Introduction
Osteoarthritis (OA) is becoming an increasing burden
to patients, communities and social care systems (1). Currently, effective therapy for OA is
limited, although exercise has been recommended as an effective
strategy for preventing or treating OA (2) Accumulating evidence indicates that the
effect of exercise on joints is intensity-dependent; a moderate
amount of exercise serves an important role in the prevention and
treatment of OA, whereas high intensity exercise is hypothesized to
induce OA (3,4).
OA is primarily characterized by progressive
articular cartilage degradation. The subchondral bone has been
reported to serve an important role in OA (5). Cartilage and its underlying
subchondral bone act as a unit, affecting one another in OA
(6). The effect of exercise has
been investigated on cartilage and the subchondral bone (4,7);
however, the interaction between cartilage and the subchondral bone
with exercise remains unclear.
Both the chondrocytes in articular cartilage and the
osteoblasts in subchondral bone are sensitive to mechanical stimuli
and can transform mechanical signals into biological responses
(8–10). It has been demonstrated that
mechanical stretch influenced the biological behavior of
osteoblasts, thereby profoundly affecting bone remodeling (8). Furthermore, Yan et al (9) demonstrated that the mechanical strains
of 2,500 με at 0.5 Hz can induce osteoblast differentiation via the
ERK signaling pathway. For chondrocyte responses to mechanical
stimulation, Agarwal et al (10) indicated that the mechanical strain
of 6% at 0.5 Hz directly suppresses the catabolism of chondrocytes,
whereas the absence of mechanical stress or high-magnitude of
strain (>10% at 0.5 Hz) causes chondrocyte catabolism and
apoptosis (11,12). However, the interaction between
chondrocytes and osteoblasts with excessive mechanical loading
remains unknown. To the best of our knowledge, no previous study
has investigated apoptosis in the communication of chondrocytes and
osteoblasts in the mechanical environment, and the underlying
mechanisms are also not completely understood. The best recognized
biochemical hallmark of both early and late stages of apoptosis is
the activation of cysteine proteases (caspases). Caspase-3 is the
most important apoptotic agent in the caspase family and when
activated by apoptotic signaling, caspase-3 is converted to cleaved
caspase-3. Detection of caspase-3 and cleaved caspase-3 in cells or
tissues is an key method for apoptosis (13).
High expression of collagen 2a (Col 2a) is a
specific phenotype of chondrocytes, while alkaline phosphatase
(ALP), collagen 1a (Col 1a) and osteocalcin (OCN) are mainly
expressed by osteoblasts (14,15).
SRY-related high mobility group-box 9 (SOX9) is a key transcription
factor in chondrogenesis and serves an important role in the
proliferation and differentiation of chondrocytes (16). Non-physiological mechanical stress
can change the phenotype of chondrocytes, transforming chondrocytes
into hypertrophic chondrocytes, secreting collagen X (Col X) and
OCN, which are rarely expressed in normal chondrocytes (17). In addition to mechanical
environmental changes, various inflammatory cytokines and proteases
are reportedly involved in the initiation and progression of
cartilage degeneration (18).
Interleukin-6 (IL-6) and prostaglandin E2 (PG E2) serve an key role
in the inflammatory response of OA. These inflammatory mediators
enhance matrix degradation and inhibit the synthesis of matrix
related proteins (19). Excessive
production of cartilage degrading enzymes such as the aggrecanases
and matrix metalloproteinases (MMPs), which are key in the
degradation of aggrecan and Coll 2a, has been demonstrated in OA
(20,21). The Wnt signaling pathway serves an
important role in the development and maintenance of cartilage and
is closely associated with OA (22). Up- or downregulation of the
classical Wnt signaling pathway displays a negative impact on
cartilage development and maintenance, eventually leading to
OA-like features (23). On the one
hand, Miclea et al (24)
demonstrated that upregulation of β-catenin in chondrocytes induced
matrix degradation and inhibited chondrocyte proliferation, leading
to OA-like features. On the other hand, Chen et al (25) reported that ablation of β-catenin in
transgenic mice decreased the proliferation of chondrocytes and
promoted chondrocyte apoptosis. Glycogen synthase kinase 3β
(GSK-3β) is another important protein in the Wnt signaling pathway.
It can lead to the degradation of β-catenin when the Wnt signaling
pathway is not activated. Inactivation of GSK-3β leads to the
accumulation of β-catenin, thus mediating proliferation and
apoptosis (26). The Wnt/β-catenin
signaling pathway is involved in transmitting the signals of
mechanical loading to cells, including chondrocytes and osteoblasts
(27–30). However, the role of Wnt/β-catenin
signaling in the interaction between chondrocytes and osteoblasts
under the mechanical environment remains unknown.
Therefore, the present study investigated the
effects of excessive mechanical stretch on chondrocytes and
osteoblasts. The effects of stretched osteoblasts on the metabolism
and apoptosis of chondrocytes were investigated, and the role of
Wnt/β-catenin signaling in the indirect chondrocyte-osteoblast
co-culture model was assessed. The results of the present study may
further the current understanding of the mechanisms underlying
excessive loading in OA, thus aiding with improving the prevention
and treatment of OA.
Materials and methods
Culture and mechanical stretching of
osteoblasts
A total of 16 rats were obtained from the
Experimental Animal Center of Southern Medical University
(Guangzhou, China), housed in rooms with a maintained temperature
of 22–25°C and humidity of 50±5% under a 12-h light-dark cycle and
provided food and water ad libitum. All experiments were
performed in accordance with protocols approved by the Animal
Ethics Committee of Nanfang Hospital, Southern Medical University
(approval no. NFYY-2016-128; Guangzhou, China). Neonatal male
Sprague-Dawley (SD) rats (age, 3–5 days; weight, 8–15 g, n=8) used
for the isolation of primary osteoblasts were euthanized by
decapitation. Male SD rats (age, 18–20 days; weight, 45–52 g, n=8)
used for the isolation of articular chondrocytes were anesthetized
by intraperitoneal injection of 7% chloral hydrate (350 mg/kg)
followed by sacrifice by cervical dislocation. No signs of
peritonitis were observed after the administration of chloral
hydrate.
Primary osteoblasts were isolated from the cortical
bone of the calvaria of neonatal SD rats by successive enzymatic
digestion. Cells from passages 2–4 were used in the present study.
