Introduction
Neural tube defect (NTD) is a serious congenital
defect that occurs during brain development. NTDs are caused by
closure anomalies during the development of the neural tube in the
embryonic stage, and are mainly manifested in the absence of the
brain, brain expansion, meningeal membrane expansion and recessive
spinal bifida (1,2). Neural stem cells (NSCs) are key cells
involved in nerve tube closure and these cover the surface of nerve
tubes (3). NSC proliferation,
differentiation and migration serve a pivotal role in the normal
closure of nerve tubes, and abnormal differentiation will lead to
brain abnormalities (4,5). NSCs are the least committed cells in
the nervous system and have self-renewal and pluripotent functional
properties, as well as produce all three basic neuroectoderm
lineages (6). NSCs produce neurons,
astrocytes and oligodendrocytes in a region-appropriate and
stage-appropriate manner throughout their lifespan (7).
In various cell fate regulatory pathways, the Notch
signal pathway serves a precise and complex regulatory role in cell
proliferation and differentiation and in embryonic development
(8). The Notch signaling pathway is
an evolutionarily conserved mechanism that functions in multiple
cell determination processes during metazoan development and in
adults (8). The core elements of
the vertebrate Notch signaling system include the Notch receptors
[Notch 1 (N1)-Notch 4], Delta (Delta l-Delta 4), Serrate/Jagged
(Jagged 1-Jagged 2) ligands and the DNA binding protein RBPjk/CBF1
(9). This system allows neighboring
cells to communicate with each other via local short-range
intercellular interactions, amplifying and consolidating molecular
differences which, eventually, manifest as different cell fates.
Therefore, disruption of this pathway has functional consequences
for multiple different tissues and cell fates (10). The current model for Notch signaling
assumes that after ligand binding to the Notch receptor via its
extracellular domain, the intracellular domain of the ligand is
ubiquitinated, triggering its endocytosis, which is activated by
the action of presenilin γ-secretase enzymes (8).
All-trans retinoic acid (atRA) is a normal
metabolite of retinoic acid present in the body and is an important
factor in embryonic development (11). Previous studies have reported that
excess atRA leads to the occurrence of NTDs (12,13).
However, its specific molecular mechanism remains to be
elucidated.
The present study demonstrated the role of N1 in
embryonic brain tissue from mice (in vivo) and in NSCs
(in vitro) treated with atRA. Furthermore, the regulatory
effect of N1 on the differentiation of NSCs and the molecular
mechanism of brain abnormality caused by atRA treatment were
identified. These results may provide a novel direction for the
application of NSCs and clinical prevention of brain
abnormality.
Materials and methods
Reagents
Anti-N1 (cat. no. ab52627), anti-presenilin1 (PS1;
cat. no. ab16244), anti-Nestin (cat. no. ab22035),
anti-neurofilament (NF; cat. no. ab204893), anti-glial fibrillary
acidic protein (GFAP; cat. no. ab10062), anti-galactocerebroside
(GALC; cat. no. ab232972) and anti-β tubulin (cat. no. ab6046) were
obtained from Abcam. All other reagents and chemicals were
purchased from Sigma-Aldrich (Merck KGaA) unless stated
otherwise.
Source of tissue
In total, 24 mice were purchased from the
Experimental Animal Center of Shandong University. Mice were housed
in conditions at 23±1°C with a constant humidity of 60±10% under a
12 h light/dark cycle, and had free access to food and water. The
research was approved by the Medical Ethics Committee of Shandong
Provincial Hospital Affiliated to Shandong First Medical
University.
Tissue preparation
Female C57BL/6 mice (age, 10–12 weeks; weight, 25–30
g) were mated with mature males (age, 7–8 weeks; weight, 18–25 g),
and the detection of a vaginal plug was designated as embryo day 0
(E0). A total of 24 mice were used. According to a study by
Seegmiller et al (14), on
E7, 12 female mice in the treatment group were administered with
all-trans atRA (100 mg/kg; Sigma-Aldrich; Merck KGaA)
dissolved in corn oil via oral gavage. The other 12 female mice in
the control group were administered with the same volume of corn
oil. All pregnant mice were sacrificed via cervical dislocation on
E11, E13, E15 and E17. The embryos were anesthetized with 10%
chloral hydrate (350 mg/kg body weight) via intraperitoneal
injection and perfused intracardially with normal saline. No signs
of peritonitis, pain or discomfort were observed in the embryos.
The mice brains were obtained for hematoxylin and eosin (H&E)
staining, as well as immunohistological and western blot
analyses.
