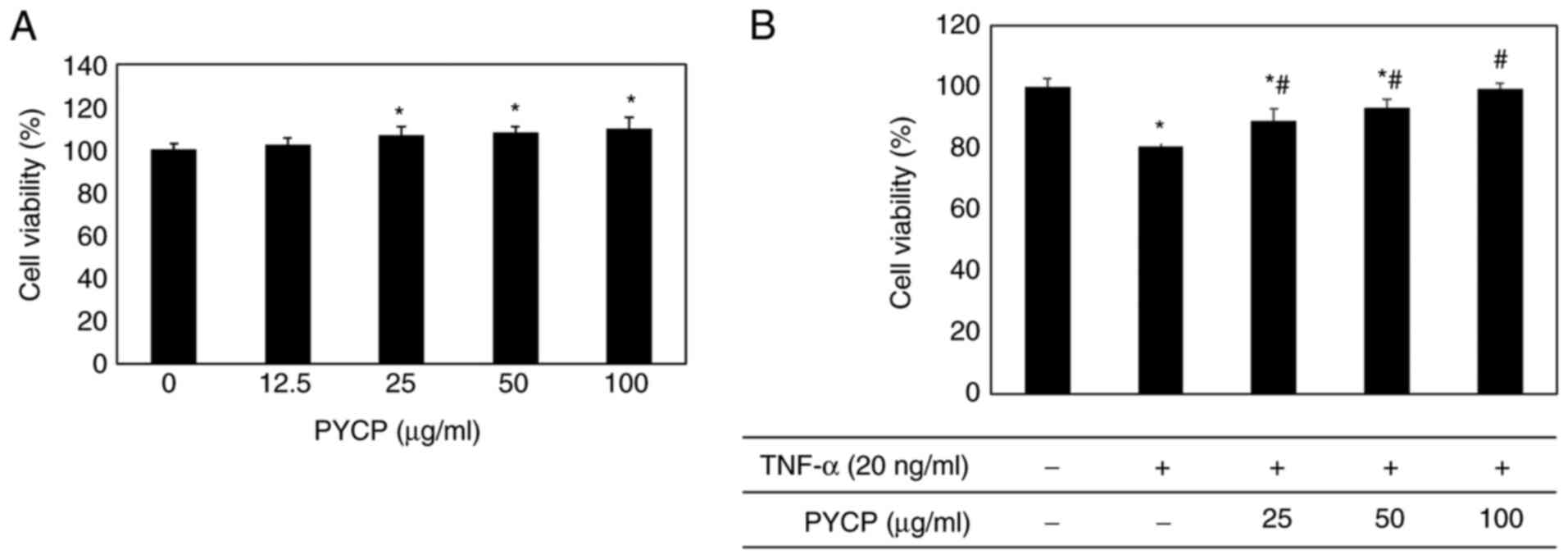
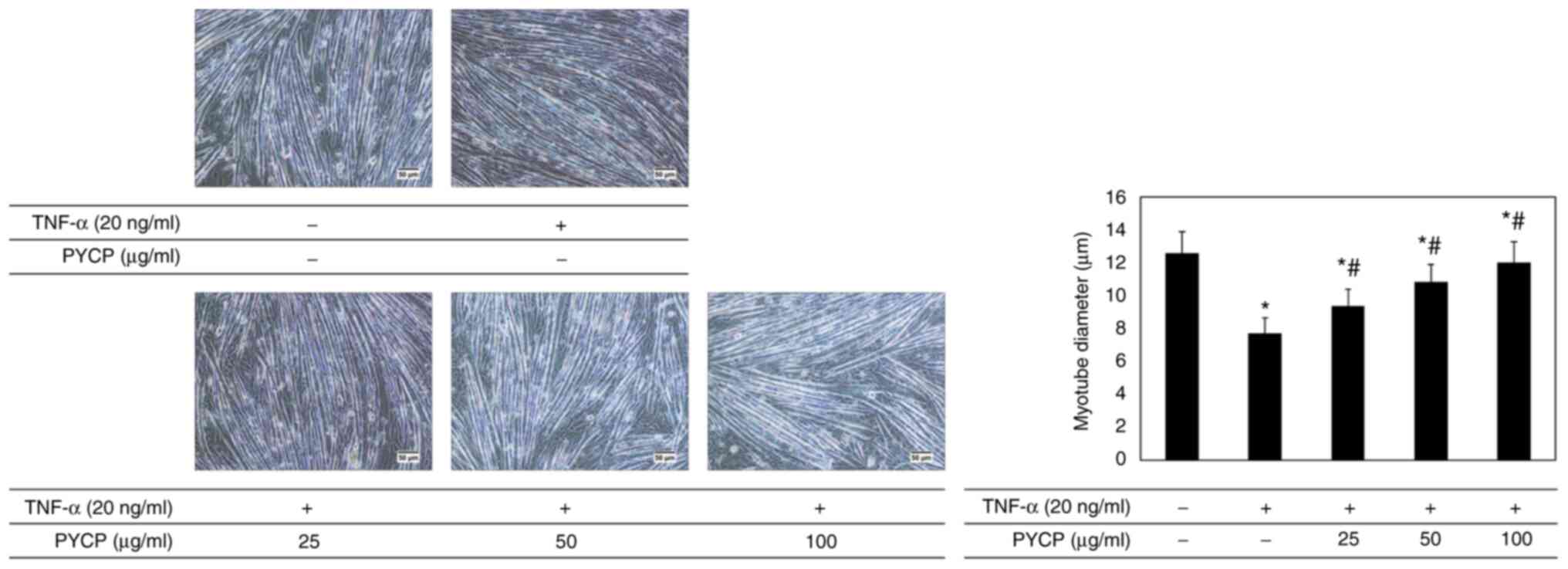
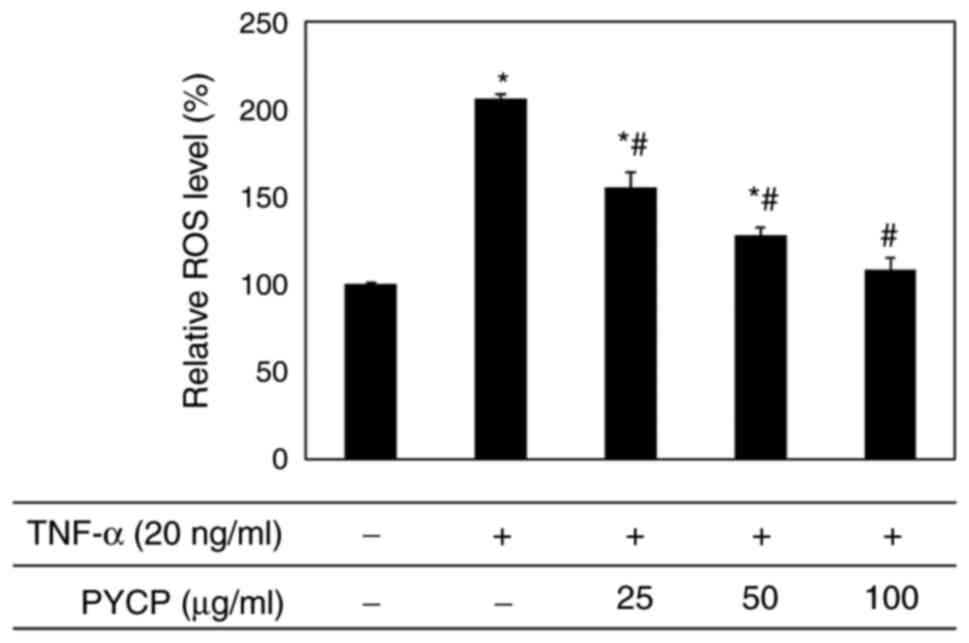
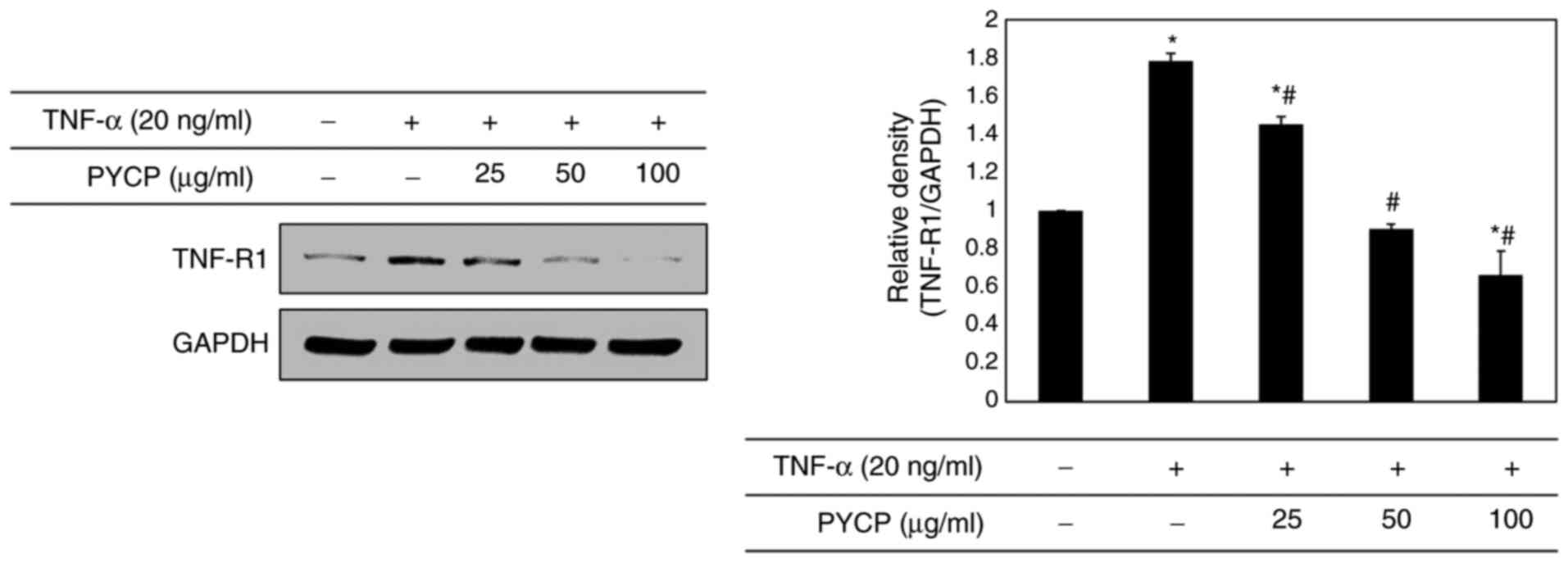
|
1
|
Tolosa L, Morla M, Iglesias A, Busquets X,
Llado J and Olmos G: IFN-gamma prevents TNF-alpha-induced apoptosis
in C2C12 myotubes through down-regulation of TNF-R2 and increased
NF-kappaB activity. Cell Signal. 17:1333–1342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lecker SH, Solomon V, Mitch WE and
Goldberg AL: Muscle protein breakdown and the critical role of the
ubiquitin-proteasome pathway in normal and disease states. J Nutr.
129 (1S Suppl):227S–237S. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiaffino S, Dyar KA, Ciciliot S, Blaauw
B and Sandri M: Mechanisms regulating skeletal muscle growth and
atrophy. FEBS J. 208:4294–4314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kandarian SC and Jackman RW: Intracellular