Osteoblasts were subjected to mechanical stretching using a
Flexcell FX-5000™ Flexercell Tension System (Flexcell International
Corp.), as previously described (6,31), at
a mechanical strain of 20% and a frequency of 1 Hz for 24 h. For
the control group, osteoblasts were collected and placed onto
plates without mechanical stretching (un-stretched group).
Following mechanical stretching for 24 h, osteoblasts from
stretched (Scm) and un-stretched (Ucm) conditioned medium were
collected and centrifuged at 1,000 × g for 10 min at 37°C.
Subsequently, the supernatant was transferred to a fresh tube and
stored at −80°C to be used for subsequent experiments. Osteoblasts
were collected for RNA extraction. The morphology of osteoblasts
before and after stretching was observed using an inverted
microscope (Olympus Corporation).
Culture and stimulation of
chondrocytes
Rat articular chondrocytes were isolated by
enzymatic digestion of articular cartilage from SD rats as
previously described (22).
Briefly, articular cartilage was cut into small pieces, then the
pieces were digested with 0.25% Trypsin-EDTA solution and 0.2%
collagenase type II (Sigma-Aldrich; Merck KGaA), respectively. To
avoid phenotype loss, chondrocyte from passage 1–2 were used in the
present study. Before applying the mechanical stretch, cells were
starved by incubation in Dulbecco's modified Eagle's medium (DMEM,
Gibco; Thermo Fisher Scientific, Inc.) containing 1% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) for 12 h,
followed by incubation in culture medium supplemented with 0.1%
FBS. Subsequently, a mechanical stretch of 20% elongation was
applied to the chondrocytes at a frequency of 1 Hz for 24 h
(stretched group for chondrocytes). For the co-culture,
chondrocytes were stimulated with Scm or Ucm at 37°C for 24 h.
Then, 10 µM XAV-939 (Sigma-Aldrich; Merck KGaA) was added to the
chondrocytes at 37°C for 24 h to assess the role of the
Wnt/β-catenin signaling pathway. After 24 h, stimulated
chondrocytes were retained and harvested for extraction of RNA or
proteins. The chondrocytes without co-culture were defined as the
control group. The morphology of chondrocytes before and after
stretching was observed using an inverted microscope (Olympus
Corporation).
Cytokine assay
Levels of total rat MMP 13 (cat. no. NBP3-06931),
MMP 3 (cat. no. NBP3-06894), IL-6 (cat. no. R6000B) and PG E2 (cat.
no. NBP3-00461) in supernatants were measured using ELISA kits
(Novus Biologicals). Samples were analyzed in serial dilutions in
duplicate and measured against standard curves according to the
manufacturer's instructions.
Flow cytometric analysis of cell
death
To determine early apoptosis/necroptosis, a
fluorescent dye Annexin V-FITC/PI Apoptosis Detection kit (Abcam)
was used according to the manufacturer's protocol. Briefly, treated
chondrocytes were harvested and washed once in ice-cold PBS. After
resuspension in 500 µl binding buffer, 5 µl Annexin V-FITC and 5 µl
PI solution were added for 30 min at 4°C in the dark. Subsequently,
samples were analyzed by a BD FACSCanto™ II (BD Biosciences) flow
cytometer using BD FACSDiva software (version 6.1.3; BD
Biosciences).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from monolayer cultured
chondrocytes and osteoblasts using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). First-strand cDNA
synthesis was conducted using a PrimeScript RT Reagent kit
according to the manufacturer's instructions (Takara Biotechnology
Co., Ltd.). Subsequently, qPCR was performed using SYBR Premix Ex
Taq (Takara Biotechnology Co., Ltd.) and the following
thermocycling conditions: After an initial denaturation at 95°C for
30 sec, 40 cycles of a two-cycle procedure (denaturation at 95°C
for 15 sec, annealing and extension at 60°C for 32 sec) were
performed. mRNA expression levels were normalized to the internal
reference gene GAPDH. The forward and reverse primer sequences are
presented in Table I. The
difference between the mean Cq values of the gene of interest and
the housekeeping gene was labelled ΔCq, and the difference between
ΔCq and the Cq value of the calibrator sample was labelled ΔΔCq.
The 2−ΔΔCq method was used to determine relative mRNA
expression levels (32).
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequences
(5′-3′) |
|---|
| GAPDH | F:
GGCACAGTCAAGGCTGAGAATG |
|
| R:
ATGGTGGTGAAGACGCCAGTA |
| Col 1a | F:
CATGTTCAGCTTTGTGGACC |
|
| R:
TTAGGGACCCTTAGGCCATT |
| ALP | F:
AACAACCTGACTGACCCTTC |
|
| R:
TCCACTAGCAAGAAGAAGCC |
| OCN | F:
GACTGCATTCTGCCTCTCTG |
|
| R:
ATTCACCACCTTACTGCCCT |
| Cyclin D1 | F:
CGTACCCTGACACCAATCTC |
|
| R:
TGAAGTAAGAAACGGAGGGC |
| β-catenin | F:
TAAATGACGAGGACCAGGTG |
|
| R:
CACTATGGCAGACACCATCT |
| MMP 13 | F:
CTCTTGAGCTGGACTCATTG |
|
| R:
CTGCAAACTGGAAGTCTTCC |
| Col X | F:
TGCTGCTATTGTCCTTGAAC |
|
| R:
ACCTTGCTCTCCTCTTAGTG |
| Col 2a | F:
CCCAGAACATCACCTACCAC |
|
| R:
GGTACTCGATGATGGTCTTG |
| SOX 9 | F:
GGGCTCTGTGCTCTACTCCA |
|
| R:
AGGTCTGGTGAGCTGTGTGT |
| ADAMTS 5 | F:
TGTGCCGTGATTGAAGATGA |
|
| R:
TCATGAGAGAGGCCAAGGA |
Western blotting
Total protein was isolated from chondrocytes using
lysis buffer containing 1 M Tris HCl (pH 8), 5 M NaCl, 20% Triton
X-100, 0.5 M EDTA and a protease inhibitor cocktail (Roche
Diagnostics). The cell lysate was clarified by centrifugation
(12,000 × g for 10 min at 4°C) and protein concentrations were
determined using a bicinchoninic acid protein assay (Sigma-Aldrich;
Merck KGaA). Protein (10 µg) was separated via SDS-PAGE (12% gel),
and the separated proteins were subsequently transferred to a
nitrocellulose membrane. Then, the membrane was blocked in TBS with
0.1% Tween-20 containing 5% non-fat milk at 37°C for 1 h. The
membranes were incubated overnight at 4°C with primary antibodies
targeted against: Caspase 3 (1:1,000; cat. no. 9662; Cell Signaling
Technology, Inc.), cleaved caspase 3 (1:1,000; cat. no. 9661; Cell
Signaling Technology, Inc.), β-catenin (1:1,000; cat. no. sc-7963;
Santa Cruz Biotechnology, Inc.), GSK-3β (1:1,000; cat. no. 9315;
Cell Signaling Technology, Inc.) and β-actin (1:1,000; cat. no.
