Introduction
Cervical cancer (CC) is one of the most common
gynecological neoplasms among women worldwide (1). The incidence and mortality rates of CC
have been increasing every year in developed and developing
countries (2,3). Despite the fact that encouraging
improvements have been achieved in CC chemotherapy, radiotherapy
and surgical therapies, the 5-year survival rate of patients with
CC remains unsatisfactory (4).
Therefore, it is essential to identify effective therapeutic
targets and discover molecular mechanisms in CC progression.
Circular RNAs (circRNAs/circs), a novel class of
non-coding RNAs, are featured with a continuous closed loop without
5′-3′ polarity or a polyadenylated tail (5). In recent years, accumulating evidence
has confirmed that circRNAs have significant implications in
numerous tumors (6). circRNAs have
been reported to be involved in the biological behaviors of tumor
cells, including proliferation, survival, apoptosis and metastasis
(7,8). For example, hsa_circ_0000263
facilitates CC progression by regulating CC cell proliferation and
migration (9). Ou et al
(10) revealed that circ_angiomotin
like 1 (AMOTL1) could promote CC growth by promoting AMOTL1
expression. Moreover, a study by Wang et al (11) reported that hsa_circ_0101119
expression was notably upregulated in the peripheral blood of
patients with cervical squamous cell carcinoma. However, to the
best of our knowledge, the role of hsa_circ_0101119 on CC has not
yet been evaluated.
Recently, an increasing number of researchers have
paid attention to the application of circRNAs in cancer diagnosis
(12,13). The main mechanisms via which
circRNAs serve a role in cancer are by directly interacting with
microRNAs (miRNAs) or RNA binding proteins (RBPs) (8,14–16).
Eukaryotic initiation factor 4A-3 (EIF4A3) is one of the RBPs that
is a core component of the exon junction complex (17). The roles of EIF4A3 are reported to
be complex and important in numerous diseases, and the abnormal
structure and function of EIF4A3 directly lead to the changes of
its downstream biological effects (18).
Members of the transcription elongation factor
A-like (TCEAL) gene family contain TFA domains and may function as
nuclear phosphoproteins that modulate transcription in a promoter
context-dependent manner (19–21).
At present, TCEAL7 is frequently deregulated in tumors, and its
decreased expression often correlates with malignant clinical
process and poor prognosis in multiple cancer types, including
gastric cancer and non-small cell lung cancer (22,23).
Moreover, Chien et al (24)
revealed that TCEAL6 has a sequence similarity to TCEAL7.
Interestingly, TCEAL6 is reported to be lowly expressed in the
early stage of CC (25). However,
whether hsa_circ_0101119 affects the progression of CC via an
interaction with EIF4A3 to regulate TCEAL6 expression remains
unknown. The present study aimed to investigate the function of
hsa_circ_0101119 in CC, and its regulatory mechanism that is
associated with EIF4A3 and TCEAL6.
Materials and methods
Bioinformatics analysis
Gene expression data matrix (GSE102686) was derived
from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/), which
included five CC samples and five adjacent non-tumorous samples
(26). The present study identified
the top five hsa_circRNAs with the highest differential expression,
and selected hsa_circ_0101119 as the research target. Then,
RNA-Protein Interaction Prediction (RPISeq; version 1.0; http://pridb.gdcb.iastate.edu/RPISeq/)
was used to predict the interaction probabilities of RNA-binding
protein EIF4A3 with hsa_circ_0101119 and TCEAL6.
Cell culture and transfection
The four human CC cell lines (C-33A, SiHa, CaSki and
HeLa) and the normal human cervical epithelial cell line, HcerEpic,
were supplied by VCANBIO Cell & Gene Engineering Co., Ltd. All
the cells were cultured in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% (v/v) FBS (Invitrogen;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.), and cultured at 37°C
in a humidified 5% CO2 incubator.
Short hairpin (sh)RNA targeting EIF4A3 (sh-EIF4A3)
and TCEAL6 (sh-TCEAL6), small interfering (si)RNA targeting
hsa_circ_0101119 (si-hsa_circ), pcDNA3.1-TCEAL6 (pcDNA-TCEAL6) and
their correspond negative controls (sh-NC, si-NC and pcDNA-NC) were
purchased from Shanghai GenePharma Co., Ltd. The sh-NC was a
plasmid containing a non-targeting (scramble) shRNA sequences, and
the si-NC was a non-targeting (scramble) siRNA sequence. The
sequences were as follows: Sh-EIF4A3, 5′-GGAAGACATGACTAAAGTGGA−3′;
sh-TCEAL6, 5′-GGAGAAGGGATCCGGTAGATT−3′; sh-NC,
5′-GGTAGTGGACGATGAGACAGT−3′; si-hsa_circ,
5′-ATGAGCAGCCATACACTGCTT−3′; si-NC, 5′-GCTCTACTTCGACGACAAGAT−3′.
According to the manufacturer's instruction, the cells were
transfected with sh-EIF4A3 (20 µl/ml), sh-TCEAL6 (20 µl/ml),
si-hsa_circ (50 nM) or pcDNA-TCEAL6 (4 µg) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. Finally, the transfection
efficiency was determined via reverse transcription-quantitative
PCR (RT-qPCR).
Colony formation assay
The transfected SiHa and HeLa cells (500 cells/well)
were seeded into 6-well plates. Then, the cells were grown for 14
days at 37°C. Next, 4% paraformaldehyde was used to fix colonies
for 15 min at room temperature and 0.1% crystal violet was used to
stain colonies for 30 min at room temperature. Finally, the numbers
of colonies containing >500 cells were assessed using a light
microscope (magnification, ×100).
5-Ethynyl-20-deoxyuridine (EdU)
incorporation assay
The EdU assay was carried out with the EdU
labeling/detection kit (Guangzhou RiboBio Co., Ltd.) according to
the manufacturer's instructions. The transfected SiHa and HeLa
cells were incubated with 50 µM EdU solution for 2 h at room
temperature. Then, the cells were fixed with 4% paraformaldehyde
for 30 min at room temperature, followed by permeabilized with 0.5%
Triton X-100 at room temperature and stained with anti-EdU working
solution for 30 min in the dark at room temperature. Finally, DAPI
(Sigma-Aldrich; Merck KGaA) was used for staining the cell nucleus
at room temperature for 30 min, and the EdU-positive cells were
observed using fluorescent microscopy (magnification, ×200).
Flow cytometry
The apoptosis of SiHa and HeLa cells was detected
using the Annexin V-PI kit (Beyotime Institute of Biotechnology).
The transfected SiHa and HeLa cells were collected and resuspended
in the binding buffer. Subsequently, the cells were labeled with
Annexin V-FITC and PI. Finally, cell apoptosis was detected using a
flow cytometry (FACSCalibur; BD Biosciences), and then data were
analyzed using FlowJo software (version v7.6.5; FlowJo LLC).