H&E staining
Embryo brains from atRA-treated and control mice on
E11, 13, 15 and 17 were fixed with 4% paraformaldehyde at room
temperature for 15 min, snap frozen in liquid nitrogen-cooled
isopentane and embedded in Optimal Cutting Temperature, followed by
storage at −20°C. Cryostat sections (thickness, 7 µm) were prepared
and immersed in Harris' hematoxylin at 4°C for 5 min to stain all
nuclei. Sections were then washed with cold running tap water for
10 min, counterstained with eosin at 4°C for 2 min and washed for
10 min with cold running tap water. The sections were visualized
and images captured using a Leica (DMRAZ) confocal microscope
(magnification, ×40; Leica Microsystems, Inc.).
Immunolocalization of N1 in fetal
mice
For immunostaining of cryosections, cryostat
sections were prepared as aforementioned, and then were fixed for
15 min at room temperature with 4% paraformaldehyde and
permeabilized for 15 min in 0.2% Triton X-100. After three washes
with PBS, the sections were incubated in room temperature for 2 h
with a primary antibody directed against N1 (1:100 in PBS).
Following three 10-min washes in PBS, the sections were incubated
in room temperature for 2 h with appropriate Alexa Fluor 594
secondary antibodies (1:1,000 in PBS; cat. no. A30008; Molecular
Probes; Thermo Fisher Scientific, Inc.). After an additional three
washes with PBS, the sections were mounted in Hydromount containing
bis-benzimide (Hoechst 33342; 1:500 of 1 mg/ml stock solution; BDH
Chemicals Ltd.) to visualize the nuclei. Substitution of the
primary antibodies with same species non-immune serum (Abcam)
served as the negative controls. The sections were visualized and
images captured using a Leica (DMRAZ) microscope (magnification,
×40; Leica Microsystems, Inc.) and a fluorescent image analysis
software (Quantimet 500; Leica Microsystems, Inc.).
Western blotting for embryonic
tissues
Western blot analysis was used to further confirm N1
expression in fetal mice. Total protein was isolated from
atRA-treated or control embryonic brain tissues as previously
described (15) using RIPA lysis
buffer (Thermo Fisher Scientific, Inc.) supplemented with protease
and phosphatase inhibitors at 4°C for 30 min. Proteins were then
centrifuged at 15,000 × g at 4°C for 30 min. Subsequently, 10%
SDS-PAGE was used to separate equal amounts (30 µg) of extracted
protein samples, which were then transferred to nitrocellulose
membranes (EMD Millipore). The membranes were blocked with 5%
evaporated skimmed milk at 37°C for 1 h and incubated with primary
antibodies against N1 and β-tubulin (1:1,000) in 5% evaporated
skimmed milk at 4°C for 12 h. Membranes were then incubated with
horseradish peroxidase-conjugated secondary antibody (1:2,000; cat.
no. 7074s; Cell Signaling Technology, Inc.) in 5% evaporated
skimmed milk at 37°C for 1 h. An ECL kit (Cell Signaling
Technology, Inc.) and the ImageQuant Las 4000 mini system (Cytiva)
was used to observe the protein bands. β-tubulin was used as an
internal control. ImageJ version 1.48 u software (Nationals
Institutes of Health) was used to semiquantify the signal
intensities.
Isolation and culture of neural stem
cells
Embryonic brains were isolated from female mice at
E18.5. In brief, 10% chloral hydrate (350 mg/kg body weight) was
used as an anesthetic in pregnant mice at E18.5. No signs of
peritonitis, pain or discomfort were observed in the embryos.
Pregnant mice were sacrificed via cervical dislocation, and fetal
mouse brain tissue was removed and placed in a flat dish containing
4% D-Hanks liquid. The brain tissue was cut into 1 mm3
sections. After placing the tissue block in a centrifuge tube, the
D-Hanks solution was absorbed and 0.125% trypsin was added at 37°C
and 5% CO2. The digestion was oscillated for 20 min and
then 10% FBS in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.)
at 4°C was used to terminate differentiation for 20 min. DMEM-F12
medium with 20 ng/ml bFGF and 20 ng/ml EGF added to suspend cells,
which were cultured in incubators at 37°C and 5% CO2.
The following day, fresh bFGF and EGF were added. The liquid was
changed after 3.5 days, and the culture was passed from days
7–9.
Co-immunolabelling for
confirmation
Cultures were fixed with 1% paraformaldehyde at room
temperature for 20 min and washed twice with PBS for 5 min. Cells
were incubated with 0.2% Triton X-100 (v/v PBS) for 20 min at room
temperature to permeabilize the cell membranes, and were then
washed twice in PBS (10 min each). The cells were incubated with
primary antibodies against N1 with Nestin (N1 + Nestin; 1:100), NF
(N1 + NF; 1:100), GFAP (N1 + GFAP; 1:100) and GALC (N1 + GALC;
1:100) for 2 h at room temperature, followed by a 2-h incubation
with Alexa Fluor 488 (1:1,000; cat. no. A32723; Molecular Probes;
Thermo Fisher Scientific, Inc.) and 594 secondary antibodies
(1:1,000; cat. no. A30008; Molecular Probes; Thermo Fisher
Scientific, Inc.) at room temperature. After an additional three
washes with PBS, the cells were mounted in Hydromount containing
bis-benzimide as aforementioned. Substitution of the primary
antibodies with same species non-immune serum (Abcam) served as
negative controls. The cells were visualized and images were
captured using a Leica fluorescent microscope (magnification, ×100;
Leica Microsystems, Inc.) and a fluorescent image analysis software
(Quantimet 500; Leica Microsystems, Inc.).