sc-47778; Santa Cruz Biotechnology, Inc.). Subsequently, the
membranes were incubated with a horseradish peroxidase-conjugated
secondary antibody at 37°C for 2 h. (Goat Anti-Mouse IgG; 1:3,000;
cat. no. CW0102; CoWin Biosciences; Goat Anti-Rabbit IgG; 1:3,000;
CW0103; CoWin Biosciences) Proteins bands were developed by
enhanced chemiluminescence (EMD Millipore) and visualized by
exposure to X-ray film. The bands were quantified by the
densitometry with ImageJ software (version 1.51; National
Institutes of Health). β-actin was used as the loading control.
Statistical analysis
To confirm the reproducibility of the results, the
experiments were repeated three times. The results are presented as
the mean ± standard deviation. Statistical analyses were performed
using SPSS software (version 16.0; SPSS, Inc.). Data were analyzed
using the unpaired Student's t-test or one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cellular phenotypes of osteoblasts and
chondrocytes
To compare the cellular phenotypes of osteoblasts
and chondrocytes, RT-qPCR was performed to detect the expression
levels of bone and cartilage-associated genes. The expression
levels of Col 1a and ALP mRNA were lower in chondrocytes compared
with osteoblasts, respectively (1.00±0.04 vs. 0.22±0.09 and
1.00±0.03 vs. 0.15±0.02, both P<0.05; Fig. 1A and B). However, the expression
levels of Col 2a and SOX 9 mRNA were significantly higher in
chondrocytes compared with osteoblasts, respectively (1.00±0.05 vs.
3.10±0.34 and 1.00±0.04 vs. 2.45±0.21, P<0.05; Fig. 1C and D). Prior to mechanical
stretching, chondrocytes and osteoblasts were cobblestone-like and
fibroblast-like, respectively (Fig. 2A
and C). Cells became notably longer and were oriented
perpendicularly to the axis of the external strain following
mechanical stretch (Fig. 2B and
D).
Excessive mechanical stretch inhibits
osteoblast osteogenesis
In order to investigate the effect of excessive
mechanical stretch on osteoblasts, the present study analyzed
alterations in osteogenesis- and proliferation-associated genes of
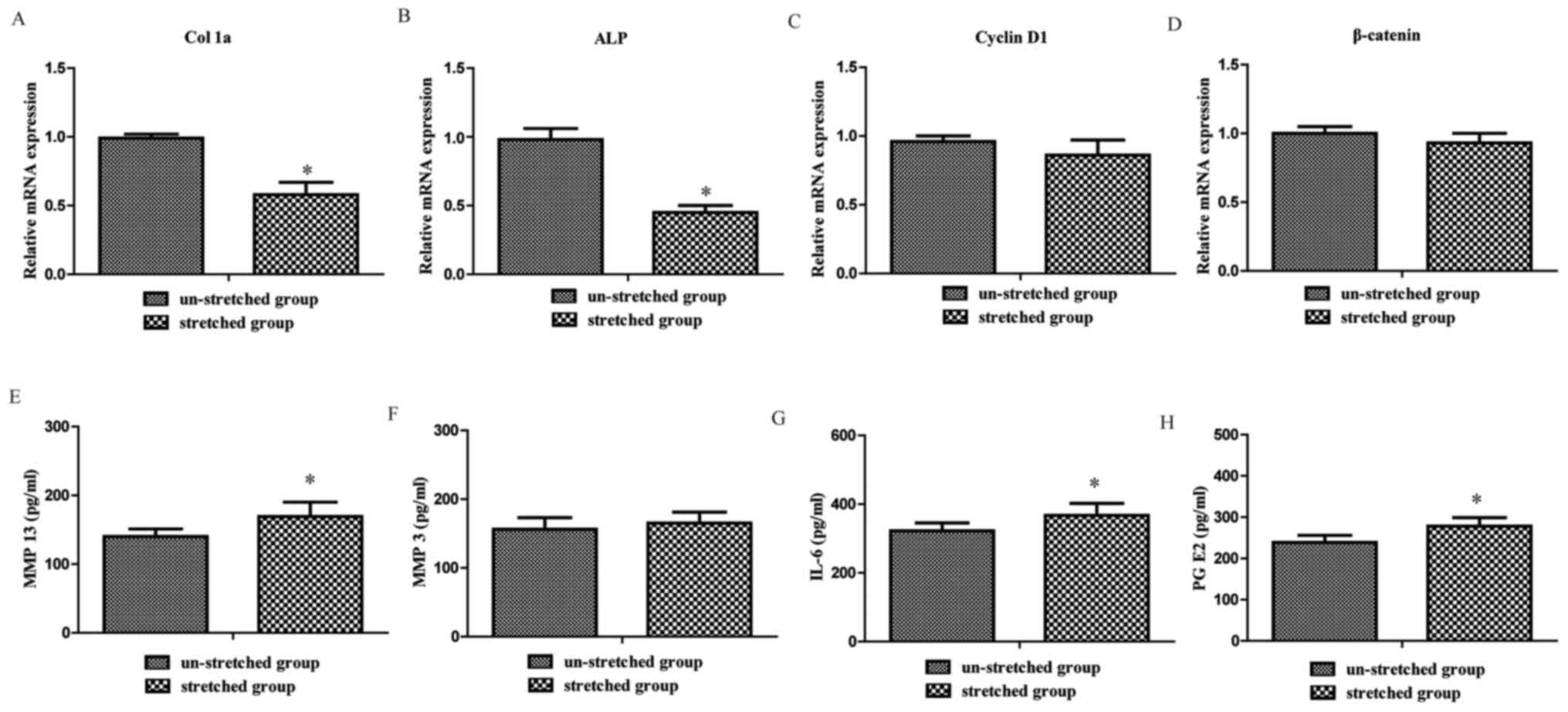
osteoblasts. The RT-qPCR results revealed that Col 1a and ALP
expression levels in the stretched group were significantly
decreased compared with the un-stretched group, respectively
(1.00±0.03 vs. 0.58±0.09 and 1.00±0.08 vs. 0.45±0.55, both
P<0.05; Fig. 3A and B). However,
no significant difference was observed between the stretched and
un-stretched groups for Cyclin D1 and β-catenin gene expression
levels (1.00±0.04 vs. 0.86±0.11 and 1.00±0.05 vs. 0.93±0.07, both
P>0.05; Fig. 3C and D). These
results indicated that excessive mechanical stretch inhibited
osteoblast osteogenesis.