Transwell assay
Transwell chambers with 8-µm pores were obtained
from Corning, Inc. A total of 48 h after transfection, the SiHa and
HeLa cells were suspended into serum-free medium and seeded into
the upper chamber without Matrigel (BD Biosciences). Next, 500 µl
medium with 10% FBS was added to the lower chamber as the
chemoattractant. After a 24-h incubation at room temperature, the
migrated cells were fixed with methanol at 37°C for 30 min and
stained with 0.1% crystal violet at 37°C for 30 min, following
which they were imaged and calculated using an inverted microscope
(magnification, ×100). Moreover, the cell invasion assay was
performed at the same time with the aforementioned steps, except
that the upper chamber was coated with Matrigel at room temperature
overnight.
RNA immunoprecipitation (RIP)
assay
The RIP assay was performed using an EZ-Magna RIP™
RNA kit (MilliporeSigma) to determine the endogenous interaction
between EIF4A3 and hsa_circ_0101119 or TCEAL6, according to the
manufacturer's protocols. Briefly, the cells were cross-linked by
treating with formaldehyde for 10 min at room temperature. After
washing with cold PBS, cells were incubated in 4 ml cell lysis
buffer for 15 min in ice. After nuclear extraction using a Dounce
homogenizer (Wheaton; DWK Life Sciences), the chromatin was sheared
by sonication (25% power, 4.5 sec impact, 9 sec clearance, 14
times) at room temperature to obtain DNA fragments in a range
between 1,000-200 bp. Following DNase treatment for 30 min at 37°C,
the cell lysates were incubated with RIP buffer containing the
magnetic beads conjugated with anti-EIF4A3 antibody for 2 h at 4°C
with gently shaking. The anti-IgG antibody served as the control.
The immunoprecipitated RNA was released by the reverse
cross-linking using proteinase K at 4°C overnight. Finally, the
immunoprecipitated RNA was extracted using a RNeasy Min Elute
Cleanup kit (Qiagen, Inc.) and then analyzed using an RT-qPCR
assay.
RNA pull down assay
The interaction between hsa_circ_0101119 and EIF4A3
was assessed using the Magnetic RNA-Protein Pull-Down kit (Pierce;
Thermo Fisher Scientific, Inc.), following the manufacturer's
instructions. Biotin-labeled hsa_circ_0101119 or antisense RNA,
which was supplied by Genepharm, Inc., was co-incubated with the
magnetic beads and the protein extract of SiHa or Hela cells. The
pull-down protein was measured via western blotting.
RT-qPCR
Total RNA from cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. Subsequently,
cDNA was synthesized and amplified from total RNA (2 µg) using the
One Step PrimeScript cDNA Synthesis kit (Takara Bio, Inc.)
according to the manufacturer's protocols. Then, cDNA samples (1
µl) were subjected to a qPCR reaction system in presence of SYBR
Premix Ex Taq II kit (Takara Bio, Inc.). GAPDH was used as an
endogenous reference. All primers were designed by Shanghai
GenePharma Co., Ltd. and were as follows: Hsa_circ_0101119 forward,
5′-AAGCACACCAGCTTCTCCTC-3′ and reverse, 5′-GCGTGCTGGCATAGGATTTG-3′;
EIF4A3 forward, 5′-CCCTCACCACAATGACAGCA-3′ and reverse,
5′-TGACCCACGCAGGTTAAACA-3′; TCEAL6 forward,
5′-CAGCCGCCATTTCTTTCCAG-3′ and reverse, 5′-GGAAACATCTGACCTCCGCA-3′;
and GAPDH forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′. The relative expression levels were
calculated using the 2−ΔΔCq method (27). The RT-qPCR reaction conditions were
as follows: Initial denaturation at 95°C for 3 min, followed by 40
cycles of 95°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec.
Western blot analysis
Total proteins from transfected SiHa and HeLa cells
were extracted using the cell lysis buffer (Cell Signaling
Technology, Inc.) with a protease inhibitor. An enhanced BCA
protein assay kit (Beyotime Institute of Biotechnology) was used to
quantify the protein concentration. The protein sample (50 µg) was
separated by 10% SDS-PAGE, electrophoretically transferred onto
PVDF membranes (MilliporeSigma) and blocked with 5% skimmed milk at
room temperature for 2 h. Then, the membrane was incubated with the
primary antibody overnight at 4°C and then incubated with the
HRP-labeled secondary antibody (anti-mouse IgG, cat. no. 7076,
1:5,000; anti-rabbit IgG, cat. no. 7074, 1:5,000) at room
temperature for 2 h. Finally, the bands were visualized using an
ECL detection kit (Thermo Fisher Scientific, Inc.). The following
antibodies were used: Bax (cat. no. 2772; 1:1,000), caspase-3 (cat.
no. 14220; 1:1,000), Bcl-2 (cat. no. 3498; 1:1,000), E-cadherin
(cat. no. 3195; 1:500), N-cadherin (cat. no. 14215; 1:1,000),
Vimentin (cat. no. 5741; 1:1,000) from Cell Signaling Technology,
Inc.; MMP-2 (cat. no. ab92536; 1:1,000), MMP-9 (cat. no. ab228402;
1:500), EIF4A3 (cat. no. ab180573; 1:500) from Abcam; and TCEAL6
(cat. no. ag11732; 1:500) from ProteinTech Group, Inc. The density
of the protein bands was semi-quantitated using ImageJ software
(version V1.8.0; National Institutes of Health).
