Introduction
The intestinal mucosal barrier serves an important
role in the development of liver failure (1); in patients with acute liver failure
(ALF), the function of the intestinal barrier is compromised
following severe structural damage to the mucosa (2). As a result, bacteria,
lipopolysaccharide (LPS) and bacterial metabolites are more likely
to invade the bloodstream, which has been shown to aggravate liver
damage (3). The integrity of the
intestinal barrier relies on a variety of mucosal structural
components that are tightly regulated during homeostasis and
disease (4). Tight junctions are
one of the most important components of the intestinal mucosal
barrier and serve as determinants of intestinal mucosal
permeability (4). Notably, the
dysfunction of tight junctions and the subsequent increased
permeability have been demonstrated to facilitate the translocation
of bacteria and microbial products, which induces severe
inflammatory responses in the target organ (5).
A previous study reported that the intestinal mucosa
tight junctions and intestinal barrier in mice with ALF were
impaired (6). The major molecular
pathways regulating the intestinal barrier function include the
Ca2+/myosin light chain kinase (MLCK), protein kinase
C/phosphatase inhibitory protein-17 and RhoA/Rho-associated kinase
(ROCK) signaling pathways (7–9);
however, a previous study reported that only MLCK and ROCK were
involved in the destruction of tight junctions, further regulating
intestinal function (9). MLCK and
ROCK are two important kinases, which phosphorylate MLC in
vitro and in vivo (10,11).
The phosphorylation of MLC is a common intermediate in the
pathophysiological regulation of intestinal barrier function; it
mediates actin contraction and cytoskeletal rearrangements, which
results in mucosal barrier dysfunction (12). However, in different cell types,
MLCK- or ROCK-mediated MLC phosphorylation does not occur in an
identical manner (10,11,13),
and the underlying mechanisms of MLC phosphorylation and its
contribution to the disease pathogenesis in patients with ALF
remains poorly understood. Thus, the present study aimed to
investigate these processes using a mouse model of ALF.
Materials and methods
Reagents
D-galactosamine (D-GalN; cat. no. G0500) and LPS
(cat. no. L2630) were purchased from Sigma-Aldrich; Merck KGaA. The
MLCK inhibitor ML-7 (cat. no.S8388) and ROCK inhibitor Y-27632
(cat. no. S1049) were purchased from Selleck Chemicals. The
monoclonal rabbit anti-MLCK antibody (cat. no. ab76092),monoclonal
rabbit anti-ROCK antibody (cat. no. ab45171),monoclonal rabbit
anti-occludin antibody (cat. no. ab167161) and polyclonal rabbit
anti-zonula occludens-1 (ZO-1) antibody (cat. no. ab96587) were all
purchased from Abcam. Monoclonal mouse anti-phosphorylated (p)-MLC
antibody (cat. no. 3675), polyclonal rabbit anti-MLC antibody (cat.
no. 3672) and the monoclonal rabbit anti-GAPDH antibody (cat. no.
2118) were purchased from Cell Signaling Technology, Inc. The
polyclonal rabbit anti-p-MLC antibody (cat. no. PAB 19884) and the
ELISA kits for tumor necrosis factor (TNF)-α (cat. no. MU30030) and
interleukin (IL)-6 (cat. no. MU30044) were purchased from Bioswamp
Life Science Lab.
Animal studies
As there are no facilities for keeping animals in
the Central Hospital of Wuhan, Tongji Medical College Huazhong
University of Science and Technology, animal experiments were
conducted at Model Animal Research Institute of Wuhan Myhalic
Biotechnology Co., Ltd. All animal procedures in the present study
were approved by the Ethics Committee of Model Animal Research
Institute of Wuhan Myhalic Biotechnology Co., Ltd. (approval no.
HLK-20180621-01). Animal welfare was considered during the
experiment, including efforts to minimize suffering and distress.