Excessive mechanical stretch increases
the levels of inflammatory cytokines in the supernatants of
osteoblasts
The present study investigated the mechanism
underlying the effect of stretch-stimulated osteoblasts on
chondrocytes. ELISAs were performed to assess the levels of
inflammatory cytokines released from osteoblasts. The results
revealed that MMP 13 (140.5±11.2 vs. 169.7±21.1 pg/ml), IL-6
(322.2±23.6 vs. 367.2±35.4 pg/ml) and PG E2 (239.7±17.6 vs.
278.1±21.5 pg/ml) levels were significantly upregulated in the
supernatants of osteoblasts subjected to mechanical stretch
compared with the un-stretched group (P<0.05; Fig. 3E, G and H). By contrast, no
significant difference between the stretched and un-stretched
groups was observed for MMP 3 (156.2±17.1 vs. 165.1±16.3 pg/ml;
P>0.05; Fig. 3F).
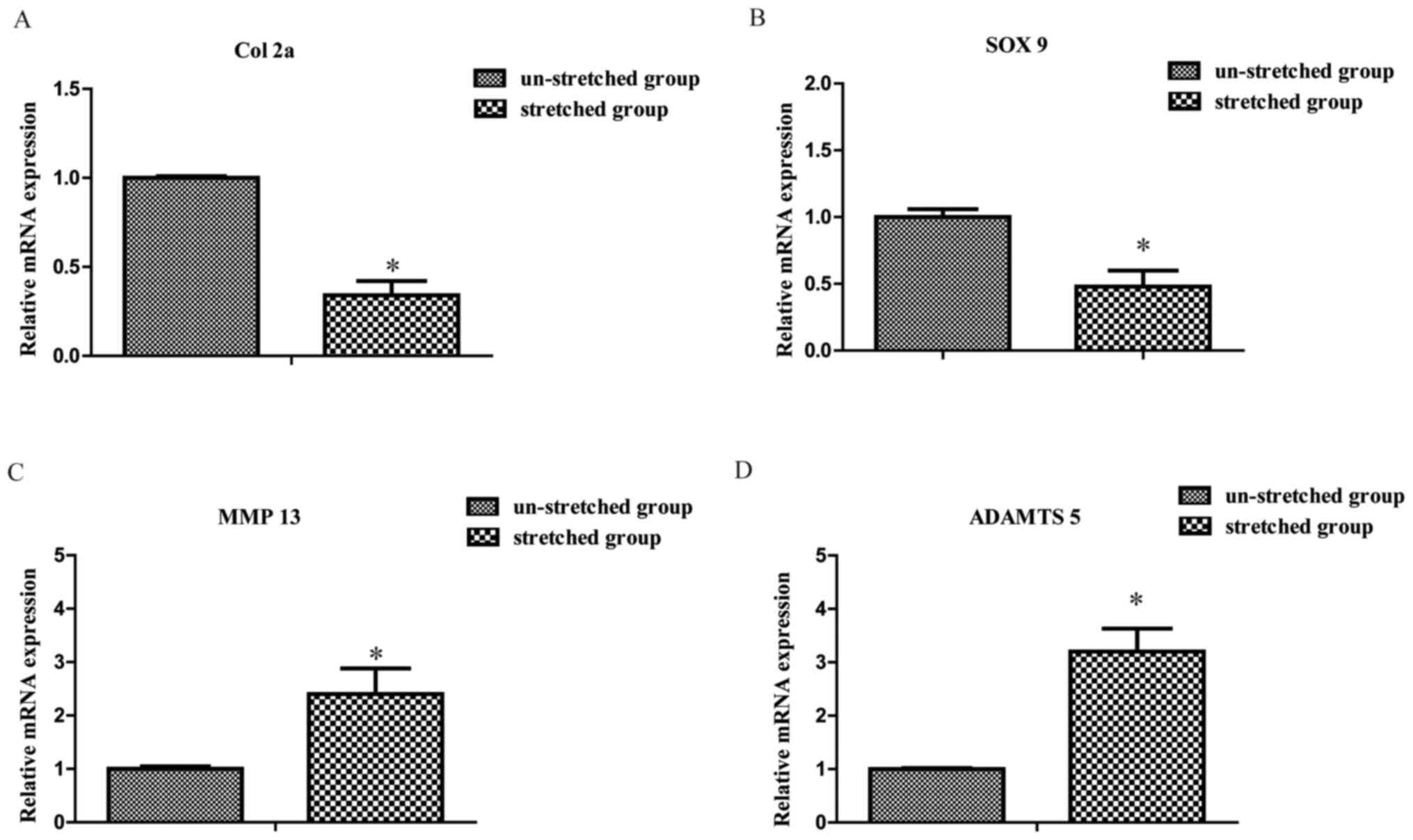
Excessive mechanical stretch induces
chondrocyte catabolism
Compared with the un-stretched group, the mRNA
expression levels of Col 2a and SOX 9 were significantly decreased
in the stretched group, respectively (1.00±0.01 vs. 0.34±0.08 and
1.00±0.06 vs. 0.48±0.12, P<0.05; Fig. 4A and B). By contrast, the expression
levels of MMP 13 and a disintegrin and metalloproteinase with
thrombospondin-like motifs 5 (ADAMTS 5) were significantly
increased in the stretched group compared with the un-stretched
group, respectively (1.00±0.05 vs. 2.40±0.48 and 1.00±0.02 vs.