Statistical analysis
Data are presented as the mean ± SEM of ≥3
experiments. All data in this study were examined using SPSS 22.0
software (IBM, Corp.) and GraphPad Prism 8.0 software (GraphPad
Software, Inc.). The differences between groups were assessed using
an unpaired or paired Student's t-test or one-way ANOVA with a
Tukey's post hoc test. The correlation between EIF4A3 and TCEAL6
was analyzed via Pearson correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
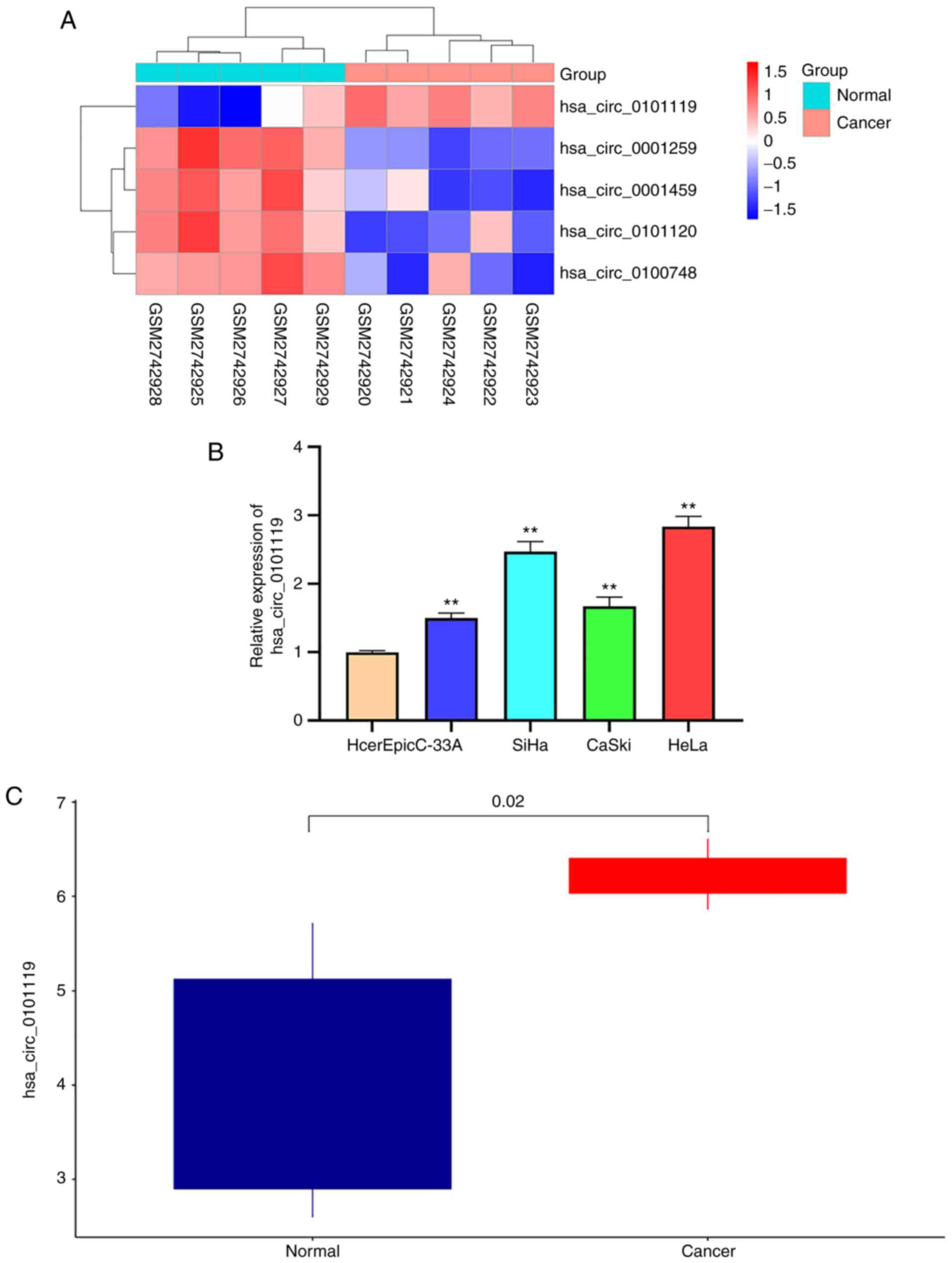
hsa_circ_0101119 is highly expressed
in CC tissues and cells
To investigate circRNA effects in CC, the most
differentially expressed circRNAs were analyzed in GSE102686
(Fig. 1A). hsa_circ_0101119 was
found to be the most significantly upregulated circRNA (Fig. 1A). Moreover, it was identified that
the expression level of hsa_circ_0101119 was notably upregulated in
CC cells and tissues (Fig. 1B and
C), further suggesting that hsa_circ_0101119 was highly
expressed in CC.
Knockdown of hsa_circ_0101119 inhibits
the proliferation of SiHa and HeLa cells
As shown in Fig. 2A,
RT-qPCR was used to verify the transfection efficiency of
hsa_circ_0101119 knockdown. Then, the effects of hsa_circ_0101119
on CC cells were assessed using EdU and colony formation assays.
The results of EdU assay demonstrated that hsa_circ_0101119
knockdown markedly reduced the proliferation of SiHa and HeLa cells
(Fig. 2B). Moreover, colony
formation assay results suggested that the cell clones of SiHa and
HeLa cells were significantly decreased after knockdown of
hsa_circ_0101119 (Fig. 2C).
Collectively, the data indicated that the knockdown of
hsa_circ_0101119 could inhibit the proliferation of SiHa and HeLa
cells.
Knockdown of hsa_circ_0101119
facilitates the apoptosis of SiHa and HeLa cells
The results of flow cytometry demonstrated that the
knockdown of hsa_circ_0101119 significantly increased the apoptosis
of SiHa and HeLa cells (Fig. 3A).
In addition, the expression levels of apoptosis-associated proteins
(Bax, caspase-3 and Bcl-2) were detected via western blot analysis.
As shown in Fig. 3B, the expression
levels of Bax and caspase-3 in SiHa and HeLa cells were markedly
upregulated after knockdown of hsa_circ_0101119, but Bcl-2
expression was significantly downregulated. These findings
suggested that the knockdown of hsa_circ_0101119 could facilitate
the apoptosis of SiHa and HeLa cells.
