Introduction
Mycoplasma pneumoniae (M. pneumoniae)
is one of the main pathogens that cause respiratory tract
infections in humans (1,2). Outbreaks of M. pneumoniae
pneumonia (MPP) occur in 3- to 7-year cycles worldwide, and
epidemics in Korea occurred in 2015 and 2016 (3). M. pneumoniae causes respiratory
tract infections in all age groups, accounting for up to 40% of
community-acquired respiratory tract infections in children aged
>5 years (1). Although MPP is
usually a benign and self-limiting process, M. pneumoniae
infection can develop into severe, life-threatening diseases,
including refractory MPP (RMPP), acute respiratory distress
syndrome and necrotizing pneumonitis (2–4).
Pneumonia and extrapulmonary complications caused by MPP pose a
serious threat to children's health (4). In previous years, MPP has been
reported in 10–40% of cases of community-acquired pneumonia (CAP)
and increases during M. pneumoniae epidemics (5,6). MPP
was previously considered to be a self-limiting process, but RMPP
has been reported (7), which shows
no clinical or radiological response to macrolides, and may
progress to severe pneumonia and cause extrapulmonary complications
(6,7). The number of RMPP cases also tends to
increase every year; the annual incidence of MPP in respiratory
disease was <10% of cases of CAP in 2009, 20.5% in September
2010 and reached a record high of 62.5% in September 2011 (8). The usual treatment strategy used for
MPP is not considered as suitable for RMPP (7,8); thus,
an improved understanding of the pathogenesis underlying RMPP is
urgently required in order to design a more effective treatment
strategy.
Studies on RMPP have indicated that its pathogenesis
is primarily associated with the macrolide-resistant mechanism of
M. pneumoniae strains (9,10).
Matsuoka et al (11) found
that mutations in domains II and V of 23S RNA in M.
pneumoniae strains result in a decrease in the affinity of
antibiotics to bacterial ribosomes, which eventually leads to
resistance to macrolides. In addition, abnormal immune responses
caused by MPP (12) and mixed
bacterial infections may cause progression of non-refractory MPP
(NRMPP) to RMPP (13). However, due
to its complexity, the key pathogenesis of RMPP remains
unknown.
Long non-coding RNAs (lncRNAs) are >200
nucleotides in length and do not have the capacity to encode
proteins (14). The mechanism of
action underlying lncRNAs is not completely understood due to their
poorly conserved nucleotide sequence. However, previous studies
have reported that lncRNAs regulate gene expression at multiple
levels through complex mechanisms (14,15).
Circular RNAs (circRNAs) are a novel type of lncRNAs that form a
covalently closed continuous loop and thus have no 5′-3′ polarity
and no polyA tail (16).
Accumulating evidence indicates that circRNAs regulate gene
expression at both the transcriptional and post-transcriptional
levels by serving as microRNA (miRNA) sponges (16,17).
circRNAs serve a role in the development of certain diseases,
including cancer, diabetes and Alzheimer's disease (16,17–19).
Thus, circRNAs may serve as potential biomarkers or therapeutic
targets.
The present study performed lncRNA and circRNA
profiling via ribosomal (r)RNA-depleted RNA sequencing of
whole-blood samples from two patients with NRMPP, two patients with
RMPP and three healthy children (HC) to provide a comprehensive
analysis of the differences between the HC and patients with MPP,
including the main differences in RNA expression levels between
patients with NRMPP and those with RMPP. The present study aimed to
identify target genes and circRNAs that may serve as biomarkers for
the clinical diagnosis of early-stage disease, as well as providing
a theoretical basis for research into the pathogenesis of MPP.
Materials and methods
Whole-blood sample preparation
The 18 blood samples were collected from December
2018 to December 2019 in the Guangzhou Women and Children's Medical
Center (Guangdong, China) from six patients with NRMPP, six
patients with RMPP and six healthy children (HC; Table I). Among them, a cohort of seven
children (two NRMPP, two RMPP and three HC) was used for
high-throughput sequencing, and a cohort of 11 children (four
NRMPP, four RMPP and three HC) was used for validation. A blood
sample (2.0 ml) was collected from each patient. All patients were
tested with the M. pneumoniae-IgM antibody in the serum and
M. pneumoniae DNA by PCR in throat swabs on admission, and
positive cases were defined as patients with MPP. All patients with
MPP were treated with appropriate antibiotics (for example,
Azithromycin). Cases with worsening cough, infiltrates on a chest
radiograph and a fever that prolonged for >7 days were recorded
as patients with RMPP. The remaining cases were defined as patients
with NRMPP. All specimens were collected at an early stage of MPP
on admission (≤7 days of onset was defined as an early stage of
MPP) (20). The exclusion criteria
were as follows: The presence of severe concomitant diseases
(chronic pulmonary disease, cardiovascular disease, neoplasia,
kidney or liver disease, immune function deficiency and
immunodepression); and the presence of mixed infections with other
microorganisms. The HC were used as healthy controls.
 | Table I.Clinical characteristics of children
diagnosed with MPP. |
Table I.
Clinical characteristics of children
diagnosed with MPP.