signaling during skeletal muscle atrophy. Muscle Nerve. 33:155–165.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Egerman MA and Glass DJ: Signaling
pathways controlling skeletal muscle mass. Crit Rev Biochem Mol
Biol. 49:59–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kirillova I, Chaisson M and Fausto N:
Tumor necrosis factor induces DNA replication in hepatic cells
through nuclear factor kappaB activation. Cell Growth Differ.
10:819–828. 1999.PubMed/NCBI
|
|
7
|
Schutze S, Machleidt T and Kronke M:
Mechanisms of tumor necrosis factor action. Semin Oncol. 19 (2
Suppl 4):S16–S24. 1992.PubMed/NCBI
|
|
8
|
Basu A, Johnson DE and Woolard MD:
Potentiation of tumor necrosis factor-alpha-induced cell death by
rottlerin through a cytochrome-C-dependent pathway. Exp Cell Res.
278:209–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li YP: TNF-alpha is a mitogen in skeletal
muscle. Am J Physiol Cell Physiol. 285:C370–C376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li YP, Schwarts RJ, Waddell ID, Holloway
BR and Reid MB: Skeletal muscle myocytes undergo protein loss and
reactive oxygen-mediated NF-kappaB activation in response to tumor
necrosis factor alpha. FASEB J. 12:871–880. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai D, Frantz JD, Tawa NE Jr, Melendez PA,
Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ and
Shoelson SE: IKKbeta/NF-kappaB activation causes severe muscle
wasting in mice. Cell. 119:285–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mourkioti F, Kratsios P, Luedde T, Song
YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M
and Rosenthal N: Targeted ablation of IKK2 improves skeletal muscle
strength, maintains mass, and promotes regeneration. J Clin Invest.