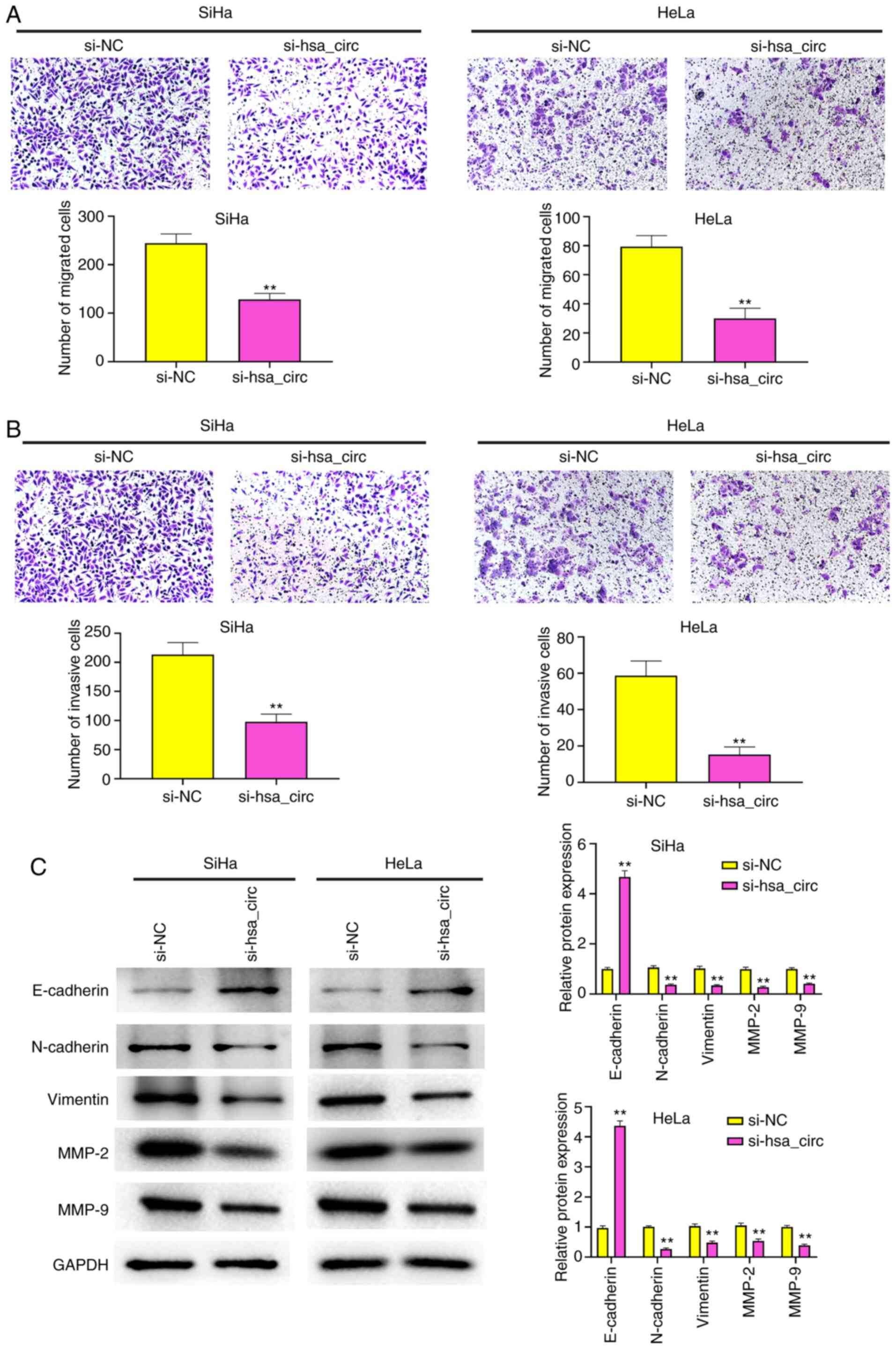
Knockdown of hsa_circ_0101119 inhibits
the migration and invasion of SiHa and HeLa cells
Knockdown of hsa_circ_0101119 significantly
decreased the migration and invasion of SiHa and HeLa cells
(Fig. 4A and B). MMPs, such as
MMP-2 and MMP-9, which are secreted by cells, are reported to
promote cancer cell invasion and migration (28). Thus, the effects of hsa_circ_0101119
knockdown on migration and invasion were determined by detecting
the expression levels of MMP-2 and MMP-9. The results demonstrated
that the knockdown of hsa_circ_0101119 significantly reduced the
expression levels of MMP-2 and MMP-9 in SiHa and HeLa cells
(Fig. 4C).
The epithelial to mesenchymal transition has been
reported to serve important roles in CC progression and metastasis
(29). The expression level of
E-cadherin in SiHa and HeLa cells was markedly increased after
knockdown of hsa_circ_0101119, but the expression levels of
N-cadherin and Vimentin were significantly decreased (Fig. 4C). These results indicated that the
knockdown of hsa_circ_0101119 could inhibit the migration and
invasion of SiHa and HeLa cells.
hsa_circ_0101119 recruits EIF4A3 to
inhibit TCEAL6 expression in CC
The bioinformatics analysis revealed that
hsa_circ_0101119 can bind to EIF4A3 (RF Classifier: 0.80; SVM
Classifier: 0.77) (Fig. 5A). Using
RIP and RNA pull down assays, it was also identified that
hsa_circ_0101119 could bind to EIF4A3 in SiHa and HeLa cells
(Fig. 5B and C). To examine whether
has_circ_0101119 could directly regulate TCEAL6 after binding with
EIF4A3, bioinformatics analysis was conducted to predict the
binding abundance between EIF4A3 and TCEAL6. The scores for RF
Classifier and SVM Classifier were 0.80 and 0.60, respectively
(Fig. 5D), suggesting that EIF4A3
likely binds to TCEAL6. Moreover, the results of RIP assay verified
this binding relationship (Fig.
5E).
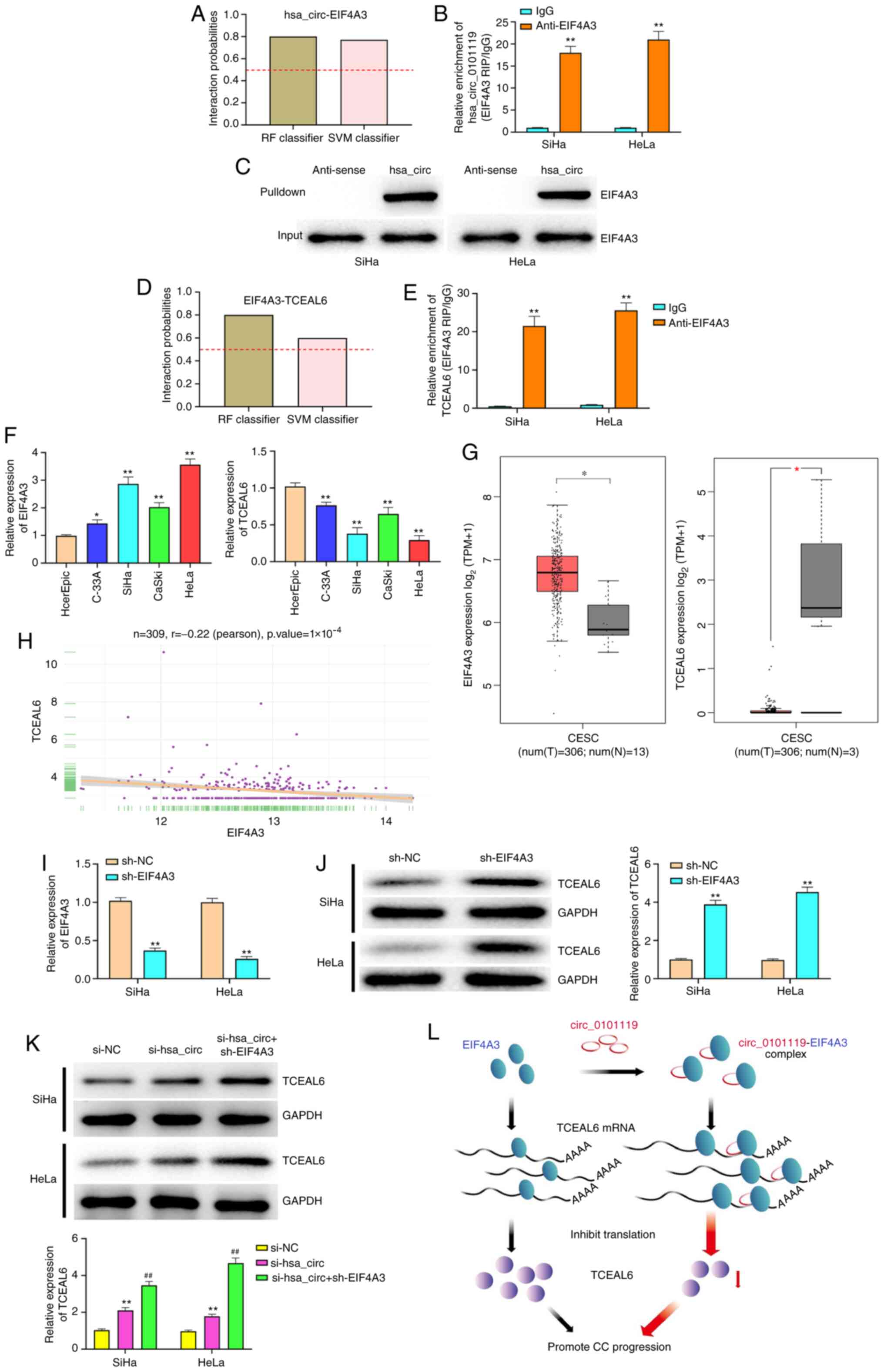 | Figure 5.hsa_circ_0101119 recruits EIF4A3 to
inhibit TCEAL6 expression in CC. (A) Bioinformatics was used to
predict the interaction probabilities of the RNA-binding protein
EIF4A3 with hsa_circ_0101119. Predictions with probabilities
>0.5 were considered ‘positive’, suggesting that the
corresponding RNA and protein are likely to interact. (B) RIP assay
using anti-EIF4A3 showed that EIF4A3 precipitated hsa_circ_0101119
in SiHa and HeLa cell lysates. (C) Pull down assay indicated that
biotin-labeled hsa_circ_0101119 interacted with EIF4A3. (D)
Bioinformatics was used to predict the interaction probabilities of
EIF4A3 with TCEAL6. (E) RIP assay using anti-EIF4A3 showed that
EIF4A3 precipitated TCEAL6 in SiHa and HeLa cell lysates. (F)
Expression levels of EIF4A3 and TCEAL6 were detected via RT-qPCR in
CC cell lines (C-33A, SiHa, CaSki and HeLa) and a normal human
cervical epithelial cell line, HcerEpic. (G) Expression levels of
EIF4A3 and TCEAL6 in CC tissues and normal tissues, according to
the analysis of TCGA. (H) Correlation between EIF4A3 and TCEAL6 in
CC samples from TCGA. (I) After transfection with sh-EIF4A3,
RT-qPCR was used to detect EIF4A3 expression in SiHa and HeLa
cells. (J) After transfection with sh-EIF4A3, western blotting was
performed to detect the expression level of TCEAL6 in SiHa and HeLa
cells. (K) After co-transfection with si-hsa_circ_0101119 and
sh-EIF4A3, western blotting was performed to measure the expression
level of TCEAL6 in SiHa and HeLa cells. (L) A proposed model
