Introduction
Systemic lupus erythematosus (SLE) is a severe
autoimmune disorder that affects several organs, such as the skin,
kidney, heart, joints, and the central nervous system (1,2).
Despite an imperfect understanding of the pathogenesis of SLE,
significant gains have been made in recent years. One of the
principal pathophysiological characteristics of SLE is the
dysfunction of various immunocyte populations, including
macrophages, dendritic cells, neutrophils and peripheral blood
mononuclear cells (PBMCs) such as B cells and CD4+ T
cells, resulting in changes in inflammatory and immune responses
(3). Increased understanding of the
pathogenesis of SLE can help to develop targeted immunotherapy in
treating patients with SLE (4).
Circular RNAs (circRNAs) are a novel class of
noncoding RNA molecules formed by a covalently closed loop
structure without a 5′cap or 3′poly A tail; they are widely
expressed in mammals (5). Growing
evidence has shown that circRNAs play critical roles in the course
of multiple diseases (6). Some
studies have shown that circRNAs play important roles in SLE
(7,8). CircRNAs can act as novel biomarkers
for SLE; for example, circRNA_002453 might serve as a biomarker for
diagnosing lupus nephritis (9);
circPTPN22 may function as a potential activity indicator in SLE
(10). CircRNAs such as circIBTK
(11), hsa_circ_0045272 (12) and hsa_circ_0012919 (13) are also involved in SLE disease
progression. Nevertheless, few studies have been done on circRNAs
in SLE, and their roles in SLE are unclear.
Therefore, the present study investigated the
expression and function of circRNAs in SLE. Hsa_circ_0010957 is
encoded within the SEPN1 gene locus and is highly expressed in
SLE-derived CD4+ T cells, as demonstrated by circRNA
microarray analysis (13). The
expression of hsa_circ_0010957 was verified in SLE-derived
CD4+ T cells and healthy controls (HCs), and its
function in SLE progression was investigated.
Materials and methods
Subjects
Thirty patients diagnosed with SLE (three males and
twenty-seven females; mean age, 32.78±7.42) and twenty-five age-
and sex-matched HCs (two males and twenty-three females; mean age,
33.12±7.31) were recruited from Jinhua Municipal Central Hospital.
This study was approved by the Human Ethics Committee of Jinhua
Municipal Central Hospital and written informed consents were
obtained from all participants in this study. The diagnostic
criteria for SLE were according to the 2012 Systemic Lupus
International Collaborating Clinic (SLICC) revised criteria for
classification of SLE (14). The
SLEDAI scoring system was applied to assess disease activity, and
SLEDAI score >4 was considered as active disease.
CD4+ T cell separation,
culture and treatment
PBMCs were separated from peripheral venous blood
using Ficoll-Paque (Sigma-Aldrich; Merck KGaA). CD4+ T
cells were selected by Miltenyi beads (Miltenyi Biotec GmbH). Cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 20% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) in an incubator at 37°C containing 5%
CO2. CD4+ T cells were treated with
interleukin (IL)-6 (10 ng/ml, 24 h; cat. no. HY-P7044,
MedChemExpress) (15).
Transfection
Human T Cell Nucleofector kit (Lonza Group, Ltd.)
was used to transfect hsa_circ_0010957 small interfering (si)RNAs
(50 nM), microRNA (miR)-125b mimics (50 nM), miR-125b inhibitor (50
nM), STAT3 siRNA (50 nM) and corresponding negative control (NC)
(50 nM) into CD4+ T cells. hsa_circ_0010957 siRNAs and
NC siRNA, miR-125b mimics and NC mimics, miR-125b inhibitor and NC
inhibitor were purchased from Shanghai GenePharma Co., Ltd. The
mimic and inhibitor-NCs used in the present study were
non-targeting sequences (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
antisense, 5′-ACGUGACACGUUCGGAGAATT-3′. Target sequences (5′→3′) of
hsa_circ_0010957 siRNA: siRNA#1, AGAGAAGACTAACGTCCATCA; siRNA#2,
GAAGACTAACGTCCATCACAT; siRNA#3, GACTAACGTCCATCACATCAA. STAT3 siRNA
(cat. no. sc-29493) and control siRNA (cat. no. sc-37007) were
purchased from Santa Cruz Biotechnology, Inc. Cells were harvested
48 h after transfection at 37°C, and transfection efficacy was then
assessed by reverse transcription-quantitative PCR (RT-qPCR).