Western blot analysis for the
differentiation of NSCs after treatment with atRA
The 5th-generation nerve spheres were arranged in
two groups (experimental and control group) and the medium was
discarded after centrifugation in 1,000 × g at room temperature for
10 min. Induced-differentiation media (DMEM with 20 ng/ml bFGF, 20
ng/ml EGF and N2 additives) with or without 1 µmol/l atRA was added
at 37°C to nerve sphere on days 1, 3, 5 and 7. The culture medium
was replaced every 24 h for western blotting.
Proteins isolated from atRA-treated or control
cultured NSCs were analyzed using western blot analysis, as
aforementioned. The primary antibodies used were N1, PS1, NF, GFAP
and GALC (1:1,000). β-tubulin (1:1,000) was used as an internal
control.
Statistical analysis
The statistically significant differences between
groups were analyzed using one-way ANOVA followed with Tukey's
multiple comparison test or unpaired Student's t-test, using
GraphPad Prism version 4.0 software (GraphPad Software, Inc.). Data
are presented as the mean ± SD. All experiments were repeated ≥3
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
atRA induces brain abnormality in
mouse embryos
atRA was administered to female mice on E7. H&E
staining identified the morphological changes in the mouse brain of
atRA-treated embryos and controls at E11, 13, 15 and 17 (Fig. 1). On E11, the embryos exposed to
atRA showed earlier symmetric brain matter formation compared with
those in the control group, based on visual observation. On E13,
brain matter grew more apparently between atRA-treated and control
embryos. On E15, meninges nearly contacted with the opposing side
in atRA-exposed embryos, whereas control embryos exhibited
uncontacted meninges. On E17, the meninges and brain matter
remained unformed and the medial edge epithelium was uncontacted in
untreated embryos; however, in atRA-exposed embryos, the two sides
of meninges were contacted and the brain matter was already
developed.
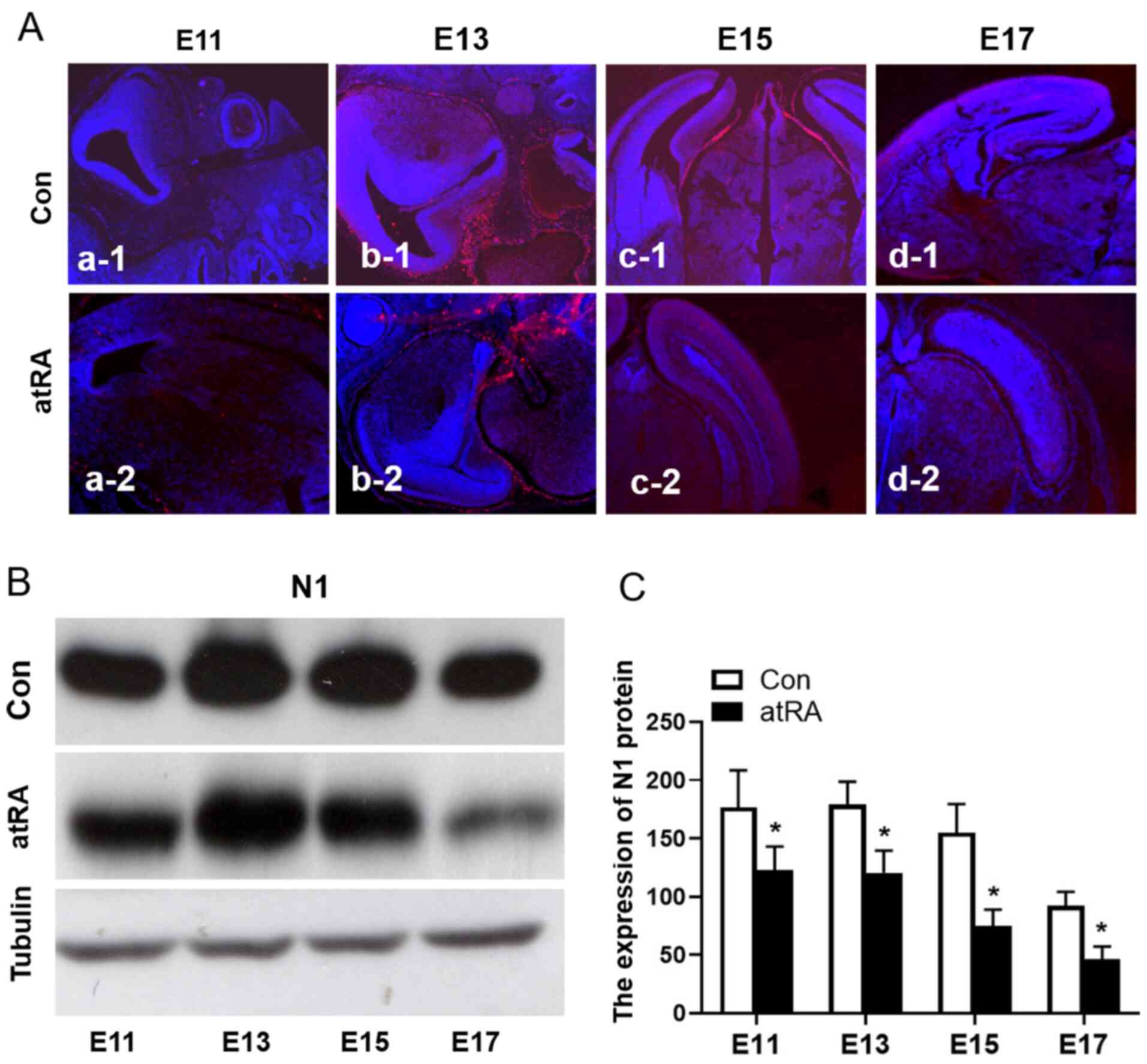
N1 expression is decreased in mouse
brains from atRA-treated embryos
To identify the effects of atRA-induced Notch
signaling events on brain development, the expression of N1 in the
brains of mice from atRA-treated and untreated E11 and E17 embryos
was detected (Fig. 2Aa-1-d-2). The
expression of N1 was further confirmed via western blotting
(Fig. 2B), the expression of N1 was
significantly downregulated in embryos treated with atRA compared