whereby hsa_circ_0101119 sequesters EIF4A3 away from TCEAL6 mRNA,
in turn suppressing TCEAL6 mRNA translation. **P<0.01 vs. IgG
group (B and E); *P<0.05, **P<0.01 vs. HcerEpic cells group
(F); *P<0.05 vs. normal tissues group (G); **P<0.01, vs.
sh-NC group (I and J); **P<0.01 vs. sh-NC group,
##P<0.01, vs. si-hsa_circ group. (K) RIP, RNA
immunoprecipitation; RT-qPCR, reverse transcription-quantitative
PCR; TCGA, The Cancer Genome Atlas; sh, short hairpin RNA; NC,
negative control; si, small interfering RNA; circ, circular RNA;
EIF4A3, eukaryotic initiation factor 4A-3; TCEAL6, transcription
elongation factor A-like 6; T, tumor; N, normal; CC, cervical
cancer. |
It was found that EIF4A3 was highly expressed and
TCEAL6 was lowly expressed in CC cells (Fig. 5F). Similarly, the analysis of The
Cancer Genome Atlas (TCGA) dataset also indicated that EIF4A3
expression was significantly upregulated and TCEAL6 expression was
downregulated in CC tissues (Fig.
5G). Moreover, the analysis of TCGA dataset revealed that
EIF4A3 expression was negatively correlated with TCEAL6 expression
in CC tissues (Fig. 5H).
As shown in Fig. 5I,
RT-qPCR was used to verify the transfection efficiency of EIF4A3
knockdown. The results demonstrated that knockdown of EIF4A3
significantly increased the expression level of TCEAL6 in SiHa and
HeLa cells (Fig. 5J), further
suggesting that EIF4A3 expression was negatively associated with
TCEAL6 expression in CC. In addition, it was found that knockdown
of hsa_circ_0101119 significantly elevated TCEAL6 expression in
SiHa and HeLa cells (Fig. 5K).
Compared with the si-hsa_circ group, the co-transfection of
si-hsa_circ and sh-EIF4A3 significantly increased the expression
level of TCEAL6 in SiHa and HeLa cells (Fig. 5K). The schematic diagram depicts
that hsa_circ_0101119 regulates TCEAL6 expression by enhancing the
binding of EIF4A3 to TCEAL6 mRNA, which inhibits TCEAL6 translation
(Fig. 5L).
TCEAL6 overexpression inhibits the
proliferation, migration and invasion, and promotes the apoptosis
of SiHa and HeLa cells
As shown in Fig. 6A,
RT-qPCR was used to verify the transfection efficiency of TCEAL6
overexpression. To verify the effect of TCEAL6 overexpression,
colony formation assays (Fig. 6B),
flow cytometry (Fig. 6C) and
Transwell assays (Fig. 6D and E)
were performed. The results indicated that TCEAL6 overexpression
significantly inhibited the proliferation, migration and invasion,
while it promoted the apoptosis of SiHa and HeLa cells (Fig. 6B-E).
Knockdown of TCEAL6 reverses the
effects of hsa_circ_0101119 knockdown on the proliferation,
apoptosis, migration and invasion of HeLa cells
As presented in Fig.
7A, the transfection efficiency of sh-TCEAL6 was assessed via
RT-qPCR. The results demonstrated that the proliferation, cell
clones, migration and invasion of HeLa cells were significantly
reduced in the si-hsa_circ + sh-NC group compared with the si-NC +
sh-NC or si-hsa_circ + sh-TCEAL6 groups, while apoptosis was
significantly elevated (Fig. 7B-E).
In addition, compared with the si-NC + sh-NC or si-hsa_circ +
sh-TCEAL6 groups, the proliferation, cell clones, migration and
invasion of HeLa cells were significantly increased in the si-NC +
sh-TCEAL6 group, but apoptosis was significantly decreased
(Fig. 7B-E). These results
indicated that the knockdown of TCEAL6 could reverse the effects of
hsa_circ_0101119 knockdown on the proliferation, apoptosis,
migration and invasion in HeLa cells.