RT-qPCR
TRIzol LS reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to isolate total RNA from
CD4+ T cells. cDNAs were synthesized with a PrimeScript
RT-PCR kit (Takara Bio, Inc.; 37°C for 15 min and 85°C for 5 sec.
RT-qPCR was performed with SYBR Premix Ex Taq (Takara Bio, Inc.)
using the following thermocycling conditions: 95°C for 2 min,
followed by 40 cycles of 95°C for 20 sec, 60°C for 30 sec and 72°C
for 20 sec. Primers for hsa_circ_0010957 were: Forward,
5′-AGAGAAGACTAACGTCCATCACA-3′; reverse, 5′-TGGACGGGTCTTCAAAGGTG-3′.
β-actin was used as an internal reference: Forward,
5′-GTGGCCGAGGACTTTGATTG-3′; reverse, 5′-CCTGTAACAACGCATCTCATATT-3′.
miR-125b was detected using TaqMan Human MicroRNA assays (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and normalized to the
U6 small nuclear RNA (U6 snRNA). The data were analyzed by
2−ΔΔCq method (16).
Enzyme-linked immunosorbent assay
(ELISA)
IL-18, IL-6 and IL-17 concentrations were measured
in the cell supernatants and serum of patients with SLE by ELISA
using Human IL-18 ELISA kit (cat. no. ab215539; Abcam), human IL-6
ELISA kit (cat. no. ab46042; Abcam) and human IL-17 ELISA kit (cat.
no. ab119535; Abcam), respectively.
Luciferase reporter assay
Wild-type and mutant sequences of hsa_circ_0010957
or STAT3 3′UTR containing miR-125b binding sites were synthesized
and inserted into the downstream of firefly luciferase reporter
vector pmirGLO (Promega Corporation). The luciferase and Renilla
luciferase reporter vectors were co-transfected into
CD4+ T cells with miR-125b mimics using Human T Cell
Nucleofector kit (Lonza Group, Ltd.) according to the
manufacturer's protocol. After 48 h culture, luciferase activity
was quantified with a Dual Luciferase Reporter Assay kit (Promega
Corporation) according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla
luciferase.
Western blotting
Total protein was extracted from CD4+ T
cells using RIPA buffer (cat. no. P0013B; Beyotime Biotechnology)
and quantified using the BCA Protein Assay Kit (Beyotime
Biotechnology). Equal amounts of protein (20 µg/lane) were resolved
on a 10% SDS-denaturing polyacrylamide gel and transferred to PVDF
membranes. The membranes were subsequently blocked by 5% fat-free
buffered milk for 2 h at room temperature and then incubated
overnight at 4°C with antibodies to STAT3 (cat. no. ab119352;
1:2,000; Abcam), STAT3 (phospho Y705; cat. no. ab76315; 1:1,000;
Abcam) and β-actin (cat. no. ab8226; 1:2,000; Abcam). The membranes
were then washed with PBS-T solution (PBS with 0.1% Tween-20) and
incubated with HRP-labeled Goat Anti-Rabbit IgG (cat. no. A0208;
1:1,000; Beyotime Institute of Biotechnology) and HRP-labeled Goat
Anti-Mouse IgG (cat. no. A0216; 1:1,000; Beyotime Institute of
Biotechnology) at room temperature for 2 h. Protein bands were
visualized using a high sensitivity ECL chemiluminescence Kit (cat.
no. P0018M; Beyotime Institute of Biotechnology). ImageJ software
(version 1.8.0; National Institutes of Health) was used for
densitometry.
Statistical analysis
Data analysis was performed using SPSS 19.0
statistical software (SPSS, Inc.). All experiments were performed
in triplicate, and data are reported as mean ± standard deviation
(SD). Differences between three or more groups were analyzed by
one-way analysis of variance following Tukey's test. Differences
between two groups were analyzed using paired (for paired groups)
or unpaired (for unpaired groups) Student's t-test, when
applicable. Non-parametric method was used to analyze the AUC (Area
Under Curve) of receiver operating characteristic (ROC) curves.