| A, NRMPP |
|---|
|
|---|
|
|
|
| Laboratory
findingsb | Radiology
findingsc |
|---|
|
|
|
|
|
|
|---|
| Case | Age | Sex | Fever
durationa, days | WBC,
1×109/l (5–12d) | Hb, g/l
(105–145d) | Plt,
1×109/l (140–440d) | HsCRP, mg/l
(<5d) | ALT, U/l
(0–40d) | LDH, U/l
(159–322d) | ALB, g/l
(40–55d) | Consolidation | Hydrothorax |
|---|
| 1 | 1 year, 11
months | Male | 4 | 5.7 | 115 | 399 | 46.6 | 27 | 295 | 39.5 |
|
|
| 2 | 2 years, 5
months | Female | 7 | 6.4 | 120 | 264 | 1.7 | 16 | 390 | 40.1 |
|
|
| 3 | 11 years | Female | 5 | 10.9 | 105 | 143 | 60.4 | 13 | 400 | 25.3 | Yes |
|
| 4 | 4 years, 1
month | Male | 7 | 4.7 | 122 | 261 | 30.4 | 12 | 316 | 39.5 | Yes |
|
| 5 | 3 years, 2
months | Female | 6 | 4.9 | 110 | 278 | 16.0 | 8 | 211 | 36.7 |
|
|
| 6 | 7 years, 2
months | Male | 5 | 9.2 | 116 | 276 | 24.0 | 11 | 239 | 39.4 | Yes |
|
|
| B, RMPP |
|
|
|
|
| Laboratory
findingsb | Radiology
findingsc |
|
|
|
|
|
|
| Case | Age | Sex | Fever
durationa,
days | WBC,
1×109/l (5–12d) | Hb, g/l
(105–145d) | Plt,
1×109/l (140–440d) | HsCRP, mg/l
(<5d) | ALT, U/l
(0–40d) | LDH, U/l
(159–322d) | ALB, g/l
(40–55d) |
Consolidation |
Hydrothorax |
|
| 7 | 1 year, 9
months | Female | 9 | 12.2 | 100 | 196 | 154.6 | 119 | 739 | 24.8 | Yes |
|
| 8 | 6 years | Female | 13 | 5.8 | 102 | 233 | 155.8 | 23 | 1,470 | 25.3 | Yes | Yes |
| 9 | 3 years, 10
months | Female | 7 | 10.1 | 106 | 495 | 31.9 | 48 | 332 | 28.1 | Yes | Yes |
| 10 | 9 years, 1
month | Female | 12 | 9.7 | 113 | 254 | 35.0 | 719 | 1,442 | 32.8 | Yes | Yes |
| 11 | 5 years, 3
months | Male | 10 | 7.4 | 119 | 319 | 25.3 | 25 | 651 | 28.2 | Yes | Yes |
| 12 | 4 years, 2
months | Male | 9 | 5.9 | 101 | 322 | 73.1 | 45 | 941 | 26.4 | Yes |
|
The present study was approved by the Ethics
Committee at Guangzhou Women and Children's Medical Center. The
parents or legal guardians of the patients signed written informed
consent forms and agreed to its content.
rRNA-depleted RNA sequencing
For rRNA-depleted RNA sequencing, two of the six
patients with NRMPP, two of the six patients with RMPP and three of
the six HC were screened out as typical cases and sent to Annoroad
Gene Technology Co, Ltd. The total RNA of each sample was isolated
using TRIzol® reagent (cat. no. MFCD00213058;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The purity, concentration and integrality
of the RNA were determined using a NanoPhotometer®
spectrophotometer (IMPLEN), Qubit®3.0 Fluorometer
(Thermo Fisher Scientific, Inc.) and Agilent 2100 RNA Nano 6000
Assay kit (cat. no. 41105500; Agilent Technologies, Inc.),
respectively. Subsequently, 3 µg RNA from each sample was loaded
and the rRNA was removed using Ribo-Zero™ Gold kits (cat. no.
MRZG126; Epicentre; Illumina, Inc.). NEBNext® Ultra™
Directional RNA Library Prep kit for Illumina® (cat. no.
E7420S; New England BioLabs, Inc.) was used to generate the
sequencing libraries, which were then sequenced through the
Illumina HiSeq platform (Illumina, Inc.). The sequencing type was
eukaryotic common transcriptome. The sequencing direction was P5 to
P7, then P7 to P5. After removing the low-quality and polluted
reads, clean reads were obtained and mapped to the reference genome
sequence using Hierarchical Indexing For Spliced Alignment Of
Transcripts 2 (version 2.05) (21).
The detected reads were mapped to the known mRNA and lncRNA. HTSeq
(version 0.6.0) was used to represent the expression level of each
gene (22). The loading
concentration of the final library was 2 nM. DESeq2 Rpackage
(version 1.18.0) was used to perform differential expression
analysis between the comparative groups (23), and the genes with P<0.05 were
considered to be differentially expressed. The unmapped reads were
identified as circRNA candidates using Find_circ (version 1.2) and
CircRNA Identifier 2 software (version 2.0.1) (23,24).
Functional analysis of the differentially expressed genes (DEGs)
was performed using Gene Ontology (GO)seq (version 1.0) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) Orthology-Based Annotation
System (version 2.0) (24–28).
Reverse transcription-quantitative PCR
(RT-qPCR)
To verify the reliability of the sequencing results,
RT-qPCR was performed to determine the expression levels of
screened genes (Table SI). EditSeq
software (version 7.10, DNASTAR, Inc.) was used to design the
specific primers and the β-actin gene was selected as a
standardization control. Briefly, total RNA from blood samples was
reverse transcribed into cDNA using the QuantiTect Reverse
Transcription kit (Qiagen GmbH) according to the manufacturer's
instructions. Subsequently, qPCR was performed using a DNA Engine
Chromo 4 real-time system (Bio-Rad Laboratories, Inc.) with a
TaqMan™ Copy Number Assay kit (Thermo Fisher Scientific, Inc.). The
sequences of the primers are listed in Table II. β-actin (forward,
5′-GAGGTATCCTGACCCTGAAGTA-3′ and reverse,
5′-CACACGCAGCTCATTGTAGA-3′) was used as an internal reference.
Thermocycling conditions were as follows: 95°C for 10 min, followed
40 cycles of 95°C for 15 sec and 60°C for 60 sec). The expression
levels were calculated using the 2−ΔΔCq method (29).
 | Table II.Primers used for reverse
transcription-quantitative PCR. |
Table II.