3.20±0.43, both P<0.05; Fig. 4C and
D).
Stretch-stimulated osteoblasts induce
the catabolism and apoptosis of co-cultured chondrocytes
In order to investigate the effects of excessively
stressed osteoblasts on the metabolism and apoptosis of
chondrocytes, the present study established an indirect
osteoblast-chondrocyte co-culture model. As presented in Fig. 5B and C, the mRNA expression levels
of Col 2a and SOX 9 in the Scm group were significantly decreased
compared with the control group, respectively (1.00±0.01 vs.
0.36±0.03 and 1.00±0.02 vs. 0.34±0.03, both P<0.05). Moreover,
the mRNA expression levels of MMP 13 (Fig. 5A), Col X (Fig. 5D), OCN (Fig. 5E) and ADAMTS 5 (Fig. 5F) were significantly increased in
the Scm group compared with the control group, respectively
(1.00±0.02 vs. 2.20±0.21 and 1.00±0.03 vs. 1.78±0.12, 1.00±0.03 vs.
1.95±0.11 and 1.00±0.01 vs. 1.86±0.11, all P<0.05). Compared
with the control group, the flow cytometry results revealed that
the apoptosis rate was significantly increased in the Scm group
(4.50±0.33% vs. 14.30±0.90%, P<0.05; Fig. 5G and H). Furthermore, compared with
the control group, the protein expression levels of cleaved caspase
3/caspase 3 were significantly upregulated in the Scm group
(1.00±0.01 vs. 2.78±0.22, P<0.05; Fig. 5I and J).
 | Figure 5.Inhibition of the Wnt signaling
pathway mitigates mechanically stretched osteoblast-mediated
effects on the catabolism and apoptosis of chondrocytes. mRNA
expression levels of (A) MMP 13, (B) Col 2a, (C) SOX 9, (D) Col X,
(E) OCN and (F) ADAMTS 5 were determined. mRNA expression levels of
MMP 13, Col X, OCN and ADAMTS 5 in the Scm group were significantly
increased compared with the control group (chondrocytes without
co-culture). Expression levels of Col 2a and SOX 9 were
significantly lower in the Scm group compared with the control
group. Scm-mediated effects were significantly inhibited by
XAV-939. Cell apoptosis was (G) assessed by flow cytometry and (H)
quantified by FACS analysis after staining with Annexin V and PI.
The results demonstrated that the rate of apoptosis in the Scm
group was significantly increased compared with the control group,
which was significantly reversed by XAV-939. Protein expression
levels were (I) determined via western blotting and semi-quantified
for (J) cleaved caspase 3/caspase 3, (K) GSK-3β and (L) β-catenin.
Protein expression levels of β-catenin and cleaved caspase
3/caspase 3 in the Scm group were significantly higher compared
with the control group, whereas GSK-3β protein expression levels in
the Scm group were significantly lower compared with the control
group. Scm-mediated effects were partly inhibited by XAV-939. Data
were analyzed using one-way ANOVA followed by Tukey's post hoc
test. *P<0.05. MMP, matrix metalloproteinase; Col, collagen;
OCN, osteocalcin; ADAMTS 5, a disintegrin and metalloproteinase
with thrombospondin-like motifs 5; Scm, stretched conditioned
medium; Ucm, un-stretched conditioned medium; PI, propidium iodide;
SOX 9, SRY-associated high mobility group-box gene 9. |
Wnt/β-catenin signaling pathway is involved in the
mechanism underlying stretched osteoblast-mediated alterations to
the metabolism and apoptosis of chondrocytes. The present study
further investigated the effect of stretched osteoblasts on the
metabolism and apoptosis of chondrocytes. The western blotting
results demonstrated that the protein expression levels of
β-catenin in the Scm group were significantly higher compared with
the control group (1.00±0.02 vs. 1.86±0.11, P<0.05; Fig. 5I and L), whereas the protein
expression levels of GSK-3β in the Scm group were significantly
lower in the Scm group compared with the control group (1.00+0.03
vs. 0.48±0.05, P<0.05; Fig. 5I and
K). By using the Wnt inhibitor XAV-939, the present study also
investigated whether the Wnt/β-catenin signaling pathway was
involved in stretched osteoblast-mediated effects on chondrocytes.
The results indicated that the Wnt inhibitor notably mitigated
stretched osteoblast-mediated effects on chondrocytes (all
P<0.05; Fig. 5A-L), suggesting
that the Wnt/β-catenin signaling pathway was involved in stretched
osteoblast-mediated effects on the metabolism and apoptosis of the
chondrocytes.
Discussion
Articular cartilage exists in a complex environment
with various mechanical stresses that serve important roles in
regulating the metabolism of articular chondrocytes (11,33).
OA induced by mechanical force is characterized by decreased
chondrocyte proliferation and degradation of the extracellular
matrix (10–11). Bleuel et al (17) reported that a mechanical stretch of
3–10% protects chondrocytes from catabolism, whereas excessive
mechanical stretch causes chondrocyte catabolism. Consistent with
these previous results, the results of the present study
demonstrated that a mechanical stretch of 20% induced chondrocyte
catabolism, demonstrating that excessive mechanical stretch
resulted in the catabolism of chondrocytes.
Subchondral bone serves an important role in the
development of OA. A moderate amount of mechanical load has been
reported to serve an important role in maintaining bone balance and
bone mass (5–6). However, bone cells are not always in a
state of physiological stress; in some cases, the bone tissue is
subjected to the overloaded mechanical environment (34,35).
Our previous studies demonstrated that high intensity exercise
induced decreased mineralization of subchondral bone and stiffer
trabecular bone, which adversely affected the overlying articular
cartilage (36,37). In addition, Tang et al
(38) suggested that the effect of
mechanical stretch (0, 6, 12 and 18%) on osteoblasts occurred in a
magnitude-dependent manner; proper mechanical stretch promotes
osteoblast proliferation and differentiation, whereas high
mechanical stretch inhibits osteoblast proliferation and
differentiation. Fushiki et al (31) considered 18% mechanical stretch as
high magnitude stretch, Lin et al (39) considered 23% mechanical stretch as
excessive mechanical stretch for osteoblasts, Zhang et al
(40) used 20% mechanical stretch
as excessive mechanical stretch for smooth muscle cells, and Yao
et al (7) used 20%
mechanical stretch as excessive mechanical stretch for osteoblasts.