Spearman's analysis was used to test correlation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Hsa_circ_0010957 expression is
increased in SLE and can serve as a biomarker
Previous microarray analyses have shown that
hsa_circ_0010957 is upregulated in SLE-derived CD4+ T
cells (12), but the level of
hsa_circ_0010957 had not been verified by RT-qPCR. SLE-derived
CD4+ T cells and cells derived from healthy volunteers
demonstrated higher expression of hsa_circ_0010957 in SLE (Fig. 1A). There was also a difference in
hsa_circ_0010957 expression between patients with active SLE and
those with inactive disease (Fig.
1B), but there was no significant difference between patients
with inactive disease and HCs (Fig.
1B). A receiver operating characteristic curve showed the
diagnostic value of hsa_circ_0010957 for differentiating patients
with SLE from HCs (Fig. 1C) and
differentiating patients with active SLE from those with inactive
disease (Fig. 1D). Thus, increased
hsa_circ_0010957 expression might be a prospective biomarker for
SLE.
Hsa_circ_0010957 absorbs miR-125b in
SLE-derived CD4+ T cells
A common mechanism of circRNA function is to act as
miRNA sponges to regulate target miRNA activity (17,18).
Putative miRNA targets of hsa_circ_0010957 were searched using the
StarBase database (http://starbase.sysu.edu.cn/index.php) (Fig. 2A) and identified miR-125b, which has
previously been associated with SLE (19,20).
Luciferase reporter assays were used to test the putative
interaction (Fig. 2B).
Overexpression of miR-125b decreased wild-type hsa_circ_0010957
activity but did not affect the mutant vector (Fig. 2C). Indeed, hsa_circ_0010957
knockdown led to increased miR-125b expression (Fig. 2D). miR-125b expression was also
decreased in SLE-derived CD4+ T cells (Fig. 2E) and was lower in patients with
active SLE compared with those with inactive disease (Fig. 2F). Moreover, miR-125b expression was
negatively associated with hsa_circ_0010957 expression (Fig. 2G). Thus, hsa_circ_0010957 acts as a
miR-125b sponge. It was also found that miR-125b is a good
diagnostic marker for differentiating patients with SLE from HCs
(Fig. 2H) and differentiating
active SLE from inactive disease (Fig.
2I). These results suggested that decreased miR-125b expression
in SLE might also be a prospective biomarker for the disease.
 | Figure 2.Hsa_circ_0010957 absorbs miR-125b in
SLE-derived CD4+ T cells. (A) StarBase database showing
the potential target miRNAs of hsa_circ_0010957. (B) Potential
binding site and the mutant site of hsa_circ_0010957 and miR-125b.
(C) Luciferase assay showing overexpression of miR-125b decreased
the luciferase activity of wide-type hsa_circ_0010957 vector, while
not the mutant vector. (D) Relative miR-125b level was detected
using RT-qPCR after knockdown of hsa_circ_0010957. (E) Relative
miR-125b expression in CD4+ T cells from 30 patients
with SLE and 25 HCs tested using RT-qPCR. (F) Comparisons of
relative miR-125b expression in CD4+ T cells from 25
HCs, 18 inactive and 12 active patients with SLE. (G) Pearson's
correlation analysis of hsa_circ_0010957 and miR-125b expression in
CD4+ T cells of patients with SLE. (H and I) Receiver
operating characteristic showing diagnosis value of miR-125b in
differentiating patients with SLE from HCs (H) and differentiating
active from inactive patients with SLE. (I) Data are shown as mean
± SD *P<0.05. NS, no significance; SLE, systemic lupus
erythematosus; wt, wild-type; mut, mutant type; miR/miRNAs,
microRNA; C, negative control; RT-qPCR, reverse
transcription-quantitative PCR; HC, healthy control; AUC, area
under the curve. |
Hsa_circ_0010957 modulates
inflammatory cytokines secretion via miR-125b
To investigate the biological role of
hsa_circ_0010957 and miR-125b in SLE, first, we detected the
overexpression or silence efficiency (Fig. 3A). Knockdown of hsa_circ_0010957
significantly reduced secretion of inflammatory cytokines IL-18,
IL-6, and IL-17, while silencing miR-125b reversed these effects
(Fig. 3B-D). We also observed a
positive correlation between hsa_circ_0010957 and inflammatory
cytokines expression (Fig. 3E-G)
and a negative correlation between miR-125b and inflammatory
cytokines (Fig. 3H-J). Therefore,
we suggest hsa_circ_0010957 modulates inflammatory cytokine
secretion via miR-125b.