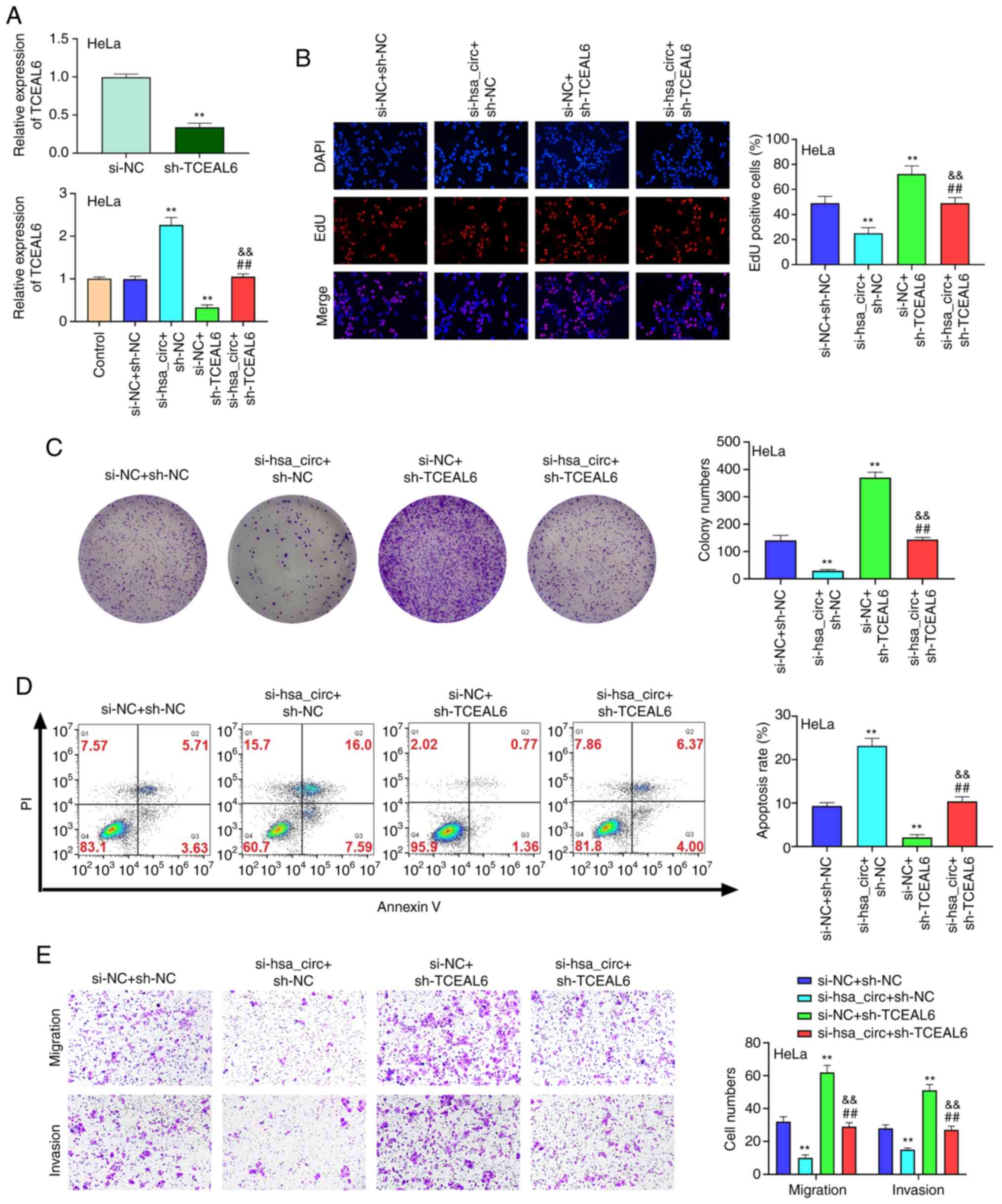 | Figure 7.Knockdown of TCEAL6 reverses the
effects of silencing hsa_circ_0101119 on the proliferation,
apoptosis and migration and invasion in HeLa cells. (A) After
transfection, the expression level of TCEAL6 was detected via
reverse transcription-quantitative PCR in HeLa cells. (B) After
co-transfection with si-hsa_circ_0101119 and sh-TCEAL6, HeLa cell
proliferation was determined using an EdU assay (magnification,
×200). (C) After co-transfection with si-hsa_circ_0101119 and
sh-TCEAL6, the colony numbers of HeLa cells were detected with a
colony formation assay (magnification, ×100). (D) After
co-transfection with si-hsa_circ_0101119 and sh-TCEAL6, flow
cytometry was used to detect HeLa cell apoptosis. (E) After
co-transfection with si-hsa_circ_0101119 and sh-TCEAL6, the
migration and invasion of HeLa cells were determined using a
Transwell assay (magnification, ×100). **P<0.01 vs. si-NC +
sh-NC group; ##P<0.01 vs. si-hsa_circ + sh-NC;
&&P<0.01 vs. si-NC + sh-TCEAL6 group. sh,
short hairpin RNA; NC, negative control; si, small interfering RNA;
circ, circular RNA; TCEAL6, transcription elongation factor A-like
6; EdU, 5-ethynyl-20-deoxyuridine; circ, circular RNA. |
Discussion
According to public reports, >450,000 patients
are diagnosed with CC and ~266,000 individuals die of CC every year
(30,31). Although different strategies have
been used for the treatment of CC, CC remains a health problem.
Therefore, it is essential to identify effective therapeutic
targets and discover the underlying molecular mechanisms in CC
progression. In the present study, it was demonstrated that
hsa_circ_0101119 could facilitate cell proliferation, migration and
invasion, and suppress apoptosis in CC via an interaction with
EIF4A3 to inhibit TCEAL6 expression.
Accumulating evidence has indicated that the
dysregulation of circRNAs is associated with the occurrence and
development of malignancies (32,33).
For example, the expression level of circ_102231 is upregulated in
lung adenocarcinoma tissues (34).