Mice were sacrificed when they lost their appetite, 20% of their
original weight, remained immobile, exhibited fast shallow
breathing, demonstrated slowed responses or were listless. Male
specific pathogen-free-class BALB/c mice (age, 6–8 weeks; weight,
20–22 g; n=26) were purchased from the Huazhong Agricultural
University Laboratory Animal Center. The total of 26 mice included
2 extras, which were purchased as substitutes in the event of
mortality. Mice were housed in pathogen-free conditions at 22°C,
50% relative humidity and under a 12-h light/dark cycle. Mice
health and behavior were monitored every 12 h. After an initial 7
days of provision of food and water ab libitum, the mice
received subsequent intervention. Mice were randomly divided into
four groups (n=6 mice/group), one of which served as the control
group. After grouping, the remaining two mice were donated to other
researchers for experiments.
Control mice were injected with PBS
intraperitoneally. ALF was induced in the remaining three groups
through an intraperitoneal injection of 380 mg/kg D-GalN and 10
µg/kg LPS, of which the doses of the drugs were determined based on
previous studies (14,15). After 3- and 6-h of induction, mice
in each of the four groups were either injected intraperitoneally
with PBS (control group), PBS (D-GalN/LPS group), 2 µg/gML-7
(D-GalN/LPS + MLCK inhibition group) or 20 µg/g Y-27632 (D-GalN/LPS
+ ROCK inhibition group). Mice health and behavior were monitored
every 4 h after intraperitoneal injection of D-GalN/LPS.
The mice were sacrificed with 100 mg/kg sodium
pentobarbital via intraperitoneal injection 12 h after
intraperitoneal injection of D-GalN/LPS; death was confirmed by
observing respiration and by using the corneal reflection method.
Blood and tissue samples were collected after the mice were
sacrificed. Briefly, the abdomen was opened, the portal vein was
exposed and blood was aspirated into a syringe. The blood samples
were allowed to stand for 4 to 5 h, prior to being centrifuged at
3,000 × g for 5 min at 4°C and stored at −80°C. The liver tissue
samples were washed in PBS to remove excess blood and the samples
were subsequently fixed in 40 g/l paraformaldehyde (PFA) for 24 h
at 4°C or frozen immediately at −80°C. The terminal 3 cm of the
small intestinal tissues were also excised and fixed in 40 g/l PFA
or frozen immediately at −80°C. It took ~2 min to collect blood and
tissue samples from each mouse.
Morphological analysis using
hematoxylin and eosin (H&E) staining and scanning electron
microscopy (SEM)
Standard H&E staining was performed to assess
the overall morphology. Briefly, the fixed liver and small
intestine tissues were fixed in 40 g/l PFA at 4°C for 12 h,
paraffin embedded and sectioned routinely for H&E staining.
Histomorphology was observed with a light microscope. For SEM, the
small intestine tissues were fixed in 2.5% glutaraldehyde at 4°C
for 6 h and 1% osmium tetroxide (OsO4) at 4°C for 2 h,
dehydrated in ethanol, dried by the critical-point method and
coated with a thin layer of gold. The intestinal mucosa was
observed by scanning electron microscope (cat. no. SNE-3000MB;
SEC).
Transmission electron microscopy
(TEM)
Mice were anesthetized with an intraperitoneal
injection of 100 mg/kg (2%) sodium pentobarbital. Mice were
sacrificed prior to small intestine collection. The small intestine
was rapidly removed from each mouse and fixed in 2.5%
glutaraldehyde. Subsequently, 1-mm2 small intestine
tissue sample was obtained using a sharp blade and fixed with 2.5%
glutaraldehyde in 0.1 M PBS at 4°C for 2–4 h. The samples were
washed three times for 10 min each in PBS and post-fixed in 1%
OsO4 at 4°C for 1 h. Subsequently, sections with a
thickness of 60 nm were dehydrated in acetone and embedded in epoxy
resin 618 at 40°C for 4 h. Areas of interest were selected from the
semi-thin sections and stained with aqueous uranyl acetate at 4°C
for 20 min, followed by lead citrate at 4°C for 15 min. The
sections were examined using an HT-7700 transmission electron
microscope (Hitachi, Ltd.).
Immunofluorescence staining
There are two major types of immunofluorescence
staining methods: i) Direct immunofluorescence staining in which
the primary antibody is labeled with fluorescence dye and ii)
indirect immunofluorescence staining in which a secondary antibody
labeled with fluorochrome is used to recognize the primary
antibody; the unlabeled antibody specific for the molecule of
interest is called the primary antibody. This section introduced
the indirect immunofluorescence staining method to detect p-MLC.
