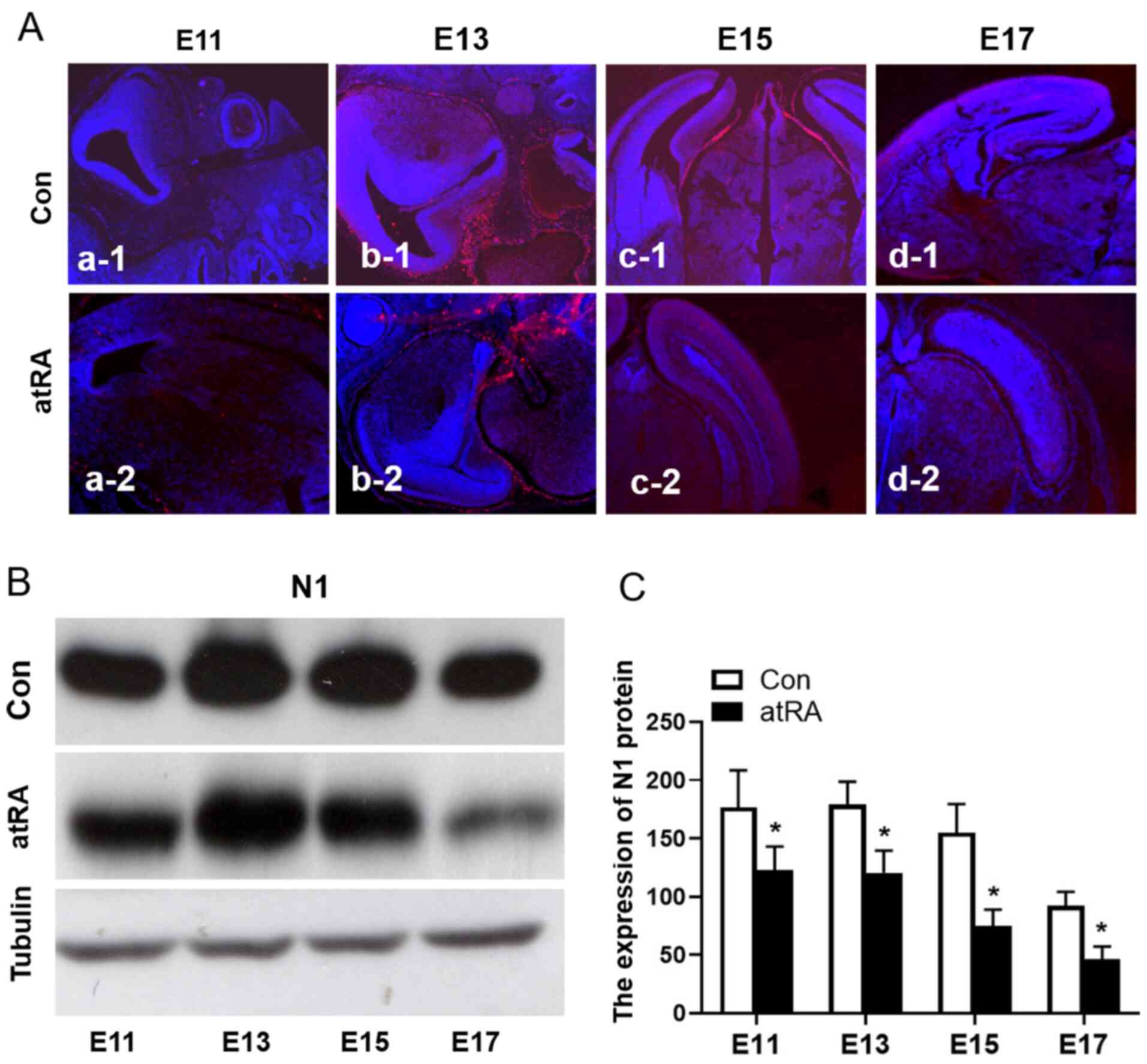
|
1
|
Zaganjor I, Sekkarie A, Tsang BL, Williams
J, Razzaghi H, Mulinare J, Sniezek JE, Cannon MJ and Rosenthal J:
Describing the Prevalence of Neural Tube Defects Worldwide: A
Systematic Literature Review. PLoS One. 11:e01515862016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Copp AJ and Greene ND: Neural tube defects
- disorders of neurulation and related embryonic processes. Wiley
Interdiscip Rev Dev Biol. 2:213–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Copp AJ, Stanier P and Greene ND: Neural
tube defects: Recent advances, unsolved questions, and
controversies. Lancet Neurol. 12:799–810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lowery LA and Sive H: Strategies of
vertebrate neurulation and a re-evaluation of teleost neural tube
formation. Mech Dev. 121:1189–1197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nikolopoulou E, Galea GL, Rolo A, Greene
ND and Copp AJ: Neural tube closure: cellular, molecular and
biomechanical mechanisms. Development. 144:552–566. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang S, Yin N and Faiola F: Human
Pluripotent Stem Cells as Tools for Predicting Developmental Neural
Toxicity of Chemicals: Strategies, Applications, and Challenges.
Stem Cells Dev. 28:755–768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daadi MM: Generation of Neural Stem Cells
from Induced Pluripotent Stem Cells. Methods Mol Biol. 1919:1–7.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andersson ER, Sandberg R and Lendahl U:
Notch signaling: Simplicity in design, versatility in function.
Development. 138:3593–3612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bray SJ: Notch signalling in context. Nat
Rev Mol Cell Biol. 17:722–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ni X, Hu G and Cai X: The success and the
challenge of all-trans retinoci acid in the treatment of
cancer. Crit Rev Food Sci Nutr. 59 (Suppl 1):S71–S80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sewell W and Kusumi K: Genetic analysis of
molecular oscillators in mammalian somitogenesis: Clues for studies
of human vertebral disorders. Birth Defects Res C Embryo Today.