Primers used for reverse
transcription-quantitative PCR.
| circRNA | Primers
(5′→3′) |
|---|
| hsa_circ_
0022808 | F:
ACTGAAGAGGATGCAGGAGC |
|
| R:
GAGGAATGTTCCCGGTCTCC |
| hsa_circ_
0006793 | F:
AGTCCCCTGCTATCACTGGT |
|
| R:
CCAGCTTCGGTCACTGAACA |
| hsa_circ_
0014390 | F:
TCTGTTGAAGATTTGAAGAACCCA |
|
| R:
CTGAGGGCTAGAGGACTGGT |
| hsa_circ_
0014305 | F:
TTCTCCCTGGCGGAGGAATA |
|
| R:
GGATGGCTGGTTTGAAGCAC |
| hsa_circ_
00216400 | F:
CTTCAGTACCAGAGCCCCAC |
|
| R:
ACCACCTCAACCGTTTCAACT |
Statistical analysis
Data were analyzed using GraphPad software (Prism
8.0; GraphPad Software, Inc.) and visualized using the ggplot2
package of R software (version 3.6.1) (30). All data are presented as the mean ±
standard deviation of three independent repeats. An unpaired
two-tailed Student's t-test was used to determine the significant
differences in lncRNAs, mRNAs and circRNAs between the three
groups. For clinical data, the non-parametric Mann-Whitney U test
was used for two-group analysis of continuous variables, and the
Kruskal-Wallis test followed by Dunn's post hoc test was used for
three-group analysis of continuous variables. Fisher's exact test
was used for the analysis of categorical variables. P-values are
two-sided and were adjusted using the Bonferroni method. P<0.05
was considered to indicate a statistically significant
difference.
Results
Characteristics of patients with
MPP
Patients with MPP displayed typical MPP clinical
symptoms and were diagnosed with pneumonia. The M.
pneumoniae IgM antibody in the serum and M. pneumoniae
DNA detected via PCR from throat swabs showed positive results. The
clinical characteristics of the cases are listed in Tables SI and SII. Abnormal findings on chest
radiographs were observed in all patients with MPP. The chest scans
primarily revealed diffuse infiltration of both lungs in NRMPP
cases. However, RMPP cases exhibited unequivocal focal or segmental
consolidation (Fig. 1A) with
pleural effusion (Fig. 1B).
Overview of rRNA-depleted RNA
sequencing analysis profiles
Upon rRNA-depleted RNA sequencing, a total of
670,528,498 raw reads (102,839,708, 98,869,356 and 93,692,076 for
the HC group; 96,341,690 and 92,905,200 for the NRMPP group; and
96,437,832 and 89,442,636 for the RMPP group) were generated.
Through filtering the raw data, 614,648,054 clean reads were
obtained and mapped to the reference genome (Fig. 2A). The error rate of the filtered
data was qualified (Fig. 2B).
A total of 611 lncRNAs (416 upregulated and 195
downregulated) and 692 mRNAs (598 upregulated and 94 downregulated)
were significantly differentially expressed (P<0.05) between the
NRMPP and HC groups (Fig. 3). A
total of 937 lncRNAs (433 upregulated and 504 downregulated) and
1,027 mRNAs (593 upregulated and 434 downregulated) were
significantly differentially expressed (P<0.05) between the RMPP
and HC groups. A total of 17 lncRNAs (4 upregulated and 13
downregulated) and 18 mRNAs (6 upregulated and 12 downregulated)
were significantly differentially expressed (P<0.05) between the
RMPP and NRMPP groups (Table
III). The significantly differentially expressed lncRNAs
between the RMPP and NRMPP groups included ENSG00000249790,
ENSG00000261026, MSTRG.215206, MSTRG.233743, MSTRG.238033,
MSTRG.238419, MSTRG.268000, MSTRG.275241 (Table III).
 | Table III.Significantly differentially
expressed long non-coding RNAs between RMPP and NRMPP groups. |
Table III.
Significantly differentially
expressed long non-coding RNAs between RMPP and NRMPP groups.
|
| Relative gene
expression levels |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Gene | RMPP | NRMPP | P-value |
Up/downregulated | Position |
|---|
|
ENSG00000249790 | 635.1540951 | 3447.840859 |
3.09×10−3 | Down |
chr12:8788257-8795789:+ |
|
ENSG00000261026 | 472.7296308 | 4.781416405 |
2.65×10−2 | Up |
chr8:22679013-22684009:− |
| MSTRG.215206 | 2391.068595 | 70.25707389 |
7.87×10−3 | Up |
chr15:48211935-48296341:− |
| MSTRG.233743 | 63.78915178 | 0 |
1.70×10−2 | Up |
chr16:15028952-15030258:− |
| MSTRG.238033 | 0 | 27.68733409 |
2.65×10−2 | Down |
chr16:34582662-34587722:+ |
| MSTRG.238419 | 0.404276272 | 87.94800239 |
2.34×10−6 | Down |
chr16:46388508-46406972:+ |
| MSTRG.268000 | 5.600197692 | 101.7282093 |
3.32×10−3 | Down |
chr17:74562930-74563656:− |
| MSTRG.275241 | 0 | 25.47228864 |
3.27×10−2 | Down |
chr18:22348684-22349256:− |
| MSTRG.299858 | 0.404276272 | 34.38883192 |
2.65×10−2 | Down |
chr19:37282731-37293921:− |
| MSTRG.32896 | 0.404276272 | 54.56033482 |
7.16×10−4 | Down |
chr1:143501630-143505359:+ |
| MSTRG.384208 | 0 | 83.06200226 |
1.08×10−6 | Down |
chr21:8219668-8220285:− |
| MSTRG.384231 | 0 | 78.99033548 |
1.45×10−6 | Down |
chr21:8446933-8448138:− |
| MSTRG.386206 | 0 | 28.50166744 |
2.23×10−2 | Down |
chr21:22451534-22451867:+ |
| MSTRG.488193 | 0 | 25.24433402 |
4.13×10−2 | Down |
chr4:146285918-146287333:+ |
| MSTRG.631597 | 321.397898 | 16.70145272 |
2.65×10−2 | Up |
chr8:32733246-32739937:+ |
| MSTRG.678280 | 0.490427811 | 39.44287588 |
9.98×10−3 | Down |
chr9:101736669-101848060:+ |
Bioinformatics analysis of sequencing
profiles
The differentially expressed mRNAs between the RMPP
and NRMPP groups (Table IV) were
ENSG00000073756, ENSG00000111788, ENSG00000122877, ENSG00000123838,
ENSG00000130656, ENSG00000165949; these were identified by
functional analysis using both the GO and KEGG databases. In the GO
analysis, the significantly differentially expressed mRNAs were
primarily enriched in ‘complement activation, classical pathway’,
‘leukocyte migration’ and ‘chemotaxis’ (Fig. 4A). In the KEGG pathway analysis, the
significantly differentially expressed mRNAs were primarily
enriched in the ‘IL-17 signaling pathway’ (Fig. 4B). RT-qPCR was used to verify
candidate genes that may be involved in pathogenesis of RMPP, such
as prostaglandin-endoperoxide synthase 2 (PTGS2), chemokine (C-X-C
motif) ligand 8 (CXCL8) and Fos-like antigen 1 (FOSL1; Fig. 4C) and the primers were designed by
EditSeq software.