with the control group (Fig. 2C;
Table I; P<0.05).
 | Table I.Data of Notch1 expression of mice
brain on E11, 13, 15 and 17 with the control media (-atRA) or atRA
media (+atRA) from western blotting. |
Table I.
Data of Notch1 expression of mice
brain on E11, 13, 15 and 17 with the control media (-atRA) or atRA
media (+atRA) from western blotting.
|
| Control | +atRA |
|
|---|
|
|
|
|
|
|---|
| Day | Exp.1 | Exp.2 | Exp.3 | Exp.1 | Exp.2 | Exp.3 | P-value |
|---|
| E11 | 131.40 | 106.80 |
88.70 |
42.30 |
81.30 | 53.90 | 0.032 |
| E13 | 192.10 | 175.60 | 136.20 | 123.20 | 111.30 | 79.50 | 0.0058 |
| E15 | 116.20 | 101.30 |
88.60 |
65.90 |
37.50 | 63.70 | 0.049 |
| E17 |
92.20 |
78.30 |
82.50 |
41.60 |
59.20 | 11.80 | 0.047 |
NSC culture shows differentiated
neural cells
The cells that grew in the culture medium continued
to undergo morphological changes. Primary NSC culture on day 0
(Fig. 3A) show a plurality of
formed neurospheres, which were attached on the bottom of the
flask. Then, 1 day after differentiation, the size of neurospheres
began to increase, and some NSCs were released from the
neurospheres. Cells that had divided by one cell grew into a
suspended growth state of nerve cloned spheres, in which numerous
cells gathered together (Fig. 3B).
After 3 days, different types of cells appear around the cloned
sphere. The edge was observed as a jagged single-cell boundary,
with extending cord-like projections and a strong three-dimensional
shape (Fig. 3C). After 7 days, the
spherical three-dimensional appearance gradually dissipated. The
cells in the cloned sphere had a tendency to migrate outwards. The
cloned sphere was completely attached and a different shape of the
cells, including neurons, astrocytes and oligodendrocytes, was
detected (Fig. 3D).
Co-expression of N1 with Nestin, NF,
GFAP and GALC in NSCs as detected using co-immunofluorescence
staining
To confirm the cell differentiation, a
co-immunofluorescence staining technique was used. The results
demonstrated there were positive co-expressions of N1 (green) with
Nestin (red), NF (red), GFAP (red) and GLAC (red) in NSCs, neurons,
astrocytes and oligodendrocytes, respectively (Fig. 4A-D).
 | Figure 4.Co-immunostaining detected the
expression of N1 with Nestin, NF, GFAP and GALC in neurons,
astrocytes and oligodendrocytes, respectively. (A-a-1-a-4) Staining
of N1 + Nestin, N1, Nestin and NSC nuclei respectively. (B-b-1-b-4)
Staining of N1 + NF, N1, NF and neuronal nuclei, respectively.