Zhang et al (35) reported
that hsa_circ_0023404 was highly expressed and exerted an oncogenic
role in CC. By analyzing the GSE102686 chip, the present study
identified that the expression level of hsa_circ_0101119 was
significantly upregulated in CC tissues compared with that in
normal tissues. In addition, it was demonstrated that
hsa_circ_0101119 expression was markedly increased in CC cells,
which was in line with a previous study by Wang et al
(11). It has also been revealed
that circRNAs can serve a critical role in different biological
processes, including proliferation, survival, apoptosis and
metastasis (36). For instance,
circ-MYB proto-oncogene like 2 is reported to facilitate the
proliferation and invasion of CC cells (37). Moreover, Chen et al (38) suggested that circRNA myosin light
chain kinase could accelerate cell proliferation and repress
apoptosis via the upregulation of Ras homolog, mTORC1 binding and
the activation of the mTOR pathway in CC. Wang et al
(39) also revealed that the
overexpression of hsa_circ_0001038 could promote cell proliferation
and invasion by regulating cyclin-M3 and metastasis-associated in
colon cancer 1 expression in CC. In the present study, it was
demonstrated that the knockdown of hsa_circ_0101119 could
significantly inhibit the proliferation, migration and invasion, as
well as facilitate the apoptosis of SiHa and HeLa cells.
Besides miRNAs, circRNAs can bind RBPs, modulate
their availability in the cells and affect the post-transcriptional
fates of RBP-interacting mRNAs (5,40). The
RBP EIF4A3 is reported to be an important regulator of
post-transcriptional regulation processes, including mRNA splicing,
transport, translation and surveillance (18). EIF4A3 has been shown to be
upregulated and identified as a diagnostic marker in some cancer
types, including breast cancer and lung cancer (18). Moreover, repression of EIF4A3 could
affect the expression levels of transcripts associated with the
cell cycle in cancer cells (41).
In the recent years, numerous studies have shown that circRNAs
serve vital roles in the development of cancer via binding EIF4A3.
For example, circ_septin9 could significantly promote
proliferation, migration and invasion, as well as inhibit apoptosis
and autophagy in triple-negative breast cancer cells via binding
EIF4A3 (42). Xu et al
(43) also reported that
circ_chromosome segregation 1 can inhibit colorectal cancer cell
proliferation by binding to EIF4A3. Furthermore, circ_nectin cell
adhesion molecule 3 was found to facilitate the proliferation of
gastric cancer cells by combining with EIF4A3 (44). Using bioinformatics analysis, the
current study predicted that hsa_circ_0101119 could bind to EIF4A3,
and this binding of hsa_circ_0101119 to EIF4A3 was confirmed using
RIP and RNA pull down assays.
Accumulating evidence has suggested that RBPs can
conduct their roles by interacting with different types of target
RNAs and forming ribonucleoprotein complexes (45,46).
The current bioinformatics analysis also identified a high binding
abundance between EIF4A3 and TCEAL6. In the present study, it was
demonstrated that EIF4A3 significantly negatively regulated TCEAL6
in CC cells. Biewenga et al (25) reported that TCEAL6 was lowly
expressed in the early stage of CC and could serve as a potential
biomarker for CC, which was confirmed by the current experiments.
Moreover, the present study identified that hsa_circ_0101119 could
recruit EIF4A3 to inhibit TCEAL6 expression in CC, as determined
via bioinformatics analysis, RIP assay, pull down assay, RT-qPCR
and western blotting. To confirm this conclusion, the effects of
co-transfection of si-hsa_circ_0101119 and sh-TCEAL6 on
proliferation, apoptosis, migration and invasion were detected in
HeLa cells. The data revealed that the knockdown of TCEAL6 could
reverse the effects of silencing hsa_circ_0101119 on the
proliferation, apoptosis, migration and invasion of HeLa cells. Of
course, further studies are required to identify the effect of
hsa_circ_0101119 and its underlying molecular mechanisms in CC
in vivo.
In conclusion, the present study identified an
oncogenic role of hsa_circ_0101119 in CC progression. It was
demonstrated that hsa_circ_0101119 could facilitate cell
proliferation, migration and invasion, and suppress apoptosis in CC
via an interaction with EIF4A3 to inhibit TCEAL6 expression. These
findings suggested that hsa_circ_0101119 may be an effective
therapeutic target for CC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS designed the study and performed the research. YW
and HL analyzed data, and wrote the paper. All authors have read
and approved the final manuscript, and met the authorship
requirements stated earlier in this document, and each author
believes that the manuscript represents honest work. All authors
confirmed the authenticity of the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J and
Forman D: Global cancer statistics. CA Cancer J Clin. 61:69–90.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kessler TA: Cervical cancer: Prevention
and early detection. Semin Oncol Nurs. 33:172–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wright JD, Chen L, Tergas AI, Burke WM,
Hou JY, Neugut AI, Ananth CV and Hershman DL: Population-level
trends in relative survival for cervical cancer. Am J Obstet
Gynecol. 213:670.e1–e7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hentze MW and Preiss T: Circular RNAs:
Splicing's enigma variations. EMBO J. 32:923–925. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han B, Chao J and Yao H: Circular RNA and
its mechanisms in disease: From the bench to the clinic. Pharmacol
Ther. 187:31–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salzman J: Circular RNA expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ebbesen KK, Kjems J and Hansen TB:
Circular RNAs: Identification, biogenesis and function. Biochim
Biophys Acta. 1859:163–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai H, Zhang P, Xu M, Yan L, Liu N and Wu
X: Circular RNA hsa_circ_0000263 participates in cervical cancer
development by regulating target gene of miR-150-5p. J Cell
Physiol. 234:11391–11400. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ou R, Lv J, Zhang Q, Lin F, Zhu L, Huang
F, Li X, Li T, Zhao L, Ren Y and Xu Y: circAMOTL1 Motivates AMOTL1
expression to facilitate cervical cancer growth. Mol Ther Nucleic
Acids. 19:50–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YM, Huang LM, Li DR, Shao JH, Xiong
SL, Wang CM and Lu SM: Hsa_circ_0101996 combined with
hsa_circ_0101119 in peripheral whole blood can serve as the
potential biomarkers for human cervical squamous cell carcinoma.
Int J Clin Exp Pathol. 10:11924–11931. 2017.PubMed/NCBI
|
|
12
|
Mao Y, Zhang L and Li Y: circEIF4G2
modulates the malignant features of cervical cancer via the
miR-218/HOXA1 pathway. Mol Med Rep. 19:3714–3722. 2019.PubMed/NCBI
|
|
13
|
Song T, Xu A, Zhang Z, Gao F, Zhao L, Chen
X, Gao J and Kong X: CircRNA hsa_circRNA_101996 increases cervical
cancer proliferation and invasion through activating TPX2
expression by restraining miR-8075. J Cell Physiol.
234:14296–14305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeck WR and Sharpless NE: Detecting and
characterizing circular RNAs. Nat Biotechnol. 32:453–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He JH, Li YG, Han ZP, Zhou JB, Chen WM, Lv
YB, He ML, Zuo JD and Zheng L: The CircRNA-ACAP2/Hsa-miR-21-5p/
tiam1 regulatory feedback circuit affects the proliferation,
migration, and invasion of colon cancer SW480 cells. Cell Physiol
Biochem. 49:1539–1550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaichian S, Shafabakhsh R, Mirhashemi SM,
Moazzami B and Asemi Z: Circular RNAs: A novel biomarker for
cervical cancer. J Cell Physiol. 235:718–724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hauer C, Curk T, Anders S, Schwarzl T,
Alleaume AM, Sieber J, Hollerer I, Bhuvanagiri M, Huber W, Hentze
MW and Kulozik AE: Improved binding site assignment by
high-resolution mapping of RNA-protein interactions using iCLIP.