 | Figure 4.Functional analysis of the DEGs
between the RMPP and NRMPP groups. (A) GO analysis of the DEGs. (B)
DEGs enriched in the ‘IL-17 signaling pathway’. (C) Validation of
DEGs via reverse transcription-quantitative PCR. For the presented
data, four NRMPP, four RMPP and three HC were used. Comparisons
between two groups were analyzed using Student's t-test. *P<0.05
and **P<0.01 vs. NRMPP group. DEGs, differentially expressed
genes; GO, Gene Ontology; MPP, M. pneumoniae pneumonia;
NRMPP, non-refractory MPP; RMPP, refractory MPP; HC, healthy
children; MPPS, severe MPP; MPPM, mild MPP; PTGS2,
prostaglandin-endoperoxide synthase 2; CXCL8, interleukin 8; FOSL1,
fos-like antigen 1; HBZ, hemoglobin subunit ζ; IGHV, immunoglobulin
heavy variable. |
 | Table IV.Significantly differentially
expressed mRNAs between RMPP and NRMPP groups. |
Table IV.
Significantly differentially
expressed mRNAs between RMPP and NRMPP groups.
|
| Relative gene
expression levels |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Gene | RMPP | NRMPP | P-value |
Up/downregulated | Gene name | Description |
|---|
|
ENSG00000073756 | 9780.85878 | 553.0410947 |
1.67×10−2 | Up | PTGS2 |
Prostaglandin-endoperoxide synthase 2 |
|
ENSG00000111788 | 9.669441835 | 103.3909011 |
3.10×10−2 | Down | DDX11L8 | Putative
ATP-dependent RNA helicase DDX11-like protein 8 |
|
ENSG00000122877 | 536.8492789 | 21.50520559 |
1.37×10−3 | Up | EGR2 | Early growth
response protein 2 |
|
ENSG00000123838 | 240.3692975 | 4.57224895 |
2.65×10−2 | Up | C4BPA | Complement
component 4 binding protein α |
|
ENSG00000130656 | 0 | 43.9105411 |
9.88×10−4 | Down | HBZ | Hemoglobin subunit
ζ |
|
ENSG00000165949 | 23.52076078 | 359.2170269 |
1.08×10−6 | Down | IFI27 | Interferon
α-inducible protein 27 mitochondrial |
|
ENSG00000169429 | 5129.9448 | 284.2008731 |
9.35×10−3 | Up | CXCL8 | Interleukin 8 |
|
ENSG00000175592 | 137.6919176 | 5.491166033 |
3.22×10−2 | Up | FOSL1 | Fos-like antigen
1 |
|
ENSG00000185736 | 0.490427811 | 190.8606578 |
1.14×10−9 | Down | ADARB2 | Double stranded
RNA-specific editase B |
|
ENSG00000188056 | 0.490427811 | 75.33804428 |
1.06×10−5 | Down | TREML4 | Trem-like
transcript 4 protein |
|
ENSG00000211653 | 82.7834293 | 501.0131059 |
5.00×10−3 | Down | IGLV1-40 | Immunoglobulin
lambda variable 1–40 |
|
ENSG00000211897 | 570.423636 | 4031.523518 |
8.08×10−5 | Down | IGHG3 | Immunoglobulin
heavy constant γ3 |
|
ENSG00000236770 | 0.404276272 | 43.86941747 |
4.53×10−3 | Down | CD300C | CMRF35-like
molecule 6 |
|
ENSG00000237973 | 1159.431015 | 6.701497833 |
4.07×10−5 | Up | COX1 | Cytochrome c
oxidase subunit 1 |
|
ENSG00000270550 | 3.664967871 | 81.06781283 |
1.81×10−3 | Down | IGHV3-30 | Immunoglobulin
heavy variable 3–30 |
|
ENSG00000282639 | 0 | 59.68597813 |
1.06×10−5 | Down | IGHV3-64D | Immunoglobulin
heavy variable 3–64D |
|
ENSG00000282651 | 0 | 108.602341 |
6.73×10−9 | Down | IGHV5-10-1 | Immunoglobulin
heavy variable 5-10-1 |
|
ENSG00000284690 | 43.90687745 | 553.5974251 |
3.09×10−3 | Down | CD300H | Protein CD300H |
In the circRNA/miRNA co-expression analysis, a total
of 1,370 circRNAs (505 upregulated and 865 downregulated) were
significantly differentially expressed (P<0.05) between the HC
and MPP groups (Fig. 5A and B). The
functions of circRNAs were associated with the known function of
the host linear transcripts and annotated by the GO and KEGG
databases. In the GO analysis of the host linear transcripts, the
differentially expressed terms were classified into three
categories. Under biological processes, the GO terms were primarily
enriched in ‘regulation of mRNA metabolic process’,
‘nucleobase-containing compound transport’ and ‘RNA localization’
(Fig. 5D). Under the category of
cellular component, the GO terms were primarily enriched in
‘nuclear speck’, ‘cell-substrate junction’ and ‘focal adhesion’
(Fig. 5E). Under the category of
molecular function, the GO terms were primarily enriched in
‘ubiquitin-like protein transferase activity’, ‘ribonucleoprotein
complex binding’ and ‘enhancer binding’ (Fig. 5F). In the KEGG pathway analysis, the
significant DEGs were primarily enriched in ‘Herpes simplex virus 1
infection’, ‘viral carcinogenesis’ and ‘RNA transport’ (Fig. 5C). The top 11 significantly
differentially expressed circRNAs between the HC and MPP groups are
listed in Table V.
 | Table V.Significantly differentially
expressed circRNAs between HC and MPP groups. |
Table V.