81:111–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu D, Xue J, Liu Y, Gu H, Wei X, Ma W,
Luo W, Ma L, Jia S, Dong N, et al: Inhibition of NRF2 signaling and
increased reactive oxygen species during embryogenesis in a rat
model of retinoic acid-induced neural tube defects.
Neurotoxicology. 69:84–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seegmiller RE, Ford WH, Carter MW, Mitala
JJ and Powers WJ Jr: A developmental toxicity study of tretinoin
administered topically and orally to pregnant Wistar rats. J Am
Acad Dermatol. 36:S60–S66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Hajj A, Yen FT, Oster T, Malaplate C,
Pauron L, Corbier C, Lanhers MC and Claudepierre T: Age-related
changes in regiospecific expression of Lipolysis Stimulated
Receptor (LSR) in mice brain. PLoS One. 14:e02188122019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cearns MD, Escuin S, Alexandre P, Greene
ND and Copp AJ: Microtubules, polarity and vertebrate neural tube
morphogenesis. J Anat. 229:63–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McShane SG, Molè MA, Savery D, Greene ND,
Tam PP and Copp AJ: Cellular basis of neuroepithelial bending
during mouse spinal neural tube closure. Dev Biol. 404:113–124.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leung KY, Pai YJ, Chen Q, Santos C,
Calvani E, Sudiwala S, Savery D, Ralser M, Gross SS, Copp AJ, et
al: Partitioning of one-carbon units in folate and methionine
metabolism is essential for neural tube closure. Cell Rep.
21:1795–1808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nau H, Hauck RS and Ehlers K: Valproic
acid-induced neural tube defects in mouse and human: Aspects of
chirality, alternative drug development, pharmacokinetics and
possible mechanisms. Pharmacol Toxicol. 69:310–321. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin L, Zhang L, Li Z, Liu JM, Ye R and Ren
A: Placental concentrations of mercury, lead, cadmium, and arsenic
and the risk of neural tube defects in a Chinese population. Reprod
Toxicol. 35:25–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren A, Qiu X, Jin L, Ma J, Li Z, Zhang L,
Zhu H, Finnell RH and Zhu T: Association of selected persistent
organic pollutants in the placenta with the risk of neural tube
defects. Proc Natl Acad Sci USA. 108:12770–12775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanakasabai S, Pestereva E, Chearwae W,
Gupta SK, Ansari S and Bright JJ: PPARγ agonists promote
oligodendrocyte differentiation of neural stem cells by modulating
stemness and differentiation genes. PLoS One. 7:e505002012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mohlin S, Kunttas E, Persson CU, Abdel-Haq
R, Castillo A, Murko C, Bronner ME and Kerosuo L: Maintaining
multipotent trunk neural crest stem cells as self-renewing
crestospheres. Dev Biol. 447:137–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Urata Y, Yamashita W, Inoue T and Agata K:
Spatio-temporal neural stem cell behavior leads to both perfect and
imperfect structural brain regeneration in adult newts. Biol Open.
7:bio0331422018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Li R, He Q, Li WI, Niu B, Cheng
N, Zhou R, Zhang T, Zheng X and Xie J: All-trans-retinoic
acid alters Smads expression in embryonic neural tissue of mice. J
Appl Toxicol. 29:364–366. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SH, Shin JH, Shin MH, Kim YS, Chung
KS, Song JH, Kim SY, Kim EY, Jung JY, Kang YA, et al: The effects
of retinoic acid and MAPK inhibitors on phosphorylation of Smad2/3
induced by transforming growth factor β1. Tuberc Respir Dis
(Seoul). 82:42–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryu HW, Park CW and Ryu KY: Disruption of
polyubiquitin gene Ubb causes dysregulation of neural stem cell
differentiation with premature gliogenesis. Sci Rep. 4:70262014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ying M, Wang S, Sang Y, Sun P, Lal B,
Goodwin CR, Guerrero-Cazares H, Quinones-Hinojosa A, Laterra J and
Xia S: Regulation of glioblastoma stem cells by retinoic acid: Role
for Notch pathway inhibition. Oncogene. 30:3454–3467. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Filippis L, Zalfa C and Ferrari D:
Neural Stem Cells and Human Induced Pluripotent Stem Cells to Model
Rare CNS Diseases. CNS Neurol Disord Drug Targets. 16:915–926.
2017.PubMed/NCBI
|
|
30
|
Dunnett SB and Rosser AE: Cell
transplantation for Huntington's disease Should we continue? Brain
Res Bull. 72:132–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Pauley AM, Myers RL, Shuang R,
Brashler JR, Yan R, Buhl AE, Ruble C and Gurney ME: SEL-10
interacts with presenilin 1, facilitates its ubiquitination, and
alters A-beta peptide production. J Neurochem. 82:1540–1548. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duggan SP and McCarthy JV: Beyond
γ-secretase activity: The multifunctional nature of presenilins in
cell signalling pathways. Cell Signal. 28:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Strooper B, Annaert W, Cupers P, Saftig
P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS,
Ray WJ, et al: A presenilin-1-dependent gamma-secretase-like
protease mediates release of Notch intracellular domain. Nature.
398:518–522. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng Z, Li X, Fu M, Zhu K, Long L, Zhao X,
Chen Q, Deng DYB and Wan Y: Inhibition of Notch1 signaling promotes
neuronal differentiation and improves functional recovery in spinal
cord injury through suppressing the activation of Ras homolog
family member A. J Neurochem. 150:709–722. 2019. View Article : Google Scholar : PubMed/NCBI
|