According to the experimental design in the present study, 20%
mechanical stretch was selected as excessive mechanical stretch.
The results of the present study demonstrated that an excessive
mechanical stretch of 20% inhibited osteogenic differentiation of
osteoblasts.
Articular cartilage and the underlying subchondral
bone have been considered as a functional unit (41). Yao et al (7) reported that mechanical stimulation
altered the cartilage metabolism by directly affecting the
subchondral bone during exercise and mechanical loading.
Chondrocytes in the cartilage and osteoblasts in the subchondral
bone are the primary cells involved in communication under
mechanical conditions (42).
Previous studies have performed numerous co-culture experiments to
investigate the interactions between chondrocytes and osteoblasts
(43,44). Co-culture can be divided into direct
co-culture and indirect co-culture. Direct co-culture models permit
cell-cell contact and paracrine interactions between osteoblast and
chondrocytes in a 3-dimensional culture. The direct co-culture
model has previously been used to determine the effects of
co-culture on the phenotypic maintenance of osteoblasts and
chondrocytes (43). On the other
hand, indirect co-culture models allow cells to share medium
without direct contact, which has also been used to investigate the
interactions between osteoblasts and chondrocytes in previous
studies (7,39). In the present study, an indirect
chondrocyte-osteoblast co-culture model was established to
investigate the effect of osteoblasts on excessive stretched
chondrocytes. Conditioned medium from Scm and Ucm osteoblasts was
collected to stimulate articular chondrocytes. Consistent with
previous studies (39,45), the Scm group displayed significantly
decreased mRNA expression levels of Col 2a and SOX 9 compared with
the control group. Furthermore, the mRNA expression levels of MMP
13, OCN and Col X were significantly upregulated in the Scm group
compared with the control group. OCN and Col X are factors of the
hypertrophic chondrocyte phenotype (35). These results indicated that
excessive stretched osteoblasts inhibited chondrocyte matrix
synthesis and promoted a hypertrophic change in chondrocytes.
Furthermore, the results of the present study demonstrated that the
apoptosis rate was significantly increased in the Scm group
compared with the control group. Collectively, the results
suggested that subchondral osteoblasts adversely affected
chondrocytes under excessive mechanical loading.
There are molecular communications between articular
cartilage and subchondral bone, which may be altered during the
development of OA (34,35,44).
Previous studies have reported that excessive mechanical
stimulation increases the production of cytokines and MMPs by
chondrocytes (11,46). In addition, a previous report
indicated that excessive stimulation increases the expression of
MMP 13, leading to bone remodeling (47). High magnitude stretch applied to
osteoblasts increases PG E2 production, which is critical for bone
remodeling (48). Consistent with
previous studies, the results of the present study demonstrated
that, compared with the un-stretched group, excessive mechanical
stretch significantly promoted osteoblast production of MMP 13,
IL-6 and PG E2, which have been reported as factors for OA
(49). These findings suggested
that excessive mechanical stretch may induce the catabolism and
apoptosis of chondrocytes via an osteoblast-associated
mechanism.
The Wnt/β-catenin signaling pathway serves an
important role in the development and maintenance of cartilage, but
its detailed function remains controversial (22,23).
During Wnt signaling, GSK-3β causes the degradation of newly
synthesized β-catenin. Inactivation of GSK-3β leads to the
accumulation of β-catenin, thus mediating proliferation and
apoptosis (25,27,50).
The results of the present study revealed that the protein
expression levels of β-catenin were significantly upregulated in
the Scm group compared with the control group, and the effect of
stretched osteoblasts on chondrocytes was partly alleviated by Wnt
inhibitor XAV-939. Compared with the Scm group, chondrocytes in the
Scm + XAV-939 group displayed significantly decreased mRNA
expression levels of MMP 13, ADAMTS 5, Col X and OCN. Meanwhile,
the mRNA expression levels of Col 2a and SOX 9 were significantly
upregulated. These findings suggested that blocking the
Wnt/β-catenin signaling pathway can promote the anabolism of
chondrocytes and inhibit chondrocyte hypertrophy. These results
demonstrated that excessive mechanically stretched osteoblasts
affected the characteristics of chondrocytes via the Wnt/β-catenin
signaling pathway.
The present study had a number of limitations.
First, osteoblasts were isolated from rat calvaria rather than the
subchondral bone; however, a previous study demonstrated that
osteoblasts from calvaria or long bones respond similarly to
mechanical strain (41), ensuring
the validity of the model in the present study. Secondly, the
results of the present study demonstrated that high mechanical
stretch increased MMP 13 and ADAMTS 5 expression levels by direct
mechanical stretching of chondrocytes and indirect co-culture with
stretched osteoblast conditioned culture medium, but it is unknown
which happened first. Third, a previous study demonstrated that
chondrocytes can alter the characteristics of osteoblasts in a
co-culture model, suggesting that stretched chondrocytes may affect
osteoblasts (51). Both
chondrocytes and osteoblasts are subjected to mechanical loading in
the human body. Similar to a number of previous studies (7,39,45),
the present study primarily focused on the effect of mechanically
stretched osteoblasts on chondrocytes. Further investigations are
required to understand the effect of mechanically stretched
osteoblasts on mechanically stretched chondrocytes, as well as
their interactions. Forth, Dickkopf1 (DKK1) is a canonical
Wnt/β-catenin inhibitor, but it has also been reported to serve as
an inducer of AKT (52). Our
preliminary experiment suggested that DKK1 may partly alleviate the
effect of stretched osteoblasts on chondrocytes (data not shown);
however, further investigation is required to understand whether or
not this effect is due to the activation of AKT signaling.
In conclusion, the findings of this study indicated
that excessive mechanical stretch promoted chondrocyte catabolism
and inhibited osteoblast osteogenesis. In addition, alterations in
chondrocyte can be induced by stressed osteoblasts, providing a
possible explanation for the onset and progression of OA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81572219 and
81871848), the Fujian Medical Innovation Program (grant no.