116:2945–2954. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar A, Takada Y, Boriek AM and Aggarwasl
BB: Nuclear factor-kappaB: Its role in health and disease. J Mol
Med (Berl). 82:434–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guttridge DC, Albanese C, Reuther JY,
Pestell RG and Baldwin AS Jr: NF-kappaB controls cell growth and
differentiation through transcriptional regulation of cyclin D1.
Mol Cell Biol. 19:5785–5799. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitin N, Kudla AJ, Konieczny SF and
Taparowsky EJ: Differential effects of Ras signaling through
NFkappaB on skeletal myogenesis. Oncogene. 20:1276–1286. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berkes CA and Tapscott SJ: MyoD and the
transcriptional control of myogenesis. Semin Cell Dev Biol.
16:585–595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Hwang JY, Park HB, Yadav D, Oda T
and Jin JO: Porphyran isolated from Pyropia yezoensis
inhibits lipopolysaccharide-induced activation of dendritic cells
in mice. Carbohydr Polym. 229:1154572020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee MK, Choi JW, Choi YH and Nam TJ:
Pyropia yezoensis protein prevents dexamethasone-induced
myotube atrophy in C2C12 myotubes. Mar Drugs. 16:4972018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim IH, Kwon MJ, Jung JH and Nam TJ:
Protein extracted porphyra yezoensis prevents cisplatin-induced
nephrotoxicity by downregulating the MAPK and NF-κB pathways. Int J
Mol Med. 41:511–520. 2018.PubMed/NCBI
|
|
20
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Activation of the mTOR signaling pathway in breast cancer MCF-7
cells by a peptide derived from porphyra yezoensis. Oncol Rep.
33:19–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi JW, Kwon MJ, Kim IH, Kim YM, Lee MK
and Nam TJ: Pyropia yezoensis glycoprotein promotes the M1
to M2 macrophage phenotypic switch via the STAT3 and STAT6
transcription factors. Int J Mol Med. 38:666–674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim CR, Kim YM, Lee MK, Kim IH, Choi YH
and Nam TJ: Pyropia yezoensis peptide promotes collagen
synthesis by activating the TGF-β/Smad signaling pathway in the
human dermal fibroblast cell line HS27. Int J Mol Med. 39:31–38.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JW, Kim IH, Kim YM, Lee MK, Choi YH
and Nam TJ: Protective effect of Pyropia yezoensis
glycoprotein on chronic ethanol consumption-induced hepatotoxicity
in rats. Mol Med Rep. 14:4881–4886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Castillero E, Alamdari N, Lecker SH and
Hasselgren PO: Suppression of atrogin-1 and MuRF1 prevents
dexamethasone-induced atrophy of cultured myotubes. Metabolism.
62:1495–1502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Menconi M, Gonnella P, Petkova V, Lecker S
and Hasselgren PO: Dexamethasone and corticosterone induce similar,
but not identical, muscle wasting responses in cultured L6 and
C2C12 myotubes. J Cell Biochem. 105:353–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malafarina V, Uriz-Otano F, Iniesta R and
Gil-Guerrero L: Sarcopenia in the elderly: Diagnosis,
physiopathology and treatment. Maturitas. 71:109–114. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kob R, Bollheimer LC, Bertsch T, Fellner
C, Djukic M, Sieber CC and Fischer BE: Sarcopenic obesity:
Molecular clues to a better understanding of its pathogenesis?
Biogerontology. 16:15–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jensen GL: Inflammation: Roles in aging
and sarcopenia. JPEN J Parenter Enteral Nutr. 32:656–659. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wing SS, Lecker SH and Jagoe RT:
Proteolysis in illness-associated skeletal muscle atrophy: From
pathways to networks. Crit Rev Clin Lab Sci. 48:49–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ventadour S and Attaix D: Mechanisms of
skeletal muscle atrophy. Curr Opin Rheumatol. 18:631–635. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen G and David V: TNF-R1 signaling: A
beautiful pathway. Science. 296:1634–1635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li YP, Atkins CM, Sweatt JD and Reid MB:
Mitochondria mediate tumor necrosis factor-alpha/NF-kappaB
signaling in skeletal muscle myotubes. Antioxid Redox Signal.