Significantly differentially
expressed circRNAs between HC and MPP groups.
| Gene | Log2FoldChange | P-value | Best
transcript | Gene symbol |
Upregulated/downregulated |
|---|
|
hsa_circ_0002171 | 10.42052522 | 0.029534 | NM_198567 | C5orf25 | Up |
|
hsa_circ_0001535 | 7.929259862 | 0.0002309 | NM_001101801 | FAM13B | Up |
|
hsa_circ_0007261 | 2.669813966 | 0.0254796 | NM_031921 | ATAD3B | Up |
|
hsa_circ_0018432 | 2.28731687 | 0.0254796 | NM_194298 | SLC16A9 | Up |
|
hsa_circ_0014535 | −1.810846752 | 0.0242991 | NM_002004 | FDPS | Down |
|
hsa_circ_0002316 | −1.891464967 | 0.0254796 | NM_145243 | OMA1 | Down |
|
hsa_circ_0004327 | −1.973037708 | 0.0429052 | NM_007124 | UTRN | Down |
|
hsa_circ_0019868 | −2.107107635 | 0.0026471 | NM_014631 | SH3PXD2A | Down |
|
hsa_circ_0001726 | −2.465790354 | 0.002007 | NM_032164 | ZNF394 | Down |
|
hsa_circ_0026176 | −2.760620461 | 0.0254796 | NM_003217 | TMBIM6 | Down |
|
hsa_circ_0021682 | −4.772727352 | 0.0242991 | NM_024662 | NAT10 | Down |
A total of 156 circRNAs (85 upregulated and 71
downregulated) were significantly differentially expressed
(P<0.05) between the NRMPP and RMPP groups (Fig. 6A-C). A total of 24 circRNAs were
identified as the most significantly differentially expressed
circRNAs between the NRMPP and RMPP groups. In the GO analysis, GO
terms were primarily enriched in ‘positive regulation of myeloid
cell differentiation’ and ‘positive regulation of hemopoiesis’
(Fig. 6D). The screened circRNAs
(Table VI) were primarily enriched
in ‘colorectal cancer’, ‘hepatitis B’ and ‘apoptosis’ (Fig. 6E). The top five upregulated circRNAs
were selected for further validation by performing RT-qPCR
(Fig. 6F), indicating that these
circRNAs may serve as potential biomarkers for RMPP.
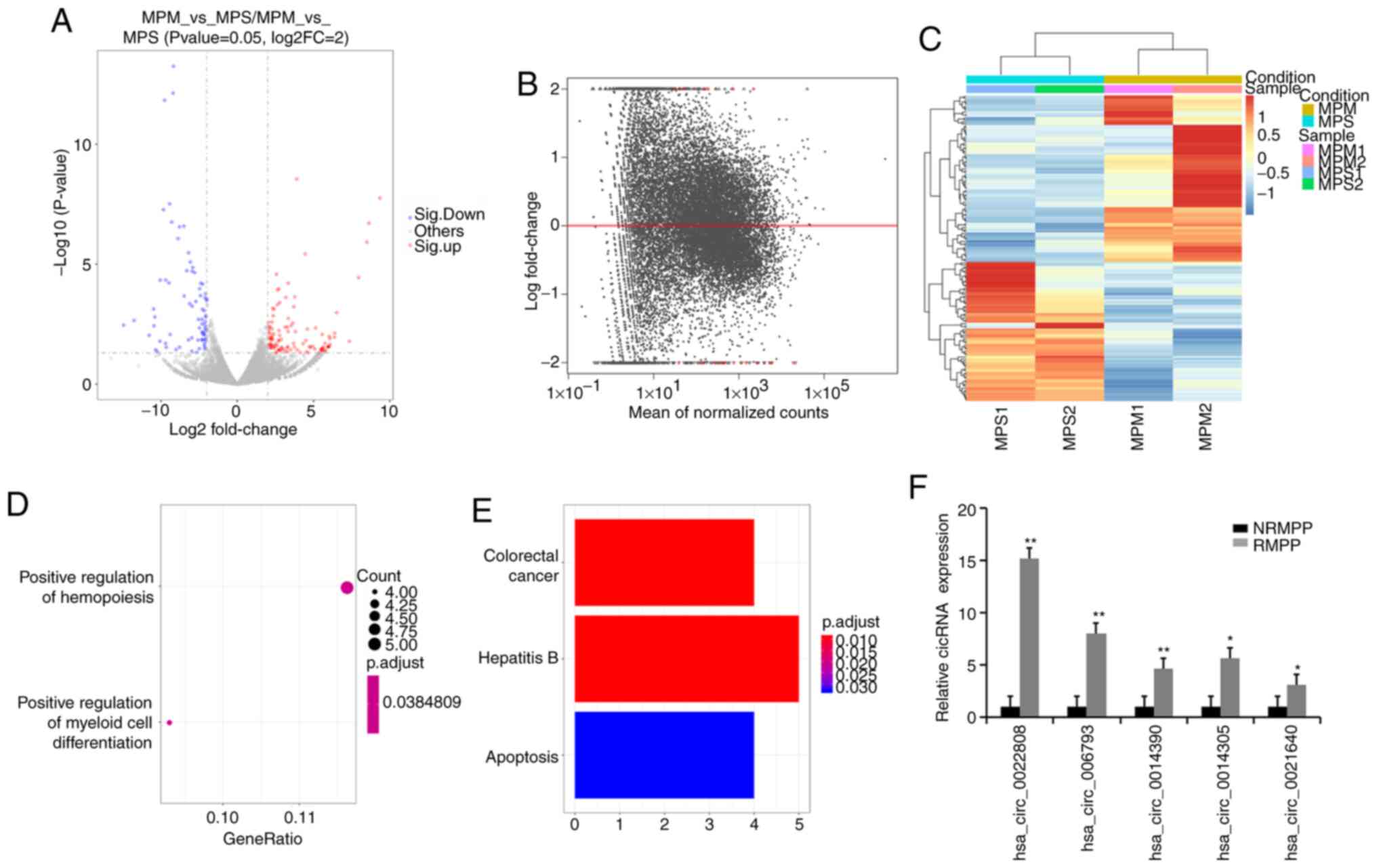 | Figure 6.Functional analysis of the circRNAs
between the NRMPP and RMPP groups. (A) Volcano plot, (B) Lateral
view of the volcano plot and (C) cluster map of significantly
differentially expressed circRNAs between the NRMPP and RMPP
groups. (D) Most enriched Gene Ontology terms. (E) Most enriched
Kyoto Encyclopedia of Genes and Genomes pathways between the NRMPP
and RMPP groups. (F) Validation of the expression of the selected
circRNAs via reverse transcription-quantitative PCR. For the
presented data, four NRMPP, four RMPP and three HC were used.