2017-CX-25), the Medical Health Science and Technology Project of
Guangzhou (grant no. 20191A011081) and the Medical Research Fund of
Science and Technology of Guangdong (grant no. A2018549).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GXN designed the study. CXS, SYL and SYX performed
the experiments and interpreted the data. CXS and SYL collected the
data. CXS, SYL and WTZ analyzed the data. GXN, CXS, SYL, WTZ and
SYX wrote the manuscript. All authors read and approved the final
version of the manuscript. GXN and CXS confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Nanfang Hospital, Southern Medical University
(approval no. NFYY-2016-128; Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bowden JL, Hunter DJ, Deveza LA, Duong V,
Dziedzic KS, Allen KD, Chan PK and Eyles JP: Core and adjunctive
interventions for osteoarthritis: Efficacy and models for
implementation. Nat Rev Rheumatol. 16:434–447. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qian J, Liang J, Wang Y and Wang H: Effect
of passive motion on articular cartilage in rat osteoarthritis. Exp
Ther Med. 8:377–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ni GX, Liu SY, Lei L, Li Z, Zhou YZ and
Zhan LQ: Intensity-dependent effect of treadmill running on knee
articular cartilage in a rat model. BioMed Res Int.
2013:1723922013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldring MB and Goldring SR: Articular
cartilage and subchondral bone in the pathogenesis of
osteoarthritis. Ann N Y Acad Sci. 1192:230–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldring SR and Goldring MB: Changes in
the osteochondral unit during osteoarthritis: Structure, function
and cartilage-bone crosstalk. Nat Rev Rheumatol. 12:632–644. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao Z, Chen P, Wang S, Deng G, Hu Y, Lin
Q, Zhang X and Yu B: Reduced PDGF-AA in subchondral bone leads to
articular cartilage degeneration after strenuous running. J Cell
Physiol. 234:17946–17958. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanchez C, Pesesse L, Gabay O, Delcour JP,
Msika P, Baudouin C and Henrotin YE: Regulation of subchondral bone
osteoblast metabolism by cyclic compression. Arthritis Rheum.
64:1193–1203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan YX, Gong YW, Guo Y, Lv Q, Guo C,
Zhuang Y, Zhang Y, Li R and Zhang XZ: Mechanical strain regulates
osteoblast proliferation through integrin-mediated ERK activation.
PLoS One. 7:e357092012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agarwal S, Long P, Gassner R, Piesco NP
and Buckley MJ: Cyclic tensile strain suppresses catabolic effects
of interleukin-1beta in fibrochondrocytes from the
temporomandibular joint. Arthritis Rheum. 44:608–617. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu G, Qian Y, Wu W and Li R: Negative
effects of high mechanical tensile strain stimulation on
chondrocyte injury in vitro. Biochem Biophys Res Commun. 510:48–52.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takebe K, Nishiyama T, Hayashi S,
Hashimoto S, Fujishiro T, Kanzaki N, Kawakita K, Iwasa K, Kuroda R
and Kurosaka M: Regulation of p38 MAPK phosphorylation inhibits
chondrocyte apoptosis in response to heat stress or mechanical
stress. Int J Mol Med. 27:329–335. 2011.PubMed/NCBI
|
|
13
|
Kaufmann SH, Lee SH, Meng XW, Loegering
DA, Kottke TJ, Henzing AJ, Ruchaud S, Samejima K and Earnshaw WC:
Apoptosis associated caspase activation assays. Methods.
44:262–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iannone F and Lapadula G: Phenotype of
chondrocytes in osteoarthritis. Biorheology. 45:411–413. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laowanitwattana T, Aungsuchawan S,
Narakornsak S, Markmee R, Tancharoen W, Keawdee J, Boonma N, Tasuya
W, Peerapapong L, Pangjaidee N, et al: Osteoblastic differentiation
potential of human amniotic fluid-derived mesenchymal stem cells in
different culture conditions. Acta Histochem. 120:701–712. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lefebvre V and Dvir-Ginzberg M: SOX9 and
the many facets of its regulation in the chondrocyte lineage.
Connect Tissue Res. 58:2–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bleuel J, Zaucke F, Brüggemann GP and
Niehoff A: Effects of cyclic tensile strain on chondrocyte
metabolism: A systematic review. PLoS One. 10:e01198162015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakao K, Takahashi KA, Mazda O, Arai Y,
Tonomura H, Inoue A, Saito M, Fujioka M, Takamiya H, Imanishi J, et
al: Enhanced expression of interleukin-6, matrix
metalloproteinase-13, and receptor activator of NF-kappaB ligand in
cells derived from osteoarthritic subchondral bone. J Orthop Sci.
13:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verma P and Dalal K: ADAMTS-4 and
ADAMTS-5: Key enzymes in osteoarthritis. J Cell Biochem.
112:3507–3514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruan G, Xu J, Wang K, Wu J, Zhu Q, Ren J,
Bian F, Chang B, Bai X, Han W, et al: Associations between knee
structural measures, circulating inflammatory factors and MMP13 in
patients with knee osteoarthritis. Osteoarthritis Cartilage.
26:1063–1069. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nusse R: Wnt signaling in disease and in
development. Cell Res. 15:28–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh H, Chun CH and Chun JS: Dkk-1
expression in chondrocytes inhibits experimental osteoarthritic
cartilage destruction in mice. Arthritis Rheum. 64:2568–2578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miclea RL, Siebelt M, Finos L, Goeman JJ,
Löwik CW, Oostdijk W, Weinans H, Wit JM, Robanus-Maandag EC and
Karperien M: Inhibition of Gsk3β in cartilage induces
osteoarthritic features through activation of the canonical Wnt
signaling pathway. Osteoarthritis Cartilage. 19:1363–1372. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen M, Zhu M, Awad H, Li TF, Sheu TJ,
Boyce BF, Chen D and O'Keefe RJ: Inhibition of beta-catenin
signaling causes defects in postnatal cartilage development. J Cell
Sci. 121:1455–1465. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niu Q, Li F, Zhang L, Xu X, Liu Y, Gao J
and Feng X: Role of the Wnt/β-catenin signaling pathway in the
response of chondrocytes to mechanical loading. Int J Mol Med.