Nat Commun. 6:79212015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin Y, Zhang J, Cai J, Liang R, Chen G,
Qin G, Han X, Yuan C, Liu Z, Li Y, et al: Systematic analysis of
gene expression alteration and co-expression network of eukaryotic
initiation factor 4A-3 in CANcer. J Cancer. 9:4568–4577. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeh CH and Shatkin AJ: A HeLa-cell-encoded
p21 is homologous to transcription elongation factor SII. Gene.
143:285–287. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeh CH and Shatkin AJ: Down-regulation of
rous sarcoma virus long terminal repeat promoter activity by a hela
cell basic protein. Proc Natl Acad Sci USA. 91:11002–11006. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pillutla RC, Shimamoto A, Furuichi Y and
Shatkin AJ: Genomic structure and chromosomal localization of
TCEAL1, a human gene encoding the nuclear phosphoprotein p21/SIIR.
Genomics. 56:217–220. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang CY, Chen YM, Zhao JJ, Chen YB, Jiang
SS, Yan SM, Zhao BW, Pan K, Wang DD, Lv L, et al: Decreased
expression of transcription elongation factor A-like 7 is
associated with gastric adenocarcinoma prognosis. PLoS One.
8:e546712013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orhan C, Bulut P, Dalay N, Ersen E and
Buyru N: Downregulation of TCEAL7 expression induces CCND1
expression in non-small cell lung cancer. Mol Biol Rep.
46:5251–5256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chien J, Staub J, Avula R, Zhang H, Liu W,
Hartmann LC, Kaufmann SH, Smith DI and Shridhar V: Epigenetic
silencing of TCEAL7 (Bex4) in ovarian cancer. Oncogene.
24:5089–5100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biewenga P, Buist MR, Moerland PD, Ver
Loren van Themaat E, van Kampen AH, ten Kate FJ and Baas F: Gene
expression in early stage cervical cancer. Gynecol Oncol.
108:520–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiao J, Zhang T, Jiao X, Huang T, Zhao L,
Ma D and Cui B: hsa_circ_0000745 promotes cervical cancer by
increasing cell proliferation, migration, and invasion. J Cell
Physiol. 235:1287–1295. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Inhibition of invasion and MMPs by a nutrient
mixture in human cancer cell lines: A correlation study. Ex Oncol.
32:243–248. 2010.PubMed/NCBI
|
|
29
|
Qureshi R, Arora H and Rizvi MA: EMT in
cervical cancer: Its role in tumour progression and response to
therapy. Cancer Lett. 356:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Denny L: Cervical cancer: Prevention and
treatment. Discov Med. 14:125–131. 2012.PubMed/NCBI
|
|
32
|
Dragomir M and Calin GA: Circular RNAs in
cancer-lessons learned from microRNAs. Front Oncol. 8:1792018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li M, Ding W, Sun T, Tariq MA, Xu T, Li P
and Wang J: Biogenesis of circular RNAs and their roles in
cardiovascular development and pathology. FEBS J. 285:220–232.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zong L, Sun Q, Zhang H, Chen Z, Deng Y, Li
D and Zhang L: Increased expression of circRNA_102231 in lung
cancer and its clinical significance. Biomed Pharmacother.
102:639–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, Zhao X, Zheng X and Li F:
Circular RNA hsa_circ_0023404 exerts an oncogenic role in cervical
cancer through regulating miR-136/TFCP2/YAP pathway. Biochem
Biophys Res Commun. 501:428–433. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu C, Wang Y, Li A, Zhang J, Xue F and Zhu
L: Overexpressed circ_0067934 acts as an oncogene to facilitate
cervical cancer progression via the miR-545/EIF3C axis. J Cell
Physiol. 234:9225–9232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Li H and Liang Z: circ-MYBL2
serves as a sponge for miR-361-3p promoting cervical cancer cells
proliferation and invasion. Onco Targets Ther. 12:9957–9964. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen R, Mao L, Shi R, Wang W and Cheng J:
circRNA MYLK accelerates cervical cancer via Up-Regulation of RHEB
and activation of mTOR signaling. Cancer Manag Res. 12:3611–3621.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Wang L, Wang W and Guo X:
Overexpression of circular RNA hsa_circ_0001038 promotes cervical
cancer cell progression by acting as a ceRNA for miR-337-3p to
regulate cyclin-M3 and metastasis-associated in colon cancer 1
expression. Gene. 733:1442732020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Panda AC, Abdelmohsen K, Martindale JL, Di
Germanio C, Yang X, Grammatikakis I, Noh JH, Zhang Y, Lehrmann E,
Dudekula DB, et al: Novel RNA-binding activity of MYF5 enhances
Ccnd1/Cyclin D1 mRNA translation during myogenesis. Nucleic Acids
Res. 44:2393–2408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mazloomian A, Araki S, Ohori M, El-Naggar
AM, Yap D, Bashashati A, Nakao S, Sorensen PH, Nakanishi A, Shah S
and Aparicio S: Pharmacological systems analysis defines EIF4A3
functions in cell-cycle and RNA stress granule formation. Commun
Biol. 2:1652019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zheng X, Huang M, Xing L, Yang R, Wang X,
Jiang R, Zhang L and Chen J: The circRNA circSEPT9 mediated by E2F1
and EIF4A3 facilitates the carcinogenesis and development of
triple-negative breast cancer. Mol Cancer. 19:732020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu B, Yang N, Liu Y, Kong P, Han M and Li
B: Circ_cse1l Inhibits Colorectal Cancer Proliferation by Binding
to eIF4A3. Med Sci Monit. 26:e9238762020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun HD, Xu ZP, Sun ZQ, Zhu B, Wang Q, Zhou
J, Jin H, Zhao A, Tang WW and Cao XF: Down-regulation of circPVRL3
promotes the proliferation and migration of gastric cancer cells.
Sci Rep. 8:101112018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tang W, Wang D, Shao L, Liu X, Zheng J,
Xue Y, Ruan X, Yang C, Liu L, Ma J, et al: LINC00680 and TTN-AS1
stabilized by EIF4A3 promoted malignant biological behaviors of
glioblastoma cells. Mol Ther Nucleic Acids. 19:905–921. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shibuya T, Tange TØ, Sonenberg N and Moore
MJ: eIF4AIII binds spliced mRNA in the exon junction complex and is
essential for nonsense-mediated decay. Nat Struct Mol Biol.
11:346–351. 2004. View Article : Google Scholar : PubMed/NCBI
|