Hsa_circ_0010957 regulates activation
of STAT3 signaling via miR-125b
Through the microRNA.org database, STAT3 was
identified as a potential target of miR-125b regulation (Fig. 4A). Luciferase reporter assays showed
that miR-125b overexpression inhibited the activity of wild-type
STAT3 3′-UTR but not a variant STAT3 3′-UTR (Fig. 4B). In addition, miR-125b
overexpression led to decreased STAT3 protein expression, while
miR-125b silencing had an inverse effect (Fig. 4C and D). The results showed that
hsa_circ_0010957 siRNA decreased total and phosphorylation levels
of STAT3 protein, and this effect can be rescued by miR-125b
inhibition (Fig. 4E). These results
indicated that hsa_circ_0010957 regulates STAT3 signaling
activation via miR-125b. STAT3 was also successfully silenced in
SLE-derived CD4+ T cells and showed that knockdown of
STAT3 inhibited the expression of inflammatory cytokines IL-18,
IL-6 and IL-17 (Fig. 4F-I). It was
also found that hsa_circ_0010957 could regulate the activation of
STAT3 signaling via miR-125b in CD4+ T cells from
healthy controls (Fig. S1).
 | Figure 4.Hsa_circ_0010957 regulates activation
of STAT3 signaling via miR-125b. (A) Potential binding site and the
mutant site of STAT3 3′UTR and miR-125b. (B) Luciferase assay
showing overexpression of miR-125b decreased the luciferase
activity of wide-type STAT3 3′UTR vector, while not the mutant
vector. (C and D) STAT3 protein level after transfecting miR-125b
mimics or inhibitor into CD4+ T cells of patients with
SLE. (E) STAT3 and phosphorylated STAT3 protein level after
transfecting hsa_circ_0010957 siRNA and miR-125b inhibitor into
CD4+ T cells of patients with SLE. (F) Silence
efficiency of STAT3 in CD4+ T cells of SLE detected by