Comparisons between two groups were analyzed using Student's
t-test. *P<0.05 and **P<0.01 vs. NRMPP group. circRNA,
circular RNA; MPP, M. pneumoniae pneumonia; NRMPP,
non-refractory MPP; RMPP, refractory MPP; HC, healthy children;
MPPS, severe MPP; MPPM, mild MPP. |
 | Table VI.Significantly differentially
expressed circRNAs between RMPP and NRMPP groups. |
Table VI.
Significantly differentially
expressed circRNAs between RMPP and NRMPP groups.
| Gene | Log2FoldChange | P-value | Best
transcript | Gene symbol |
Upregulated/downregulated |
|---|
|
hsa_circ_0022808 | 23.10152809 |
1.38×10−6 | NM_002689 | POLA2 | Up |
|
hsa_circ_0006793 | 7.812895001 |
4.88×10−2 | NM_001242767 | MTHFD1L | Up |
|
hsa_circ_0014390 | 6.98745233 |
3.28×10−3 | NM_014847 | UBAP2L | Up |
|
hsa_circ_0014305 | 6.373176867 |
9.65×10−3 | NM_023015 | INTS3 | Up |
|
hsa_circ_0021640 | 6.166346606 |
1.54×10−2 | NM_005898 | CAPRIN1 | Up |
|
hsa_circ_0017689 | 6.1204205 |
1.71×10−2 | NM_014688 | USP6NL | Up |
|
hsa_circ_0025915 | 6.067184847 |
1.80×10−2 | NM_025003 | ADAMTS20 | Up |
|
hsa_circ_0003781 | 5.86144273 |
12.85×10−2 | NM_001242865 | PRMT2 | Up |
|
hsa_circ_0024849 | 5.834493906 |
2.66×10−2 | NM_001143835 | NFRKB | Up |
|
hsa_circ_0015519 | 5.737917011 |
3.35×10−2 | NM_004736 | XPR1 | Up |
|
hsa_circ_0005901 | 5.506362304 |
4.75×10−2 | TCONS_00024174 | TCONS_00024174 | Up |
|
hsa_circ_0023925 | 5.506362304 |
4.75×10−2 | NM_007166 | PICALM | Up |
|
hsa_circ_0025400 | −4.680048526 |
3.23×10−2 | NM_004426 | PHC1 | Down |
|
hsa_circ_0010030 | −5.606173248 |
4.84×10−2 | NM_201628 | KAZN | Down |
|
hsa_circ_0011249 | −5.645431582 |
4.67×10−2 | NM_001020658 | PUM1 | Down |
|
hsa_circ_0004477 | −5.753705882 |
3.74×10−2 | NM_182751 | MCM10 | Down |
|
hsa_circ_0025209 | −5.791153915 |
3.88×10−2 | NM_001033714 | NOP2 | Down |
|
hsa_circ_0018110 | −5.819081289 |
3.37×10−2 | NM_004521 | KIF5B | Down |
|
hsa_circ_0010131 | −5.889114841 |
3.05×10−2 | NM_004431 | EPHA2 | Down |
|
hsa_circ_0010148 | −6.072531801 |
2.51×10−2 | NM_198546 | SPATA21 | Down |
|
hsa_circ_0026524 | −6.476462428 |
1.13×10−2 | NM_001417 | EIF4B | Down |
|
hsa_circ_0001890 | −7.728346085 |
6.81×10−3 | NM_001006617 | MAPKAP1 | Down |
|
hsa_circ_0014349 | −9.677465499 |
4.32×10−2 | NM_002870 | RAB13 | Down |
|
hsa_circ_0022807 | −15.16379666 |
4.45×10−13 | NM_002689 | POLA2 | Down |
Discussion
Although the macrolide-resistant mechanisms of M.
pneumoniae strains and excessive immunological inflammation are
the most commonly proposed mechanisms underlying RMPP (3,4), the
pathogenesis of RMPP remains to be elucidated and there is still a
lack of accurate assessment tools and biomarkers for RMPP. At
present, the common methods for estimating the severity of RMPP are
based on clinical characteristics, pulmonary imaging severity and
therapeutic effect, which are unable to ensure an effective
identification of early-stage RMPP (5,6).
Therefore, it is necessary to identify novel tools and biomarkers
for the early diagnosis of RMPP. The present study was designed to
identify target genes implicated in the pathogenesis of RMPP to
enable early diagnosis by comparing the differences between the
cases with NRMPP and those with RMPP. To the best of our knowledge,
the present study was the first to assess the differences in
lncRNAs and circRNAs between NRMPP and RMPP.