37:755–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liedert A, Wagner L, Seefried L, Ebert R,
Jakob F and Ignatius A: Estrogen receptor and Wnt signaling
interact to regulate early gene expression in response to
mechanical strain in osteoblastic cells. Biochem Biophys Res
Commun. 394:755–759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan YB, Li JM, Xiao E, An JG, Gan YH and
Zhang Y: A pilot trial on the molecular pathophysiology of
traumatic temporomandibular joint bony ankylosis in a sheep model.
Part I: Expression of Wnt signaling. J Craniomaxillofac Surg.
42:e15–e22. 2014. View Article : Google Scholar
|
|
30
|
Chen X, Guo J, Yuan Y, Sun Z, Chen B, Tong
X, Zhang L, Shen C and Zou J: Cyclic compression stimulates
osteoblast differentiation via activation of the Wnt/β-catenin
signaling pathway. Mol Med Rep. 15:2890–2896. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fushiki R, Mayahara K, Ogawa M, Takahashi
Y, Karasawa Y, Tsurumachi N, Tamura T and Shimizu N: High-magnitude
mechanical strain inhibits the differentiation of bone-forming rat
calvarial progenitor cells. Connect Tissue Res. 56:336–341. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang RK, Li GW, Zeng C, Lin CX, Huang LS,
Huang GX, Zhao C, Feng SY and Fang H: Mechanical stress contributes
to osteoarthritis development through the activation of
transforming growth factor beta 1 (TGF-β1). Bone Joint Res.
7:587–594. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Findlay DM and Atkins GJ:
Osteoblast-chondrocyte interactions in osteoarthritis. Curr
Osteoporos Rep. 12:127–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan XL, Meng HY, Wang YC, Peng J, Guo QY,
Wang AY and Lu SB: Bone-cartilage interface crosstalk in
osteoarthritis: Potential pathways and future therapeutic
strategies. Osteoarthritis Cartilage. 22:1077–1089. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Z, Liu SY, Xu L, Xu SY and Ni GX:
Effects of treadmill running with different intensity on rat
subchondral bone. Sci Rep. 7:19772017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu SY, Li Z, Xu SY, Xu L, Yang M and Ni
GX: Intensity dependent effect of treadmill running on
differentiation of rat bone marrow stromal cells. Mol Med Rep.
17:7746–7756. 2018.PubMed/NCBI
|
|
38
|
Tang L, Lin Z and Li YM: Effects of
different magnitudes of mechanical strain on Osteoblasts in vitro.
Biochem Biophys Res Commun. 344:122–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin YY, Tanaka N, Ohkuma S, Iwabuchi Y,
Tanne Y, Kamiya T, Kunimatsu R, Huang YC, Yoshioka M, Mitsuyoshi T,
et al: Applying an excessive mechanical stress alters the effect of
subchondral osteoblasts on chondrocytes in a co-culture system. Eur
J Oral Sci. 118:151–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang WM, Liu Y, Li TT, Piao CM, Liu O,
Liu JL, Qi YF, Jia LX and Du J: Sustained activation of ADP/P2ry12
signaling induces SMC senescence contributing to thoracic aortic
aneurysm/dissection. J Mol Cell Cardiol. 99:76–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu W, Chen Y, Dou C and Dong S:
Microenvironment in subchondral bone: Predominant regulator for the
treatment of osteoarthritis. Ann Rheum Dis. 80:413–422. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saltzman BM and Riboh JC: Subchondral bone
and the osteochondral unit: Basic science and clinical implications
in sports medicine. Sports Health. 10:412–418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang J, Nicoll SB and Lu HH: Co-culture
of osteoblasts and chondrocytes modulates cellular differentiation
in vitro. Biochem Biophys Res Commun. 338:762–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sanchez C, Deberg MA, Piccardi N, Msika P,
Reginster JY and Henrotin YE: Subchondral bone osteoblasts induce
phenotypic changes in human osteoarthritic chondrocytes.
Osteoarthritis Cartilage. 13:988–997. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Priam S, Bougault C, Houard X, Gosset M,
Salvat C, Berenbaum F and Jacques C: Identification of soluble
14-3-3є as a novel subchondral bone mediator involved in cartilage
degradation in osteoarthritis. Arthritis Rheum. 65:1831–1842. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sanchez C, Deberg MA, Piccardi N, Msika P,
Reginster JY and Henrotin YE: Osteoblasts from the sclerotic
subchondral bone downregulate aggrecan but upregulate
metalloproteinases expression by chondrocytes. This effect is
mimicked by interleukin-6, −1beta and oncostatin M pre-treated
non-sclerotic osteoblasts. Osteoarthritis Cartilage. 13:979–987.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sasaki K, Takagi M, Konttinen YT, Sasaki
A, Tamaki Y, Ogino T, Santavirta S and Salo J: Upregulation of
matrix metalloproteinase (MMP)-1 and its activator MMP-3 of human
osteoblast by uniaxial cyclic stimulation. J Biomed Mater Res B
Appl Biomater. 80:491–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fermor B, Gundle R, Evans M, Emerton M,
Pocock A and Murray D: Primary human osteoblast proliferation and
prostaglandin E2 release in response to mechanical strain in vitro.
Bone. 22:637–643. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Akhtar N, Khan NM, Ashruf OS and Haqqi TM:
Inhibition of cartilage degradation and suppression of PGE2 and
MMPs expression by pomegranate fruit extract in a model of
posttraumatic osteoarthritis. Nutrition. 33:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weng LH, Wang CJ, Ko JY, Sun YC and Wang
FS: Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage
destruction, and subchondral bone deterioration in osteoarthritic
knees. Arthritis Rheum. 62:1393–1402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Prasadam I, Friis T, Shi W, van Gennip S,
Crawford R and Xiao Y: Osteoarthritic cartilage chondrocytes alter
subchondral bone osteoblast differentiation via MAPK signalling
pathway involving ERK1/2. Bone. 46:226–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kimura H, Fumoto K, Shojima K, Nojima S,
Osugi Y, Tomihara H, Eguchi H, Shintani Y, Endo H, Inoue M, et al:
CKAP4 is a Dickkopf1 receptor and is involved in tumor progression.
J Clin Invest. 126:2689–2705. 2016. View Article : Google Scholar : PubMed/NCBI
|