(C-c-1-c-4) Staining of N1 + GFAP, N1, GFAP and astrocyte nuclei,
respectively. (D-d-1-d-4) Staining of N1 + GALC, N1, GALC and
oligodendrocyte nuclei, respectively. Green indicates the positive
staining of N1, Red indicates the positive staining of Nestin, NF,
GFAP or GALC. Blue indicates Hoechst-labelled nuclei. Scale bar,
100 µM. N1, Notch1; PS1, presenilin 1; NF, neurofilament; GFAP,
glial fibrillary acidic protein; GALC, galactocerebroside. |
Expression levels of N1, PS1, NF, GFAP
and GALC in NSCs with or without atRA as detected via western
blotting
Western blotting was performed to detect the
expression levels of the proteins in NSCs. The results indicated
that there were significantly decreased expression levels of N1 and
PS1, but increased expression levels of NF, GFAP and GALC in NSCs
from the atRA group compared with those in the controls (P<0.05;
Fig. 5A-E). β-tubulin was used as a
control. The results of statistical analyses have also been
detailed in Table II.
 | Figure 5.Expression levels of N1, PS1, NF,
GFAP and GALC in neural cells at D1, 3, 5 and 7 treated with the
Con or atRA media as detected by western blotting. Expression
levels of (A) N1, (B) PS1, (C) NF, (D) GFAP and (E) GALC in atRA
media have been compared with in control media. Tubulin was used as
a control. *P<0.05 Con vs. atRA. Con, control media; N1, Notch1;
PS1, presenilin 1; NF, meurofilament; GFAP, glial fibrillary acidic
protein; GALC, galactocerebroside; atRA, all-trans retinoic
acid; D, day. |
 | Table II.Data of N1, PS1, NF, GFAP and GALC
expression levels in neural cells on days 1, 3, 5 and 7 with the
control media (-atRA) or atRA media (+atRA) from western
blotting. |
Table II.
Data of N1, PS1, NF, GFAP and GALC
expression levels in neural cells on days 1, 3, 5 and 7 with the
control media (-atRA) or atRA media (+atRA) from western
blotting.
|
|
| Control | +atRA |
|
|---|
|
|
|
|
|
|
|---|
| Protein | Day | Exp.1 | Exp.2 | Exp.3 | Exp.1 | Exp.2 | Exp.3 | P-value |
|---|
| N1 | D1 | 211.30 | 169.90 | 148.70 | 123.40 | 102.30 | 142.60 |
0.019 |
|
| D3 | 197.50 | 181.20 | 158.10 | 100.20 | 123.30 | 137.90 |
0.011 |
|
| D5 | 132.20 | 181.10 | 151.30 | 73.40 | 61.70 | 89.50 |
0.0007 |
|
| D7 | 79.80 | 93.60 | 103.30 | 36.70 | 58.40 | 42.50 |
0.048 |
| PS1 | D1 | 126.30 | 135.70 | 119.80 | 96.40 | 102.70 | 97.50 |
0.011 |
|
| D3 | 125.10 | 116.70 | 106.20 | 76.60 | 104.60 | 91.30 |
0.025 |
|
| D5 | 59.90 | 78.50 | 52.40 | 21.30 | 5.10 | 12.70 | <0.0001 |
|
| D7 | 37.60 | 47.40 | 24.30 | 12.30 | 19.20 | 8.30 |
0.042 |
| NF | D1 | 18.30 | 22.40 | 29.60 | 32.50 | 18.90 | 38.60 |
0.94 |
|
| D3 | 33.10 | 31.10 | 23.50 | 103.30 | 121.60 | 96.70 | <0.0001 |
|
| D5 | 51.60 | 44.80 | 68.70 | 93.50 | 103.60 | 116.30 | 0.0004 |
|
| D7 | 71.30 | 93.20 | 78.80 | 123.70 | 138.50 | 101.20 | 0.0027 |
| GFAP | D1 | 21.10 | 28.90 | 33.80 | 15.40 | 18.70 | 28.60 |
0.88 |
|
| D3 | 67.80 | 75.70 | 58.90 | 102.30 | 88.20 | 94.30 |
0.018 |
|
| D5 | 92.50 | 78.10 | 85.30 | 98.90 | 112.30 | 123.70 |
0.024 |
|
| D7 | 82.70 | 78.30 | 104.20 | 109.50 | 128.60 | 98.60 |
0.045 |
| GALC | D1 | 62.70 | 39.80 | 31.30 | 59.70 | 70.80 | 64.20 |
0.40 |
|
| D3 | 61.80 | 38.70 | 58.50 | 49.60 | 104.60 | 86.70 |
0.16 |
|
| D5 | 73.60 | 81.80 | 85.60 | 126.40 | 116.70 | 103.80 |
0.044 |
|
| D7 | 113.50 | 92.70 | 79.80 | 148.90 | 126.60 | 142.10 |
0.010 |
Discussion
In vertebrates, NTDs originate from a failure in
morphogenetic events that occur during the neurulation process
(16). Nerve tubes and nerve
sheaths are the origins of the nervous system. Nerve epithelial
cells are NSCs that possess a variety of differentiation potentials
during the development of nerve tubes (16). The normal proliferation and
differentiation of NSCs is involved in the normal development of
nerve tubes (17). Moreover, the
development of the neural tube can be easily influenced by internal
and external factors. For example, folic acid deficiency during
pregnancy (18), use of
antiepileptic drugs (19), exposure
to heavy metals (20) and
pesticides (21) all increase the
risk of NTDs. Previous studies have reported that abnormal
development of nerve epithelium is associated with NTDs, which
occurs as the result of the differentiation of NSCs under various
regulatory mechanisms, such as convergent extension, apical
constriction, interkinetic nuclear migration and multiple signaling
pathway (22). Furthermore, NSCs
have the ability to self-renewal and diversify (23,24).
atRA is a powerful inducer that can cause NTDs.
Previous studies have revealed that atRA-induced NTDs are
associated with the abnormal expression of Smad protein (25,26).
atRA induces significant alterations in the expression of various
stemness and differentiation genes associated with neuro-glial
differentiation in NSCs (22).
However, there is little research on the effect of atRA on the
proliferation and differentiation of NSCs. Therefore, the primary
focus of the present study was to investigate the effect of atRA on
N1 expression in NSCs to identify the teratogenic mechanism of
atRA.
The Notch signaling pathway influences the
development of multiple biological functions, including
differentiation, proliferation and apoptosis (27). Notch signaling has previously been
reported to be inhibited during atRA-induced glioblastoma stem cell
growth inhibition (28). In the
present study, the effects of atRA on primary neurulations were
investigated during neurogenesis. It was identified that N1
expression was downregulated in atRA-treated E11-17 embryos
compared with that in the controls. The present results indicated
that atRA promoted neural tube differentiation via the suppression
of N1 activation.
To further investigate the effects of N1 in
atRA-induced stem cells fate, primary NSCs were extracted from the
fetal brain of 18.5-day pregnant mice. After differentiation of the
culture medium to cultivate NSCs, cells could be divided into
neurons, astrocytes and oligodendrocytes, as indicated via
morphology and immunofluorescence identification. It was
demonstrated that NSCs had self-renewal and multidirectional
differentiation capabilities, which was consistent with previous
studies (29,30). Moreover, the expression of N1 on the
cell membrane and cytoplasm of differentiated neurons, astrocytes
and oligodendrocytes was detected via co-immunofluorescence, and it
was suggested that N1 was associated with the differentiation of
NSCs.
The Notch signal pathway serves a regulatory role in
cell proliferation and differentiation (8). The ubiquitination of PS1 by C.
elegans SEL-10 targets PS1 for degradation via the
ubiquitin-proteasome system and antagonizes the activity of the
Notch signaling pathway (31).
Previous studies have reported that PS1, the main factor of the
presenilin γ-secretase enzymes, activates the downstream molecules
of the Notch signaling pathway (31–33).
In accordance with the present results of the in vivo study,
N1 expression was downregulated in atRA-treated NSCs. Furthermore,
the expression of PS1, as the target of atRA in Notch signaling,
was detected. The results demonstrated that the PS1 was also
downregulated in atRA-treated NSCs.
Based on the present results, western blotting was
conducted to further detect the expression levels of N1 and PS1 and
the differentiation of NSCs with or without atRA. In untreated
NSCs, the expression levels of both N1 and PS1 were regularly
decreased, but there were significant increases in NF, GALC and
GFAP expression levels in a time-dependent manner. It was suggested
that the decreased expression levels of N1 and PS1 were
synchronous, and when the expression of both of these factors
declined the markers of neurons, astrocytes and oligodendrocytes,
including NF, GALC and GFAP, were significantly increased.
Moreover, it was indicated that the decrease of N1 expression, to a
certain degree, significantly promoted the differentiation of NSCs.
After the treatment of NSCs with atRA, the expression levels of N1
and PS1 gradually decreased, but the expression levels of NF, GALC
and GFAP were increased significantly. Thus, the present results
demonstrated that after atRA treatment, the differentiation ability
of NSCs was increased.
In NTDs, the role of Notch1 signaling pathway is
complex. Recent research has revealed the inhibition of N1 by
suppressing the RhoA/ROCK1 signaling pathway caused a decreased in
the expression levels of its target genes, hes family bHLH
transcription factor 1 (Hes1) and Hes5, which led to the promotion
of neuronal differentiation (34).
To the best of our knowledge, the present study provided the first
evidence that N1 inhibits NSC differentiation via the activation of
PS1. After NSCs were treated with atRA, the expression of N1 was
gradually decreased via the inhibition of PS1, and the markers of
the differentiated cells, such as neurons, astrocytes and
oligodendrocytes, were significantly increased, indicating that
atRA promoted the differentiation of NSCs by inhibiting PS1.
However, the present study has some limitations. For example, the
changes of N1 expression were studied only at the protein level,
and the downstream factors associated with the Notch pathways were
not further investigated. Thus, future studies will examine the
relationship between the target genes Hes1 and Hes5 and the N1
signaling pathway in NTDs at the gene and protein levels.
Collectively, the present results provided evidence
to improve the understanding of the molecular mechanism of NTDs,
such as brain bulging, meningeal membrane bulging and recessive
spinal bifida, which are caused by increased amounts of atRA. The
present study demonstrated the role of atRA in the successful
development of the neural tube, as well as identified the common
etiology for a spectrum of idiopathic anomalies that characterize
certain human congenital disorders. These findings highlighted the
molecular and teratogenic actions of atRA and may contribute to the
development of potential novel treatments for NTDs in the
future.
In conclusion, the present study demonstrated that
atRA mainly promoted the differentiation of NSCs by inhibiting the
action of γ-secretase enzymes, which activated the Notch signal
pathway. These conclusions provide not only a theoretical basis for
the future clinical application of NSCs, but also a novel treatment
window for the prevention of fetal NTDs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shandong
Natural Foundation, China (grant no. ZR2017MH021), the National
Natural Science Foundation (grant no. 81400166) and the Key
Research and Development Program of Shandong Province (grant no.
2018GSF118157).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
NC, BH and AM designed the study and edited the
manuscript. JX, SL, HC and XZ performed the experiments. WZ and YS
analyzed the data. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
All the experiments complied with the guidance by
the Animal Use and Care of Shandong Provincial Hospital Affiliated
to Shandong First Medical University and the agents were approved
by the Ethical Committee of Animal Care and Use. The research was
approved by the Medical Ethics Committee of Shandong Provincial
Hospital Affiliated to Shandong First Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zaganjor I, Sekkarie A, Tsang BL, Williams
J, Razzaghi H, Mulinare J, Sniezek JE, Cannon MJ and Rosenthal J:
Describing the Prevalence of Neural Tube Defects Worldwide: A
Systematic Literature Review. PLoS One. 11:e01515862016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Copp AJ and Greene ND: Neural tube defects
- disorders of neurulation and related embryonic processes. Wiley
Interdiscip Rev Dev Biol. 2:213–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Copp AJ, Stanier P and Greene ND: Neural
tube defects: Recent advances, unsolved questions, and
controversies. Lancet Neurol. 12:799–810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lowery LA and Sive H: Strategies of
vertebrate neurulation and a re-evaluation of teleost neural tube
formation. Mech Dev. 121:1189–1197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nikolopoulou E, Galea GL, Rolo A, Greene
ND and Copp AJ: Neural tube closure: cellular, molecular and
biomechanical mechanisms. Development. 144:552–566. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang S, Yin N and Faiola F: Human
Pluripotent Stem Cells as Tools for Predicting Developmental Neural
Toxicity of Chemicals: Strategies, Applications, and Challenges.
Stem Cells Dev. 28:755–768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daadi MM: Generation of Neural Stem Cells
from Induced Pluripotent Stem Cells. Methods Mol Biol. 1919:1–7.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andersson ER, Sandberg R and Lendahl U:
Notch signaling: Simplicity in design, versatility in function.
Development. 138:3593–3612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bray SJ: Notch signalling in context. Nat
Rev Mol Cell Biol. 17:722–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ni X, Hu G and Cai X: The success and the
challenge of all-trans retinoci acid in the treatment of
cancer. Crit Rev Food Sci Nutr. 59 (Suppl 1):S71–S80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sewell W and Kusumi K: Genetic analysis of
molecular oscillators in mammalian somitogenesis: Clues for studies
of human vertebral disorders. Birth Defects Res C Embryo Today.
81:111–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu D, Xue J, Liu Y, Gu H, Wei X, Ma W,
Luo W, Ma L, Jia S, Dong N, et al: Inhibition of NRF2 signaling and
increased reactive oxygen species during embryogenesis in a rat
model of retinoic acid-induced neural tube defects.
Neurotoxicology. 69:84–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seegmiller RE, Ford WH, Carter MW, Mitala
JJ and Powers WJ Jr: A developmental toxicity study of tretinoin
administered topically and orally to pregnant Wistar rats. J Am
Acad Dermatol. 36:S60–S66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Hajj A, Yen FT, Oster T, Malaplate C,
Pauron L, Corbier C, Lanhers MC and Claudepierre T: Age-related
changes in regiospecific expression of Lipolysis Stimulated
Receptor (LSR) in mice brain. PLoS One. 14:e02188122019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cearns MD, Escuin S, Alexandre P, Greene
ND and Copp AJ: Microtubules, polarity and vertebrate neural tube
morphogenesis. J Anat. 229:63–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McShane SG, Molè MA, Savery D, Greene ND,
Tam PP and Copp AJ: Cellular basis of neuroepithelial bending
during mouse spinal neural tube closure. Dev Biol. 404:113–124.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leung KY, Pai YJ, Chen Q, Santos C,
Calvani E, Sudiwala S, Savery D, Ralser M, Gross SS, Copp AJ, et
al: Partitioning of one-carbon units in folate and methionine
metabolism is essential for neural tube closure. Cell Rep.
21:1795–1808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nau H, Hauck RS and Ehlers K: Valproic
acid-induced neural tube defects in mouse and human: Aspects of
chirality, alternative drug development, pharmacokinetics and
possible mechanisms. Pharmacol Toxicol. 69:310–321. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin L, Zhang L, Li Z, Liu JM, Ye R and Ren
A: Placental concentrations of mercury, lead, cadmium, and arsenic
and the risk of neural tube defects in a Chinese population. Reprod
Toxicol. 35:25–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren A, Qiu X, Jin L, Ma J, Li Z, Zhang L,
Zhu H, Finnell RH and Zhu T: Association of selected persistent
organic pollutants in the placenta with the risk of neural tube
defects. Proc Natl Acad Sci USA. 108:12770–12775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanakasabai S, Pestereva E, Chearwae W,
Gupta SK, Ansari S and Bright JJ: PPARγ agonists promote
oligodendrocyte differentiation of neural stem cells by modulating
stemness and differentiation genes. PLoS One. 7:e505002012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mohlin S, Kunttas E, Persson CU, Abdel-Haq
R, Castillo A, Murko C, Bronner ME and Kerosuo L: Maintaining
multipotent trunk neural crest stem cells as self-renewing
crestospheres. Dev Biol. 447:137–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Urata Y, Yamashita W, Inoue T and Agata K:
Spatio-temporal neural stem cell behavior leads to both perfect and
imperfect structural brain regeneration in adult newts. Biol Open.
7:bio0331422018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Li R, He Q, Li WI, Niu B, Cheng
N, Zhou R, Zhang T, Zheng X and Xie J: All-trans-retinoic
acid alters Smads expression in embryonic neural tissue of mice. J
Appl Toxicol. 29:364–366. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SH, Shin JH, Shin MH, Kim YS, Chung
KS, Song JH, Kim SY, Kim EY, Jung JY, Kang YA, et al: The effects
of retinoic acid and MAPK inhibitors on phosphorylation of Smad2/3
induced by transforming growth factor β1. Tuberc Respir Dis
(Seoul). 82:42–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryu HW, Park CW and Ryu KY: Disruption of
polyubiquitin gene Ubb causes dysregulation of neural stem cell
differentiation with premature gliogenesis. Sci Rep. 4:70262014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ying M, Wang S, Sang Y, Sun P, Lal B,
Goodwin CR, Guerrero-Cazares H, Quinones-Hinojosa A, Laterra J and
Xia S: Regulation of glioblastoma stem cells by retinoic acid: Role
for Notch pathway inhibition. Oncogene. 30:3454–3467. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Filippis L, Zalfa C and Ferrari D:
Neural Stem Cells and Human Induced Pluripotent Stem Cells to Model
Rare CNS Diseases. CNS Neurol Disord Drug Targets. 16:915–926.
2017.PubMed/NCBI
|
|
30
|
Dunnett SB and Rosser AE: Cell
transplantation for Huntington's disease Should we continue? Brain
Res Bull. 72:132–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Pauley AM, Myers RL, Shuang R,
Brashler JR, Yan R, Buhl AE, Ruble C and Gurney ME: SEL-10
interacts with presenilin 1, facilitates its ubiquitination, and
alters A-beta peptide production. J Neurochem. 82:1540–1548. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duggan SP and McCarthy JV: Beyond
γ-secretase activity: The multifunctional nature of presenilins in
cell signalling pathways. Cell Signal. 28:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Strooper B, Annaert W, Cupers P, Saftig
P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS,
Ray WJ, et al: A presenilin-1-dependent gamma-secretase-like
protease mediates release of Notch intracellular domain. Nature.
398:518–522. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng Z, Li X, Fu M, Zhu K, Long L, Zhao X,
Chen Q, Deng DYB and Wan Y: Inhibition of Notch1 signaling promotes
neuronal differentiation and improves functional recovery in spinal
cord injury through suppressing the activation of Ras homolog
family member A. J Neurochem. 150:709–722. 2019. View Article : Google Scholar : PubMed/NCBI
|