circRNAs serve important roles in regulating gene
expression by sequestering miRNAs as a sponge at the
transcriptional or post-transcriptional levels (16). Thus, circRNAs can regulate a number
of processes associated with numerous diseases, such as cancer
(16,17). M. pneumoniae possesses a
tip-like organelle that permits a highly oriented extracellular
parasitism of the respiratory epithelium to avoid clearance by
mucosal cilia and phagocytosis, and its adhesion ability is
positively correlated with pathogenicity (31). Upregulation of PTGS2 promotes
inflammation, which may indicate that more severe inflammation was
observed in the RMPP group in the present study (32). IL-8 is a chemotactic factor that
attracts neutrophils, basophils and T cells, and it is also
involved in neutrophil activation (33). FOSL1 encodes the regulator protein
and is involved in cell proliferation, differentiation and
transformation (34). The
expression of PTGS2, IL-8 and FOSL1 was significantly higher in the
RMPP group compared with the NRMPP group in the present study,
indicating that the upregulation of these proteins may participate
in the pathogenicity of RMPP. In addition, RMPP cases exhibit a
high activation level of the IL-17 signaling pathway, which may
cause an autoimmune response and disease aggravation (35). Immunoglobulin heavy variable
(IGHV)3-30, IGHV3-64D and IGHV5-10-1 belong to the V region of the
variable domain of immunoglobulin heavy chains that participate in
antigen recognition (36). In the
present study, the disappearance of IGHV3-64D and IGHV5-10-1 genes
and low expression of the IGHV3-30 gene in the RMPP group may be an
important mechanism that leads to RMPP cases due to antigen
recognition problems. However, further experiments are required to
confirm these hypotheses.
In the present circRNA/mRNA analysis, circRNA
function was found to be associated with the known function of the
host linear transcripts. Based on the circRNA/miRNA/mRNA analysis
conducted in the present study, several differentially expressed
mRNAs were identified to be associated with the differentially
expressed circRNAs. A total of 11 circRNAs were identified as the
most significantly differentially expressed circRNAs between the HC
and MPP groups. Among those, hsa_circ_0019868 [SH3 and PX
domain-containing protein 2A (SH3PXD2A) gene] may be associated
with MPP pathogenesis. SH3PXD2A is an adapter protein involved in
the invasiveness of cancer cells that mediates the neurotoxic
effect of the amyloid-β peptide (37). The higher expression in MPP groups
may enhance the invasiveness of M. pneumoniae. A total of 24
circRNAs were identified as the most significantly differentially
expressed circRNAs between the NRMPP and RMPP groups. Among their
target genes, hsa_circ_0001890 [target of rapamycin complex 2
subunit MAPKAP1 (MAPKAP1) gene], hsa_circ_0026524 (eukaryotic
translation initiation factor 4B gene), hsa_circ_0021640 [caprin-1
(CAPRIN1) gene], hsa_circ_0003781 [protein arginine
N-methyltransferase 2 (PRMT2) gene], hsa_circ_0010131 [ephrin
type-A receptor 2 (EPHA2) gene], hsa_circ_0025209 [NOP2 nucleolar
protein (NOP2) gene] and hsa_circ_0023925
[phosphatidylinositol-binding clathrin assembly protein (PICALM)
gene] may be associated with the pathogenesis of RMPP. The
identified genes display the following characteristics: MAPKAP1 is
involved in ciliogenesis, regulates cell proliferation and survival
(38), and may serve as a novel
anti-infection and antifibrogenesis genomic locus in chronic
schistosomiasis japonica (39);
NOP2 is involved in a ribosomal large subunit assembly and
regulates cell proliferation (40);
PRMT2 is involved in the regulation of proliferation and promotes
apoptosis (41); EPHA2 regulates
migration, integrin-mediated adhesion, proliferation and
differentiation of cells (42);
CAPRIN1 may regulate cell proliferation and migration in multiple
cell types (43); and PICALM serves
an important role in several processes, such as internalization of
cell receptors, synaptic transmission and removal of apoptotic
cells (44). Therefore, these genes
might be involved in the pathogenesis of RMPP, but further
investigations are required. In addition, the selected circRNAs
(hsa_circ_0022808, hsa_circ_0006793, hsa_circ_0014390,
hsa_circ_0014305 and hsa_circ_00216400) may represent valuable
markers for the diagnosis of patients with early-stage RMPP and
NRMPP. However, there were limited samples used in the present
study; therefore, future studies should use larger sample
sizes.
To conclude, the present study provided a
comprehensive analysis of the expression levels of different
lncRNAs, mRNAs and circRNAs between NRMPP and RMPP cases using
rRNA-depleted RNA-sequencing techniques. The selected genes or
circRNAs may aid with identifying the complex pathogenesis of RMPP
and determining the diagnostic and therapeutic value of circRNAs in
RMPP.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81701990 and 81802817) and
the National Science and Technology Major Project (grant no.
2018ZX10101004003001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The datasets generated and/or analyzed during the current
study are available in the Sequence Read Archive repository
(BioProject accession no. PRJNA704769; http://www.ncbi.nlm.nih.gov/bioproject/704769).
Authors' contributions
FH and GL made substantial contributions to
conception and design. FH and GL confirm the authenticity of all
the raw data. GL supervised the study. HF, DZ and DY provided the
study materials. FH, HF, DY and TS collected and assembled the
data. FH, HF, DZ and JZ analyzed and interpreted the data. FH, HF,
DZ, TS and GL wrote and gave final approval of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee at Guangzhou Women and Children's Medical Center
(Guangzhou, China; approval no. 202009600). All the parents or
legal guardians of the patients signed written informed consent
forms and agreed to its content.
Patient consent for publication
All the parents or legal guardians of the patients
signed written informed consent forms for the publication of
patient data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar S: Mycoplasma pneumoniae: A
significant but underrated pathogen in paediatric
community-acquired lower respiratory tract infections. Indian J Med
Res. 147:23–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medjo B, Atanaskovic-Markovic M, Radic S,
Nikolic D, Lukac M and Djukic S: Mycoplasma pneumoniae as a
causative agent of community-acquired pneumonia in children:
Clinical features and laboratory diagnosis. Ital J Pediatr.
40:1042014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang HJ, Song DJ and Shim JY: Mechanism of
resistance acquisition and treatment of macrolide-resistant
Mycoplasma pneumoniae pneumonia in children. Korean J
Pediatr. 60:167–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Youn YS and Lee KY: Mycoplasma
pneumoniae pneumonia in children. Clin Exp Pediatr. 55:42–47.