The small intestine tissues were paraffin-embedded, 10%
formaldehyde fixed at 4°C for 12 h and sliced with a thickness of 4
µm. The slides were washed with PBS and underwent antigen retrieval
using 0.1% trypsin at 37°C for 10 min, prior to being washed three
times with PBS. The slides were incubated with 3%
H2O2 at room temperature for 10 min, washed
three times in PBS for 3 min each and incubated with anti-p-MLC
primary antibody (cat. no. PAB 19884; Bioswamp Life Science Lab.;
1:100) overnight at 4°C. Following the primary antibody incubation,
the slides were washed three times in PBS for 5 min each and
incubated with an Alexa Fluor 594 goat anti-rabbit IgG secondary
antibody (PAB160018, Bioswamp Life Science Lab) at a 1:200 dilution
at 37°C for 1 h. After a final wash with PBS, the slides were
mounted using anti-fluorescence quenching sealing solution at 4°C
for 5 min, and stained cells were visualized using an Olympus CX41
fluorescent microscope (Olympus Corporation).
Western blotting
Total protein was extracted using 1% Triton X-100
from the small intestine tissue. Total protein was quantified using
bicinchoninic acid protein concentration assay kit (Bioswamp Life
Science Lab) and proteins were separated by SDS-PAGE. The
concentration of protein in each lane was 20 µg and 12% gel was
used. The separated proteins were transferred onto polyvinylidene
difluoride membranes and blocked with 5% non-fat milk in
2,4,6-trinitrobenzenesul-phonic acid (TNBS) for 2 h at room
temperature. The membranes were then incubated with the following
primary antibodies overnight at 4°C: Anti-MLCK (1:5,000), anti-ROCK
(1:5,000), anti-occludin (1:50,000), anti-ZO-1 (1:1,000),
anti-p-MLC (1:1,000), anti-MLC (1:1,000) and anti-GAPDH (1:1,000).
Following the primary antibody incubation, the membranes were
washed three times for 10 min each in TNBS and incubated with
corresponding horseradish peroxidase-conjugated secondary
antibodies (1:1,000) at room temperature (goat anti-rabbit IgG,
cat. no. PAB150011;goat anti-mouse IgG, cat. no. PAB150009,
Bioswamp Life Science Lab) for 1 h. Protein bands were visualized
using an electrochemiluminescence reagent (EMD Millipore) and a
Tanon 5200 automatic chemiluminescence image analysis system (Tanon
Science and Technology Co., Ltd.). Protein expression levels were
analyzed using BandScan5.0 software (Glyko, Inc.).
ELISA
After the blood samples were allowed to stand for 4
to 5 h, serum was obtained after centrifugation at 3,000 × g for 5
min at 4°C. To determine the concentrations of TNF-α and IL-6 in
the serum, TNF-α and IL-6 ELISA kits were used according to the
manufacturer's protocol. The absorbance was measured using a
Multiskan MS 352 microplate reader (Thermo Fisher Scientific, Inc.)
at a wavelength of 450 nm. All the samples were thawed once and
assayed in triplicate.
Statistical analysis
After the pre-experiment, western blotting was
repeated twice and the other tests were repeated three times.
Statistical analysis was performed using SPSS 13.0 software (SPSS,
Inc.) and quantitative data are presented as the mean ± standard
deviation. Statistical differences were determined using one-way
ANOVA followed by Tukey's post hoc analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
D-GalN/LPS induces hepatotoxicity
similar to the pathology of ALF
D-GalN/LPS is an established model of hepatotoxicity
that closely resembles clinical cases of ALF (11,12).
In the present study, D-GalN/LPS stimulation was demonstrated to
induce hepatotoxicity in mice, which was determined through the
increased inflammatory cell infiltration and the accumulating
presence of hepatocyte necrosis (Fig.
1). By contrast, the control mice exhibited normal liver
morphology, without inflammatory cell infiltration (Fig. 1). Notably, the inhibition of MLCK
with ML-7 or the inhibition of ROCK with Y-27632 markedly reduced
the D-GalN/LPS-induced hepatotoxicity and inflammatory cell
infiltration. These findings suggested that D-GalN/LPS induced ALF
in mice, and inhibition of MLCK or ROCK may be able to reduce
inflammatory cell infiltration and liver damage.