reverse transcription-quantitative PCR. (G-I) IL-18, IL-6 and IL-17
levels detected in cell supernatant after transfecting STAT3 siRNA
into CD4+ T cells of patients with SLE. Data are shown
as mean ± SD *P<0.05. NS, no significance; SLE, systemic lupus
erythematosus; wt, wild-type; mut, mutant type; UTR, untranslated
region; miR, microRNA; siRNA, small interfering RNA; NC, negative
control; IL, interleukin. |
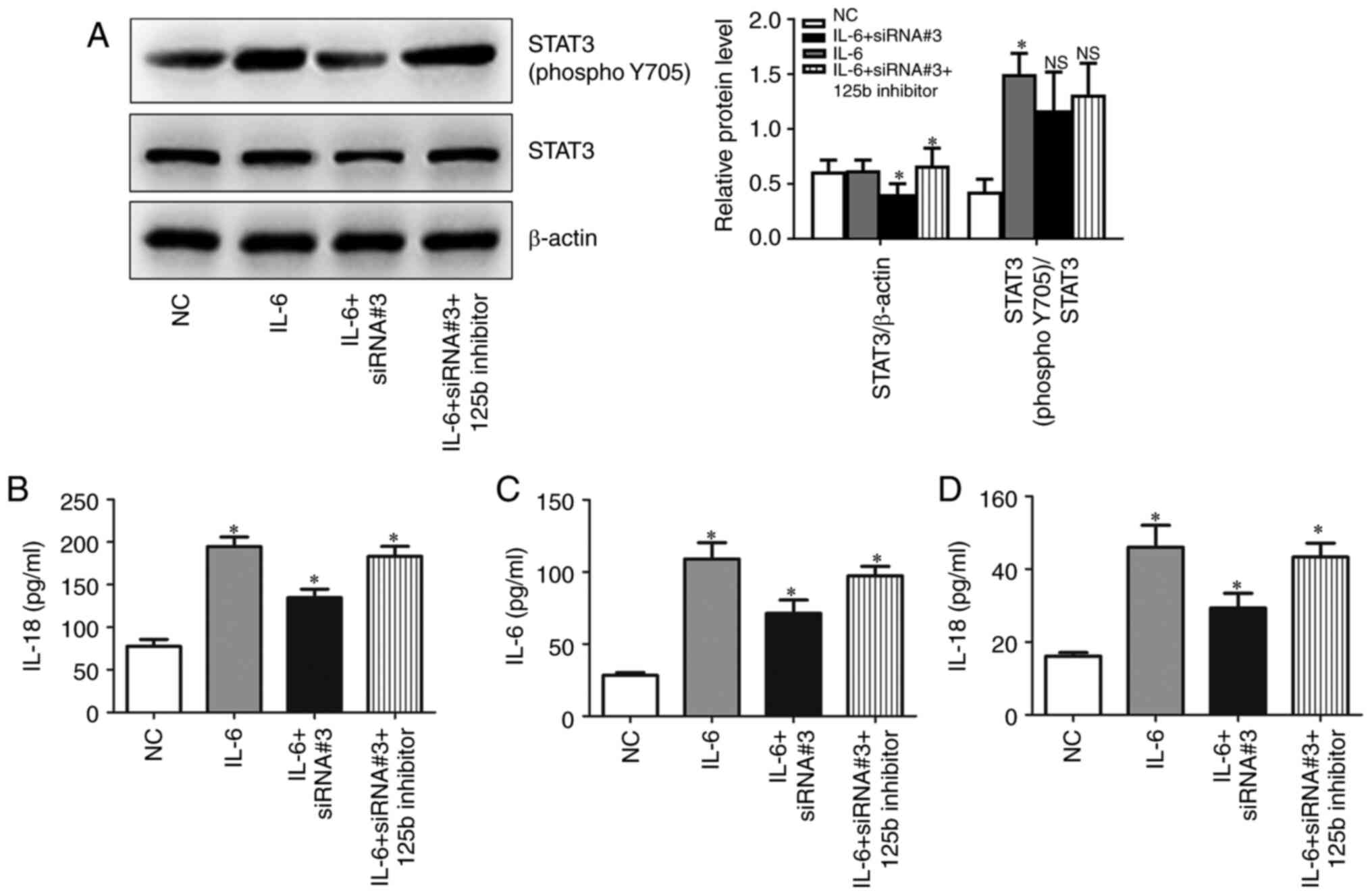
Hsa_circ_0010957 depletion blocks
IL-6-induced activation of STAT3 signaling
Given that serum IL-6 levels are increased in SLE,
and IL-6 is an established activator of STAT3 signaling, the role
of hsa_circ_0010957 in IL-6/STAT3 signaling was explored.
CD4+ T cells obtained from patients with SLE were
incubated with IL-6 and then transfected with hsa_circ_0010957
siRNA and miR-125b inhibitor. Silencing hsa_circ_0010957 blocks
IL-6-induced activation of STAT3 signaling via miR-125b (Fig. 5A). It was also observed that
silencing hsa_circ_0010957 abolished IL-6-induced secretion of
inflammatory cytokines (Fig.
5B-D).
Discussion
In recent years, circRNAs have garnered attention as
a new class of noncoding RNA. CircRNAs are potential biomarkers and
therapeutic targets for many diseases; however, few studies have
been done on circRNAs in SLE. The present study focused on
hsa_circ_0010957, whose role in SLE was unknown.
CircRNAs are prospective biomarkers for many
diseases, and it was demonstrated that hsa_circ_0010957 might serve
as a potential biomarker for SLE. The present study found that
hsa_circ_0010957 expression increases in SLE and is a good
diagnostic indicator for differentiating patients with active SLE
from inactive or no disease.