2012.
|
|
5
|
Waites KB, Xiao L, Liu Y, Balish MF and
Atkinson TP: Mycoplasma pneumoniae from the respiratory
tract and beyond. Clin Microbiol Rev. 30:747–809. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rogozinski LE, Alverson BK and Biondi EA:
Diagnosis and treatment of Mycoplasma pneumoniae in
children. Minerva Pediatrica. 69:156–160. 2017.PubMed/NCBI
|
|
7
|
Izumikawa K: Clinical features of severe
or fatal Mycoplasma pneumoniae pneumonia. Front Microbiol.
7:8002016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin JE, Cheon BR, Shim JW, Kim DS, Jung
HL, Park MS and Shim JY: Increased risk of refractory Mycoplasma
pneumoniae pneumonia in children with atopic sensitization and
asthma. Korean J Pediatr. 57:271–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bajantri B, Venkatram S and Diaz-Fuentes
G: Mycoplasma pneumoniae: A potentially severe infection. J
Clin Med Res. 10:535–544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morozumi M, Hasegawa K, Kobayashi R, Inoue
N, Iwata S, Kuroki H, Kawamura N, Nakayama E, Tajima T, Shimizu K
and Ubukata K: Emergence of macrolide-resistant Mycoplasma
pneumoniae with a 23S rRNA gene mutation. Antimicrob Agents
Chemother. 49:2302–2306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuoka M, Narita M, Okazaki N, Ohya H,
Yamazaki T, Ouchi K, Suzuki I, Andoh T, Kenri T, Sasaki Y, et al:
Characterization and molecular analysis of macrolide-resistant
Mycoplasma pneumoniae clinical isolates obtained in Japan.
Antimicrob Agents Chemother. 48:4624–4630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oishi T, Narita M, Matsui K, Shirai T,
Matsuo M, Negishi J, Kaneko T, Tsukano S, Taguchi T and Uchiyama M:
Clinical implications of interleukin-18 levels in pediatric
patients with Mycoplasma pneumoniae pneumonia. J Infect
Chemother. 17:803–806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou YJ, Wang J, Chen WJ, Shen N, Tao Y,
Zhao RK, Luo LJ, Li BR and Cao Q: Impact of viral coinfection and
macrolide-resistant mycoplasma infection in children with
refractory Mycoplasma pneumoniae pneumonia. BMC Infect Dis.
20:6332020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li MS, Liu YL, Zhang XL, Liu J and Wang P:
Transcriptomic analysis of high-throughput sequencing about
circRNA, lncRNA and mRNA in bladder cancer. Gene. 677:189–197.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P
and Wu M: CircRNA: Functions and properties of a novel potential
biomarker for cancer. Mol Cancer. 16:942017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang HD, Jiang LH, Sun DW, Hou JC and Ji
ZL: CircRNA: A novel type of biomarker for cancer. Breast Cancer.
25:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu H, Wu S, Zhu Y, Ye M, Shen J, Liu Y,
Zhang YS and Bu S: Hsa_circRNA_0054633 is highly expressed in
gestational diabetes mellitus and closely related to glycosylation
index. Clin Epigenetics. 11:222019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui X, Niu W, Kong L, He M, Jiang K, Chen
S, Zhong A, Li W, Lu J and Zhang L: hsa_circRNA_103636: Potential
novel diagnostic and therapeutic biomarker in major depressive
disorder. Biomarkers Med. 10:943–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee YC, Chang CH, Lee WJ, Liu TY, Tsai CM,
Tsai TA, Tsai CK, Kuo KC, Chen CC, Niu CK and Yu HR: Altered
chemokine profile in Refractory Mycoplasma pneumoniae
pneumonia infected children. J Microbiol Immunol Infect.
S1684-S1182(20)30090-30096. 2020.
|
|
21
|
Kim D, Langmead B and Salzberg S: HISAT: A
fast spliced aligner with low memory requirements. Nat Methods.
12:357–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anders S, Pyl PT and Huber W: HTSeq-a
Python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao Y, Wang J and Zhao F: CIRI: An
efficient and unbiased algorithm for de novo circular RNA
identification. Genome Biol. 16:42015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Young MD, Wakefield MJ, Smyth GK and
Oshlack A: Gene ontology analysis for RNA-seq: Accounting for
selection bias. Genome Biol. 11:R142010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanehisa M, Araki M, Goto S, Hattori M,
Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T
and Yamanishi Y: KEGG for linking genomes to life and the
environment. Nucleic Acids Res. 36:D480–D484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong
S, Kong L, Gao G, Li CY and Wei L: KOBAS 2.0: A web server for
annotation and identification of enriched pathways and diseases.
Nucleic Acids Res. 39:W316–W322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu S, Xie X, Lei H, Zou B and Xie L:
Identification of key circRNAs/lncRNAs/miRNAs/mRNAs and pathways in
preeclampsia using bioinformatics analysis. Med Sci Monit.
25:1679–1693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaudhry R, Ghosh A and Chandolia A:
Pathogenesis of Mycoplasma pneumoniae: An update. Indian J
Med Microbiol. 34:7–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rumzhum NN and Ammit AJ: Cyclooxygenase 2:
Its regulation, role and impact in airway inflammation. Clin Exp
Allergy. 46:397–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cavallaro EC, Liang KK, Lawrence MD,
Forsyth KD and Dixon DL: Neutrophil infiltration and activation in
bronchiolitic airways are independent of viral etiology. Pediatr
Pulmonol. 52:238–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maurus K, Hufnagel A, Geiger F, Graf S,
Berking C, Heinemann A, Paschen A, Kneitz S, Stigloher C,
Geissinger E, et al: The AP-1 transcription factor FOSL1 causes
melanocyte reprogramming and transformation. Oncogene.
36:5110–5121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu Y, Shen F, Crellin NK and Ouyang W: The
IL-17 pathway as a major therapeutic target in autoimmune diseases.
Ann N Y Acad Sci. 1217:60–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McHeyzer-Williams M, Okitsu S, Wang N and
McHeyzer-Williams L: Molecular programming of B cell memory. Nature
Rev Immunol. 12:24–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Q, Jiang S, Liu H, