical
University (approval no. 201939) and written informed consent was
provided by patients or family members of patients and the
volunteers. All surgical procedures adhered to the ethical norms of
clinical experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perico N and Remuzzi G: Chronic kidney
disease: A research and public health priority. Nephrol Dial
Transplant. 27 (Suppl 3):iii19–iii26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin Z, Gong Q, Zhou Z, Zhang W, Liao S,
Liu Y, Yan X, Pan X, Lin S and Li X: Increased plasma CXCL16 levels
in patients with chronic kidney diseases. Eur J Clin Invest.
41:836–845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grgic I, Duffield JS and Humphreys BD: The
origin of interstitial myofibroblasts in chronic kidney disease.
Pediatr Nephrol. 27:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayer MK, Price AM, Liu B, Baig S, Ferro
CJ, Townend JN, Steeds RP and Edwards NC: Diffuse myocardial
interstitial fibrosis and dysfunction in early chronic kidney
disease. Am J Cardiol. 121:656–660. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou TB, Qin YH, Lei FY, Huang WF and
Drummen GPC: Prohibitin attenuates oxidative stress and
extracellular matrix accumulation in renal interstitial fibrosis
disease. PLoS One. 8:e771872013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghosh AK, Rai R, Flevaris P and Vaughan
DE: Epigenetics in reactive and reparative cardiac fibrogenesis:
The promise of epigenetic therapy. J Cell Physiol. 232:1941–1956.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun YB, Qu X, Caruana G and Li J: The
origin of renal fibroblasts/myofibroblasts and the signals that
trigger fibrosis. Differentiation. 92:102–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jurkovicova D, Sedlakova B, Lacinova L,
Kopacek J, Sulova Z, Sedlak J and Krizanova O: Hypoxia differently
modulates gene expression of inositol 1,4,5-trisphosphate receptors
in mouse kidney and HEK 293 cell line. Ann N Y Acad Sci.
1148:421–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang G, Cheng QL, Li CL, Jia YL, Yue W,
Pei XT, Liu Y, Zhao JH, Du J and Ao QG: High glucose reduced the
repair function of kidney stem cells conditional medium to the
hypoxia-injured renal tubular epithelium cells. Beijing Da Xue Xue
Bao Yi Xue Ban. 49:125–130. 2017.(In Chinese). PubMed/NCBI
|
|
10
|
Bienholz A, Reis J, Sanli P, de Groot H,
Petrat F, Guberina H, Wilde B, Witzke O, Saner FH, Kribben A, et
al: Citrate shows protective effects on cardiovascular and renal
function in ischemia-induced acute kidney injury. BMC Nephrol.
18:1302017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng Z, Liu L, Wang Z, Cai Y, Xu Q and
Chen P: Hypoxia activates src and promotes endocytosis which
decreases MMP-2 activity and aggravates renal interstitial
fibrosis. Int J Mol Sci. 19:5812018. View Article : Google Scholar
|
|
12
|
Karakashev SV and Reginato MJ:
Hypoxia/HIF1α induces lapatinib resistance in ERBB2-positive breast
cancer cells via regulation of DUSP2. Oncotarget. 6:1967–1980.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie J, Li DW, Chen XW, Wang F and Dong P:
Expression and significance of hypoxia-inducible factor-1α and
MDR1/P-glycoprotein in laryngeal carcinoma tissue and hypoxic Hep-2
cells. Oncol Lett. 6:232–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu K, Yan Z, Wu Y, Chen Y, Qu P, Xu X,
Yuan P, Huang X, Xing J, Zhang H, et al: Transarterial
chemoembolization aggravated peritumoral fibrosis via
hypoxia-inducible factor-1α dependent pathway in hepatocellular
carcinoma. J Gastroenterol Hepatol. 30:925–932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimura K, Iwano M, Higgins DF, Yamaguchi
Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, et
al: Stable expression of HIF-1alpha in tubular epithelial cells
promotes interstitial fibrosis. Am J Physiol Renal Physiol.
295:F1023–F1029. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao D, Hu L, Lei D, Fang X, Zhang Z, Wang
T, Lin M, Huang J, Yang H, Zhou X and Zhong L: MicroRNA-196b
promotes cell proliferation and suppress cell differentiation in
vitro. Biochem Biophys Res Commun. 457:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan J, Xiao G, Peng G, Liu D, Wang Z,
Liao Y, Liu Q, Wu M and Yuan X: MiRNA-125a-5p inhibits glioblastoma
cell proliferation and promotes cell differentiation by targeting
TAZ. Biochem Biophys Res Commun. 457:171–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao N, Yu H, Yu H, Sun M, Zhang Y, Xu M
and Gao W: MiRNA-711-SP1-collagen-I pathway is involved in the
anti-fibrotic effect of pioglitazone in myocardial infarction. Sci
China Life Sci. 56:431–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patil A, Sweeney WE, Pan CG and Avner ED:
Unique interstitial miRNA signature drives fibrosis in a murine
model of autosomal dominant polycystic kidney disease. World J
Nephrol. 7:108–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patel V, Williams D, Hajarnis S, Hunter R,
Pontoglio M, Somlo S and Igarashi P: miR-17~92 miRNA cluster
promotes kidney cyst growth in polycystic kidney disease. Proc Natl
Acad Sci USA. 110:10765–10770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H,
Chen PS and Liu BC: MicroRNA-29c in urinary exosome/microvesicle as
a biomarker of renal fibrosis. Am J Physiol Renal Physiol.
305:F1220–F1227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castoldi G, Gioia C, Giollo F, Carletti R,
Bombardi C, Antoniotti M, Roma F, Zerbini G and Stella A: Different
regulation of miR-29a-3p in glomeruli and tubules in an
experimental model of angiotensin II-dependent hypertension:
Potential role in renal fibrosis. Clin Exp Pharmacol Physiol.
43:335–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Makni K, Jarraya F, Khabir A, Hentati B,
Hmida MB, Makni H, Boudawara T, Jlidi R, Hachicha J and Ayadi H:
Renal alpha-smooth muscle actin: A new prognostic factor for lupus
nephritis. Nephrology (Carlton). 14:499–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Racca MA, Novoa PA, Rodríguez I, Vedova
ABD, Pellizas CG, Demarchi M and Donadio AC: Renal dysfunction and
intragraft proMMP9 activity in renal transplant recipients with
interstitial fibrosis and tubular atrophy. Transpl Int. 28:71–78.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chow BSM, Kocan M, Bosnyak S, Sarwar M,
Wigg B, Jones ES, Widdop RE, Summers RJ, Bathgate RAD, Hewitson TD
and Samuel CS: Relaxin requires the angiotensin II type 2 receptor
to abrogate renal interstitial fibrosis. Kidney Int. 86:75–85.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kriegel AJ, Liu Y, Cohen B, Usa K, Liu Y
and Liang M: MiR-382 targeting of kallikrein 5 contributes to renal
inner medullary interstitial fibrosis. Physiol Genomics.
44:259–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ben-Dov IZ, Muthukumar T, Morozov P,
Mueller FB, Tuschl T and Suthanthiran M: MicroRNA sequence profiles
of human kidney allografts with or without tubulointerstitial
fibrosis. Transplantation. 94:1086–1094. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu XJ, Hong Q, Wang Z, Yu YY, Zou X and
Xu LH: MicroRNA21 promotes interstitial fibrosis via targeting
DDAH1: A potential role in renal fibrosis. Mol Cell Biochem.
411:181–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang B, Koh P, Winbanks C, Coughlan MT,
McClelland A, Watson A, Jandeleit-Dahm K, Burns WC, Thomas MC,
Cooper ME and Kantharidis P: miR-200a prevents renal fibrogenesis
through repression of TGF-β2 expression. Diabetes. 60:280–287.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Taylor NE, Lu L, Usa K, Cowley AW
Jr, Ferreri NR, Yeo NC and Liang M: Renal medullary microRNAs in
Dahl salt-sensitive rats: MiR-29b regulates several collagens and
related genes. Hypertension. 55:974–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou H, Hasni SA, Perez P, Tandon M, Jang
SI, Zheng C, Kopp JB, Austin H III, Balow JE, Alevizos I and Illei
GG: miR-150 promotes renal fibrosis in lupus nephritis by
downregulating SOCS1. J Am Soc Nephrol. 24:1073–1087. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fang Y, Yu X, Liu Y, Kriegel AJ, Heng Y,
Xu X, Liang M and Ding X: miR-29c is downregulated in renal
interstitial fibrosis in humans and rats and restored by HIF-α
activation. Am J Physiol Renal Physiol. 304:F1274–F1282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mezzano SA, Aros CA, Droguett A, Burgos
ME, Ardiles LG, Flores CA, Carpio D, Vío CP, Ruiz-Ortega M and
Egido J: Renal angiotensin II up-regulation and myofibroblast
activation in human membranous nephropathy. Kidney Int Suppl.
S39–S45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tunçdemir M, Demirkesen O, Oztürk M,
Atukeren P, Gümüştaş MK and Turan T: Antiapoptotic effect of
angiotensin-II type-1 receptor blockade in renal tubular cells of
hyperoxaluric rats. Urol Res. 38:71–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou L, Xue H, Yuan P, Ni J, Yu C, Huang Y
and Lu LM: Angiotensin AT1 receptor activation mediates high
glucose-induced epithelial-mesenchymal transition in renal proximal
tubular cells. Clin Exp Pharmacol Physiol. 37:e152–e157. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeisberg M and Kalluri R: Physiology of
the renal interstitium. Clin J Am Soc Nephrol. 10:1831–1840. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mimura I, Tanaka T and Nangaku M: Novel
therapeutic strategy with hypoxia-inducible factors via reversible
epigenetic regulation mechanisms in progressive tubulointerstitial
fibrosis. Semin Nephrol. 33:375–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Du R, Xia L, Ning X, Liu L, Sun W, Huang
C, Wang H and Sun S: Hypoxia-induced Bmi1 promotes renal tubular
epithelial cell-mesenchymal transition and renal fibrosis via
PI3K/Akt signal. Mol Biol Cell. 25:2650–2659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang B, Liang X, Shi W, Ye Z, He C, Hu X
and Liu S: Role of impaired peritubular capillary and hypoxia in
progressive interstitial fibrosis after 56 subtotal nephrectomy of
rats. Nephrology (Carlton). 10:351–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baek KJ, Cho JY, Rosenthal P, Alexander
LEC, Nizet V and Broide DH: Hypoxia potentiates allergen induction
of HIF-1α, chemokines, airway inflammation, TGF-β1, and airway
remodeling in a mouse model. Clin Immunol. 147:27–37. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baumann B, Hayashida T, Liang X and
Schnaper HW: Hypoxia-inducible factor-1α promotes
glomerulosclerosis and regulates COL1A2 expression through
interactions with Smad3. Kidney Int. 90:797–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kushida N, Nomura S, Mimura I, Fujita T,
Yamamoto S, Nangaku M and Aburatani H: Hypoxia-inducible factor-1α
activates the transforming growth factor-β/SMAD3 pathway in kidney
tubular epithelial cells. Am J Nephrol. 44:276–285. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang L, Yi R, Yang B, Li H, Chen H and Liu
Z: Valsartan inhibited HIF-1α pathway and attenuated renal
interstitial fibrosis in streptozotocin-diabetic rats. Diabetes Res
Clin Pract. 97:125–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma C, Wei J, Zhan F, Wang R, Fu K, Wan X
and Li Z: Urinary hypoxia-inducible factor-1alpha levels are
associated with histologic chronicity changes and renal function in
patients with lupus nephritis. Yonsei Med J. 53:587–592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matoba K, Kawanami D, Nagai Y, Takeda Y,
Akamine T, Ishizawa S, Kanazawa Y, Yokota T and Utsunomiya K:
Rho-Kinase blockade attenuates podocyte apoptosis by inhibiting the
notch signaling pathway in diabetic nephropathy. Int J Mol Sci.
18:17952017. View Article : Google Scholar
|
|
47
|
Zhu Y, Tan J, Xie H, Wang J, Meng X and
Wang R: HIF-1α regulates EMT via the Snail and β-catenin pathways
in paraquat poisoning-induced early pulmonary fibrosis. J Cell Mol
Med. 20:688–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Higgins DF, Kimura K, Iwano M and Haase
VH: Hypoxia-inducible factor signaling in the development of tissue
fibrosis. Cell Cycle. 7:1128–1132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Anorga S, Overstreet JM, Falke LL, Tang J,
Goldschmeding RG, Higgins PJ and Samarakoon R: Deregulation of
Hippo-TAZ pathway during renal injury confers a fibrotic
maladaptive phenotype. FASEB J. 32:2644–2657. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu B, Ma AQ, Yang L and Dang XM:
Atorvastatin attenuates bleomycin-induced pulmonary fibrosis via
suppressing iNOS expression and the CTGF (CCN2)/ERK signaling
pathway. Int J Mol Sci. 14:24476–24491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Montford JR and Furgeson SB: A new CTGF
target in renal fibrosis. Kidney Int. 92:784–786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun B, Xing CY, He WC, Wang NN, Yu XB,
Zhao XF, Qian J, Yang JW, Liu J and Wang XY: Expression of
connective tissue growth factor in renal interstitial fibrosis
after ureteral obstruction and effects of rapamycin thereupon:
Experiment with rats. Zhonghua Yi Xue Za Zhi. 87:562–566. 2007.(In
Chinese). PubMed/NCBI
|
|
53
|
Montgomery TA, Xu L, Mason S, Chinnadurai
A, Lee CG, Elias JA and Cantley LG: Breast regression
protein-39/chitinase 3-like 1 promotes renal fibrosis after kidney
injury via activation of myofibroblasts. J Am Soc Nephrol.
28:3218–3226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Q, Peng Z, Xiao S, Geng S, Yuan J and
Li Z: RNAi-mediated inhibition of COL1A1 and COL3A1 in human skin
fibroblasts. Exp Dermatol. 16:611–617. 2010. View Article : Google Scholar
|
|
55
|
Schauer SN, Sontakke SD, Watson ED,
Esteves CL and Donadeu FX: Involvement of miRNAs in equine follicle
development. Reproduction. 146:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin JF, Zeng H and Zhao JQ: MiR-212-5p
regulates the proliferation and apoptosis of AML cells through
targeting FZD5. Eur Rev Med Pharmacol Sci. 22:8415–8422.
2018.PubMed/NCBI
|