CircRNAs act as miRNA sponges, thus influencing
their function (17,18). In the present study, it was found
that hsa_circ_0010957 was a sponge for miR-125b in SLE. miR-125b is
downregulated in SLE and inhibits autophagy by targeting UVRAG
(19). Low expression of miR-125b,
mainly in T cells, is inversely correlated with lupus nephritis and
contributes to the pathogenesis of SLE (20). Here, it was shown that
hsa_circ_0010957 modulates the expression of inflammatory cytokines
IL-18, IL-6 and IL-17 via miR-125b. These cytokines are increased
in SLE and play vital roles in disease progression (21–23).
Subsequently, it was shown that the
hsa_circ_0010957/miR-125b axis activated STAT3 signaling via
mediating STAT3 expression. STAT3, an essential element of the
JAK-STAT signal pathway (24), was
reported to participate in the pathogenesis of lupus-susceptible
mice (25). Increased expression of
STAT3 in SLE T cells promotes chemokine-mediated cell migration
(26). It was found that knockdown
of STAT3 inhibited the secretion of IL-18, IL-6 and IL-17 and
concluded that hsa_circ_0010957/miR-125b influences the secretion
of these inflammatory cytokines via regulating STAT3 signaling.
In addition, it was revealed that silencing
hsa_circ_0010957 inhibited IL-6-induced inflammatory cytokines
secretion through restraining STAT3 signaling. High IL-6 level
presents in the serum of patients with SLE (19,20)
and is responsible for the activation of STAT3 signaling (27). Another role of hsa_circ_0010957 in
impeding the proinflammatory effect of IL-6 was also demonstrated.
The proposed model of hsa_circ_0010957/miR-125b/STAT3 signaling in
SLE is illustrated in Fig. 6.
In conclusion, the present findings show that the
increased level of hsa_circ_0010957 in SLE is involved in the
secretion of inflammatory cytokines of CD4+ T cells via
regulating miR-125b/STAT3 signaling. Hsa_circ_0010957 and miR-125b
can be used as a potential biomarker and therapeutic target for
SLE.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Jinhua Public Welfare
Technology Application Research Project (grant no. 2019-4-002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, SH and HD conceived the study and performed the
experiments. YW and XS analyzed and interpreted the data. YZ and SH
confirm the authenticity of all the raw data. YZ and SH wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Human Ethics
Committee of Jinhua Municipal Central Hospital (approval no.
2019-118) and written informed consents were obtained from all
participants in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miyagawa-Hayashino A, Yoshifuji H,
Kitagori K, Ito S, Oku T, Hirayama Y, Salah A, Nakajima T, Kiso K,
Yamada N, et al: Increase of MZB1 in B cells in systemic lupus
erythematosus: Proteomic analysis of biopsied lymph nodes.
Arthritis Res Ther. 20:132018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fanouriakis A, Tziolos N, Bertsias G and
Boumpas DT: Update οn the diagnosis and management of systemic
lupus erythematosus. Ann Rheum Dis. 80:14–25. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morel L: Immunometabolism in systemic
lupus erythematosus. Nat Rev Rheumatol. 13:280–290. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsokos GC, Lo MS, Costa Reis P and
Sullivan KE: New insights into the immunopathogenesis of systemic
lupus erythematosus. Nat Rev Rheumatol. 12:716–730. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeck WR and Sharpless NE: Detecting and
characterizing circular RNAs. Nat Biotechnol. 32:453–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z, Yang T and Xiao J: Circular RNAs:
Promising biomarkers for human diseases. EBioMedicine. 34:267–274.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsai CY, Shen CY, Liu CW, Hsieh SC, Liao
HT, Li KJ, Lu CS, Lee HT, Lin CS, Wu CH, et al: Aberrant non-coding
RNA expression in patients with systemic lupus erythematosus:
Consequences for immune dysfunctions and tissue damage.