D-GalN/LPS-induced mice exhibit
severely damaged intestinal mucosa
Compared with the control mice, the
D-GalN/LPS-induced mice exhibited signs of severe mucosal atrophy,
microvilli membrane shedding, lamina propria inflammation and edema
(Fig. 2A). In addition, SEM
revealed a poorly organized brush border with irregular and reduced
microvilli on the intestinal surface; the surface of the microvilli
was rough, uneven and incomplete (Fig.
2B). However, the inhibition of either MLCK or ROCK markedly
reduced the damage to the intestinal mucosa, with less inflammatory
cell infiltration and mild mucosal atrophy being observed in the
D-GalN/LPS+MLCK inhibition group and the D-GalN/LPS + ROCK
inhibition group compared with those in the D-GalN/LPS group
(Fig. 2A and B).
Intestinal epithelial tight junctions
are disrupted in D-GalN/LPS-induced mice
TEM analysis demonstrated that the intestinal
epithelial tight junctions of the control mice remained tight and
intact (Fig. 3). By contrast,
dilated tight junctions were observed in D-GalN/LPS-induced mice,
alongside sparse, irregular microvilli with signs of partial
shedding, and an expanded endoplasmic reticulum (Fig. 3). In both the D-GalN/LPS + MLCK
inhibition and the D-GalN/LPS + ROCK inhibition groups, the tight
junctions were revealed to be slightly dilated and the microvilli
shedding was reduced compared with those in the D-GalN/LPS group
(Fig. 3).
p-MLC expression levels are
significantly increased in D-GalN/LPS-induced mice
Immunofluorescence analysis of the intestinal tissue
confirmed the presence of increased expression levels of p-MLC in
the small intestine of D-GalN/LPS-induced mice, whereas the
inhibition of ROCK or MLCK reduced p-MLC protein expression levels
(Fig. 4A). Western blotting also
confirmed that the expression levels of p-MLC were significantly
increased in D-GalN/LPS-induced mice compared with those in the
control mice (Fig. 4B and C).
Furthermore, the expression levels of p-MLC in the D-GalN/LPS +
MLCK inhibition group and D-GalN/LPS + ROCK inhibition group were
significantly decreased compared with those in the
D-GalN/LPS-treated group, which suggested that both MLCK and ROCK
may be involved in regulating MLC phosphorylation (Fig. 4B and C).
Increased expression levels of MLCK
and ROCK are associated with tight junction protein
alternations
Since MLCK and ROCK inhibition significantly reduced
the expression levels of p-MLC in the D-GalN/LPS-induced mice, it
was subsequently investigated whether MLCK and ROCK expression
levels were increased in these mice. As hypothesized, MLCK and ROCK
expression levels were revealed to be significantly increased in
the D-GalN/LPS-induced mice compared with those in the control mice
(Fig. 5). The phosphorylation of
MLC serves a central role in regulating barrier function, thus
whether the increased p-MLC expression levels altered tight
junction protein levels was investigated. Tight junction proteins
include occludin and ZO-1. Therefore, the present study detected
the expression levels of occludin and ZO-1. Compared to those in
the control group, the protein expression levels of occludin and
ZO-1 were significantly decreased in the D-GalN/LPS-induced mice
(Fig. 5), whereas inhibition of
MLCK and ROCK significantly reduced MLCK and ROCK expression
levels, whilst simultaneously increasing occludin and ZO-1 levels
(Fig. 5).
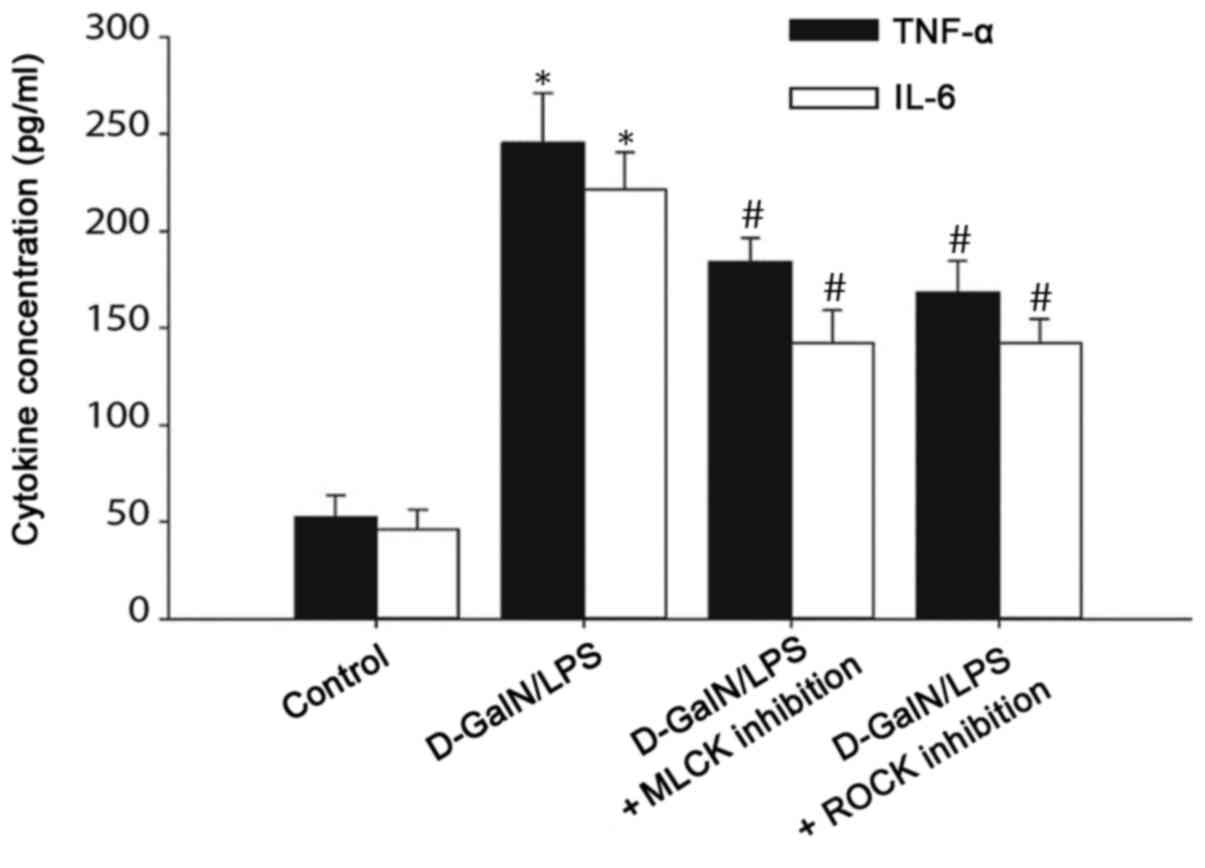
Serum TNF-α and IL-6 levels are
increased in D-GalN/LPS-induced mice
The immune response serves a pivotal role in the
pathogenesis of liver failure, and the expression of inflammatory
cytokines, including IL-6 and TNF-α, has been associated with ALF
(16). Thus, ELISA was performed to
analyze the serum levels of TNF-α and IL-6 in D-GalN/LPS-induced
mice. The serum levels of TNF-α and IL-6 were significantly
increased in the D-GalN/LPS-treated mice compared with those in the
control mice. By contrast, the inhibition of MLCK or ROCK
significantly reduced serum TNF-α and IL-6 levels compared with
those in the D-GalN/LPS group (Fig.
6). These data suggested that the tight junction dysregulation
observed in the D-GalN/LPS-induced mice may be mediated through
TNF-α and IL-6 signaling, and the inhibition of MLC phosphorylation
may be able to reduce TNF-α and IL-6 signaling.
Discussion
Epithelial tight junctions are important structures
that regulate the permeability of the intestinal epithelial barrier
(4). Increased intestinal
permeability often accompanies liver disease (2); however, the roles of permeability
defects and intestinal barrier dysfunction in disease pathogenesis
remain poorly understood. Thus, the present study aimed to define
the molecular mechanisms involved in barrier dysfunction and
increased permeability during disease progression.
D-GalN/LPS-induced acute liver injury is a
well-established in vivo model of ALF (17,18).
In this model, LPS, an endotoxin, activates macrophages and Kupffer
cells to produce TNF-α, which activates a variety of cytokines that
mediate cell death and inflammation (19). Meanwhile, D-GalN is a selective
hepatotoxin that depletes uridine nucleotides in the liver, which
is subsequently found to inhibit RNA synthesis in hepatocytes and
enhance the toxicity of TNF-α (20). In previous studies, it was reported
that mice who received D-GalN and LPS via intraperitoneal injection
to induce acute liver injury exhibited signs of rapid cell death
that closely resembled clinical cases of ALF (14,15).
In the present study, an increased prevalence of hepatocyte
necrosis and inflammatory cell infiltration was observed in the
D-GalN/LPS-induced mice. The diagnosis of ALF includes ALT, AST and
pathology (21). It was limitation
of the present study that ALT and AST were not detected. ALT and
AST detection would be more useful in the diagnosis of ALF.
The phosphorylation of MLC is a common intermediate
in the pathophysiological regulation of the mucosal barrier
(12). Previously, studies have
reported that MLC can be phosphorylated by both MLCK and ROCK
(10,11); however, although both kinases share
the same phosphorylation sites, each has been demonstrated to have
distinct roles in the spatial regulation of MLC phosphorylation
(22,23). This phenomenon has been observed in
different cells and species, thus suggesting that either the joint
or single action of MLCK and ROCK serves the leading role depending
on the cell or species type. For example, in an inflammatory bowel
disease model, the activation of MLCK led to tight junction
dysfunction and a dysregulated intestinal mucosal barrier in
early-stage disease, whereas in the advanced stages, the loss of
tight junction function was revealed to be independent of MLCK
(24). During the mycophenolic
acid-mediated barrier function test of the human colon cancer cell
line Caco-2,MLC and MLCK were also discovered to be involved in the
destruction of tight junctions and changes in the cell permeability
(25); however, whether ROCK was
involved in the destruction of the tight junctions was unclear. It
is well established that the intestinal barrier is dynamically
regulated (26). The present study
demonstrated that in ALF model mice, the expression levels of p-MLC
were significantly increased, and both MLCK and ROCK were suggested
to be involved in the phosphorylation of MLC, because the
inhibition of either of them significantly reduced p-MLC expression
levels. However, the differences between the MLCK/ROCK inhibition
groups and the control group suggested that both may be required or
that other signaling pathways may also be involved.
Cytokines are critical for intercellular
communication and mucosal homeostasis, and they are also important
drivers of intestinal inflammation and mucosal damage (27). Thus, it was hypothesized that the
inflammatory factors TNF-α and IL-6 may initially trigger tight
junction dysregulation. For example, a previous study reported that
the morphological abnormalities in the intestinal mucosa of ALF
mice were positively associated with serum TNF-α levels, whereas
TNF-α antibodies significantly prevented changes in the intestinal
tissue ultrastructure and ZO-1 expression (6). Unfortunately, this previous study did
not investigate the mechanisms of intestinal mucosal damage and
tight junction molecular changes (6). In addition, anti-TNF-α antibody
therapy is widely used to treat Crohn's disease and ulcerative
colitis, demonstrating its important role in regulating intestinal
permeability (28,29). There are several lines of evidence
indicating the effect of IL-6 on paracellular permeability; for
example, in IL-6-knockout mice, the increased intestinal
permeability to small molecules was linked to the stability of ZO-1
in tight junctions (30). In
addition, IL-6 treatment increased the permeability across
endothelial cells through inducing the aberrant localization of
ZO-1 and actin structure remodeling, of which these effects were
reversible (31). The present study
demonstrated that TNF-α and IL-6 levels were significantly
increased in the ALF model mice; unfortunately, other cytokines
were not tested in the present study, such as IL-1,IL-8 and IL-12.
Notably, the inhibition of MLCK or ROCK significantly reduced serum
levels of TNF-α and IL-6, and prevented tight junction
dysregulation. These data suggested that in ALF model mice, MLCK
and ROCK signaling may be mediated by TNF-α and IL-6. However, the
manner in which cytokines activate MLCK and ROCK remains largely
unclear. A previous study suggested that TNF-α induces increases in
intestinal permeability, which contributes to inflammation in
disease conditions; this was mediated through NF-κB activation,
involving the NF-κB p50/p65 dimer binding to the MLCK promoter
region (32). A comprehensive
understanding of the signaling cascade during disease progression
will be the focus of future studies, including immunofluorescence
analysis to detect occludin, ZO-1 and other tight junction proteins
for further elucidation of tight junction function.
In conclusion, the findings of the present study
suggested that D-GalN/LPS treatment may induce ALF, which was
accompanied by severe damage to the intestinal mucosa and barrier
function. The signal transduction pathways that regulate intestinal
barrier dysfunction were also investigated, and both MLCK and ROCK
were discovered to be responsible for MLC phosphorylation in the
ALF model mice. Increased TNF-α and IL-6 levels due to liver damage
were also demonstrated to be a possible trigger for mucosal damage,
which led to increased intestinal permeability. Thus, targeting
both MLCK and ROCK may be therapeutically effective to stabilize
barrier function and reduce bacterial translocation in patients
with ALF.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science
Foundation of Hubei Province (grant no. 2016CFB357).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
FW and DZ conceived and designed the study. FW, HL,
HZ, YL, JW and HR acquired and analyzed the data. FW, JW and DZ
confirmed the authenticity of all the raw data. FW prepared the
draft of the manuscript, including the figures. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures in the present study were
approved by the Ethics Committee of Model Animal Research Institute
of Wuhan Myhalic Biotechnology Co., Ltd. (approval no.
HLK-20180621-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vancamelbeke M and Vermeire S: The
intestinal barrier: A fundamental role in health and disease.
Expert Rev Gastroenterol Hepatol. 11:821–834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aguirre Valadez JM, Rivera-Espinosa L,
Mendez-Guerrero O, Chavez-Pacheco JL, Juarez IG and Torre A:
Intestinal permeability in a patient with liver cirrhosis. TherClin
Risk Manag. 12:1729–1748. 2016. View Article : Google Scholar
|
|
3
|
Wiest R, Lawson M and Geuking M:
Pathological bacterial translocation in liver cirrhosis. J Hepatol.
60:197–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Odenwald MA and Turner JR: The intestinal
epithelial barrier: A therapeutic target? Nat Rev Gastroenterol
Hepatol. 14:9–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelly JR, Kennedy PJ, Cryan JF, Dinan TG,
Clarke G and Hyland NP: Breaking down the barriers: The gut
microbiome, intestinal permeability and stress-related psychiatric
disorders. Front Cell Neurosci. 14:3922015.
|
|
6
|
Song HL, Lv S and Liu P: The roles of
tumor necrosis factor-alpha in colon tight junction protein
expression and intestinal mucosa structure in a mouse model of
acute liver failure. BMC Gastroenterol. 9:702009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murthy KS: Signaling for contraction and
relaxation in smooth muscle of the gut. Annu Rev Physiol.
68:345–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rattan S, Phillips BR and Maxwell PJ 4th:
RhoA/Rho-kinase: Pathophysiologic and therapeutic implications in
gastrointestinal smooth muscle tone and relaxation.
Gastroenterology. 138:13–18.e1-3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zolotarevsky Y, Hecht G, Koutsouris A,
Gonzalez DE, Quan C, Tom J, Mrsny RJ and Turner JR: A
membrane-permeant peptide that inhibits MLC kinase restores barrier
function in in vitro models of intestinal disease.
Gastroenterology. 123:163–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rattan S: Ca2+/calmodulin/MLCK pathway
initiates, and RhoA/ROCK maintains, the internal anal sphincter
smooth muscle tone. Am J Physiol Gastrointest Liver Physiol.
312:G63–G66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong Y, Wang C, Shi L, Wang L, Zhou Z,
Chen D, Wang J and Guo H: Myosin light chain kinase: A potential
target for treatment of inflammatory diseases. Front Pharmacol.
8:2922017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cunningham KE and Turner JR: Myosin light
chain kinase: Pulling the strings of epithelial tight junction
function. Ann N Y Acad Sci. 1258:34–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rigor RR, Shen Q, Pivetti CD, Wu MH and
Yuan SY: Myosin light chain kinase signaling in endothelial barrier
dysfunction. Med Res Rev. 33:911–933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Tian L and Jiang K: Propofol
attenuates inflammatory response and apoptosis to protect
d-galactosamine/lipopolysaccharide induced acute liver injury via
regulating TLR4/NF-κB/NLRP3 pathway. Int Immunopharmacol.
77:1059742019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Wang T, Liu X, Cai L, Qi J, Zhang P
and Li Y: Biochanin A protects
lipopolysaccharide/D-galactosamine-induced acute liver injury in
mice by activating the Nrf2 pathway and inhibiting NLRP3
inflammasome activation. Int Immunopharmacol. 38:324–331. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simpson KJ, Henderson NC, Bone-Larson CL,
Lukacs NW, Hogaboam CM and Kunkel SL: Chemokines in the
pathogenesis of liver disease: So many players with poorly defined
roles. Clin Sci (Lond). 104:47–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rietschel ET, Kirikae T, Schade FU, Mamat
U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova
F, et al: Bacterial endotoxin: Molecular relationships of structure
to activity and function. FASEB J. 8:217–225. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belanger M and Butterworth RF: Acute liver
failure: A critical appraisal of available animal models. Metab
Brain Dis. 20:409–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawaratani H, Tsujimoto T, Douhara A,
Takaya H, Moriya K, Namisaki T, Noguchi R, Yoshiji H, Fujimoto M
and Fukui H: The effect of inflammatory cytokines in alcoholic
liver disease. Mediators Inflamm. 2013:4951562013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Silverstein R: D-galactosamine lethality
model: Scope and limitations. J Endotoxin Res. 10:147–162. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Gao LN, Cui YL and Jiang HL:
Protective effect of danhong injection on acute hepatic failure
induced by lipopolysaccharide and d-galactosamine in mice. Evid
Based Complement Alternat Med. 2014:1539022014.PubMed/NCBI
|
|
22
|
Totsukawa G, Yamakita Y, Yamashiro S,
Hartshorne DJ, Sasaki Y and Matsumura F: Distinct roles of ROCK
(Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation
for assembly of stress fibers and focal adhesions in 3T3
fibroblasts. J Cell Biol. 150:797–806. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kassianidou E, Hughes JH and Kumar S:
Activation of ROCK and MLCK tunes regional stress fiber formation
and mechanics via preferential myosin light chain phosphorylation.
Mol Biol Cell. 28:3832–3843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su L, Nalle SC, Shen L, Turner ES, Singh
G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai PY, Fu YX, et al:
TNFR2 activates MLCK-dependent tight junction dysregulation to
cause apoptosis-mediated barrier loss and experimental colitis.
Gastroenterology. 145:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qasim M, Rahman h, Ahmed R, Oellerich M
and Asif AR: Mycophenolic acid mediated disruption of the
intestinal epithelial tight junctions. Exp Cell Res. 322:277–289.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nusrat A, Turner JR and Madara JL:
Molecular physiology and pathophysiology of tight junctions. IV.
Regulation of tight junctions by extracellular stimuli: Nutrients,
cytokines, and immune cells. Am J Physiol Gastrointest Liver
Physiol. 279:G851–G857. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andrews C, McLean MH and Durum SK:
Cytokine tuning of intestinal epithelial function. Front Immunol.
9:12702018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gibson PR: Increased gut permeability in
Crohn's disease: Is TNF the link? Gut. 53:1724–1725. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sands BE and Kaplan GG: The role of
TNFalpha in ulcerative colitis. J Clin Pharmacol. 47:930–941. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang R, Han X, Uchiyama T, Watkins SK,
Yaguchi A, Delude RL and Fink MP: IL-6 is essential for development
of gut barrier dysfunction after hemorrhagic shock and
resuscitation in mice. Am J Physiol Gastrointest Liver Physiol.
285:G621–G629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maruo N, Morita I, Shirao M and Murota S:
IL-6 increases endothelial permeability in vitro. Endocrinology.
131:710–714. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye X and Sun M: AGR2 ameliorates tumor
necrosis factor-α-induced epithelial barrier dysfunction via
suppression of NF-κB p65-mediated MLCK/p-MLC pathway activation.
Int J Mol Med. 39:1206–1214. 2017. View Article : Google Scholar : PubMed/NCBI
|