1:97–104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thoma A and Lightfoot AP: NF-κB and
inflammatory cytokine signaling: Role in skeletal muscle atrophy.
Adv Exp Med Biol. 1088:267–279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YP and Reid MB: NF-kappaB mediates the
protein loss induced by TNF-alpha in differentiated skeletal muscle
myotubes. Am J Physiol Regul Integr Comp Physiol. 279:R1165–R1170.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thaloor D, Miller KJ, Gephart J, Mitchell
PO and Pavlath GK: Systemic administration of the NF-kappaB
inhibitor curcumin stimulates muscle regeneration after traumatic
injury. Am J Physiol. 277:C320–C329. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nisr RB, Shah DS, Ganley IG and Hundal HS:
Proinflammatory NFκB signaling promotes mitochondrial dysfunction
in skeletal muscle in response to cellular fuel overloading. Cell
Mol Life Sci. 76:4887–4904. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McKinnell IW and Rudnicki MA: Molecular
mechanisms of muscle atrophy. Cell. 119:907–910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Legard GE and Pedersen BK: Muscle as an
endocrine organ. Muscle Exerc Physiol. 25:258–307. 2019.
|
|
39
|
Bhatnagar S, Panguluri SK, Gupta SK,
Dahiya S, Lundy RF and Kumar A: Tumor necrosis factor-α regulates
distinct molecular pathway and gene networks in cultured skeletal
muscle cells. PLoS One. 5:e132622010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lightfoot AP, Sakellariou GK, Nye GA,
McArdle F, Jackson MJ, Griffiths RD and McArdle A: SS-31 attenuates
TNF-α induced cytokine release from C2C12 myotubes. Redox Biol.
6:253–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kaliman P, Canicio J, Testar X, Palacin M
and Zorzano A: Insulin-like growth factor-II, phosphatidylinositol
3-kinase, nuclear factor-kappaB and inducible nitric-oxide synthase
define a common myogenic signaling pathway. J Biol Chem.
274:17437–17444. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Langen RC, Schols AM, Kelders MC, Wouters
EF and Janssen-Heininger YM: Inflammatory cytokines inhibit
myogenic differentiation through activation of nuclear
factor-kappaB. FASEB J. 15:1169–1180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dogra C, Changotra H, Mohan S and Kumar A:
Tumor necrosis factor-like weak inducer of apoptosis inhibits
skeletal myogenesis through sustained activation of nuclear
factor-kappaB and degradation of MyoD protein. J Biol Chem.
281:10327–10336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guttridge DC, Mayo MW, Madrid LV, Wang CY
and Baldwin AS Jr: NF-kappaB-induced loss of MyoD messenger RNA:
Possible role in muscle decay and cachexia. Science. 289:2363–2366.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sitcheran R, Cogswell PC and Baldwin AS
Jr: NF-kappaB mediates inhibition of mesenchymal cell
differentiation through a posttranscriptional gene silencing
mechanism. Genes Dev. 17:2368–2373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Di Marco S, Mazroui R, Dallaire P, Chittur
S, Tenenbaum SA, Radzioch D, Marette A and Gallouzi IE:
NF-kappB-mediated MyoD decay during muscle wasting requires nitric
oxide synthase mRNA stabilization, HuR protein, and nitric oxide
release. Mol Cell Biol. 25:6533–6545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roose JL, Wegener KM and Pakrasi HB: The
extrinsic proteins of photosystem II. Photosynth Res. 92:369–387.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Balint I, Bhattacharya J, Perelman A,
Schatz D, Moskovitz Y, Keren N and Schwarz R: Inactivation of the
extrinsic subunit of photosystem II, PsbU, in Synechococcus PCC
7942 results in elevated resistance to oxidative stress. FEBS Lett.
580:2117–2122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Abasova L, Deak Z, Schwarz R and Vass I:
The role of the PsbU subunit in the light sensitivity of PSII in
the cyanobacterium Synechococcus 7942. J Photochem Photobiol B.
105:149–156. 2011. View Article : Google Scholar : PubMed/NCBI
|