Biomolecules. 10:16412020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo Q, Li X, Fu B, Zhang L, Fang L, Qing
C, Guo Y, Huang Z and Li J: Expression profile and diagnostic value
of circRNAs in peripheral blood from patients with systemic lupus
erythematosus. Mol Med Rep. 23:12021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ouyang Q, Huang Q, Jiang Z, Zhao J, Shi GP
and Yang M: Using plasma circRNA_002453 as a novel biomarker in the
diagnosis of lupus nephritis. Mol Immunol. 101:531–538. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miao Q, Zhong Z, Jiang Z, Lin Y, Ni B,
Yang W and Tang J: RNA-seq of circular RNAs identified circPTPN22
as a potential new activity indicator in systemic lupus
erythematosus. Lupus. 28:520–528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Zhang C, Wu Z, Chen Y and Shi W:
CircIBTK inhibits DNA demethylation and activation of AKT signaling
pathway via miR-29b in peripheral blood mononuclear cells in
systemic lupus erythematosus. Arthritis Res Ther. 20:1182018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li LJ, Zhu ZW, Zhao W, Tao SS, Li BZ, Xu
SZ, Wang JB, Zhang MY, Wu J, Leng RX, et al: Circular RNA
expression profile and potential function of hsa_circ_0045272 in
systemic lupus erythematosus. Immunology. 155:137–149. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Wang X, Chen Y, Wu Z, Zhang C and
Shi W: The down-regulation of hsa_circ_0012919, the sponge for
miR-125a-3p, contributes to DNA methylation of CD11a and CD70 in
CD4 T cells of systemic lupus erythematous. Clin Sci (Lond).
132:2285–2298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petri M, Orbai AM, Alarcón GS, Gordon C,
Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O,
et al: Derivation and validation of the systemic lupus
international collaborating clinics classification criteria for
systemic lupus erythematosus. Arthritis Rheum. 64:2677–2686. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Zhao C, Zhang C, Mei X, Song J,
Sun Y, Wu Z and Shi W: Increased HERV-E clone 4-1 expression
contributes to DNA hypomethylation and IL-17 release from
CD4+ T cells via miR-302d/MBD2 in systemic lupus
erythematosus. Cell Commun Signal. 17:942019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao W, Qian G, Luo W, Liu X, Pu Y, Hu G,
Han L, Yuan L, Xiao A and Deng D: miR-125b is downregulated in
systemic lupus erythematosus patients and inhibits autophagy by
targeting UVRAG. Biomed Pharmacother. 99:791–797. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo X, Zhang L, Li M, Zhang W, Leng X,
Zhang F, Zhao Y and Zeng X: The role of miR-125b in T lymphocytes
in the pathogenesis of systemic lupus erythematosus. Clin Exp
Rheumatol. 31:263–271. 2013.PubMed/NCBI
|
|
21
|
Mende R, Vincent FB, Kandane-Rathnayake R,
Koelmeyer R, Lin E, Chang J, Hoi AY, Morand EF, Harris J and Lang
T: Analysis of serum interleukin (IL)-1β and IL-18 in systemic
lupus erythematosus. Front Immunol. 9:12502018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Y, Tao H, Gong Y, Chen F, Li C and
Yang X: Changes of serum IL-6, IL-17, and complements in systemic
lupus erythematosus patients. J Interferon Cytokine Res.
39:410–415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monzavi SM, Alirezaei A, Shariati-Sarabi
Z, Afshari JT, Mahmoudi M, Dormanesh B, Jahandoost F, Khoshdel AR
and Rezaie AE: Efficacy analysis of hydroxychloroquine therapy in
systemic lupus erythematosus: A study on disease activity and
immunological biomarkers. Inflammopharmacology. 26:1175–1182. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zegeye MM, Lindkvist M, Fälker K, Kumawat
AK, Paramel G, Grenegård M, Sirsjö A and Ljungberg LU: Activation
of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6
trans-signaling-mediated pro-inflammatory response in human
vascular endothelial cells. Cell Commun Signal. 16:552018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pramanik R, Jørgensen TN, Xin H, Kotzin BL
and Choubey D: Interleukin-6 induces expression of Ifi202, an
interferon-inducible candidate gene for lupus susceptibility. J
Biol Chem. 279:16121–16127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harada T, Kyttaris V, Li Y, Juang YT, Wang
Y and Tsokos GC: Increased expression of STAT3 in SLE T cells
contributes to enhanced chemokine-mediated cell migration.
Autoimmunity. 40:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|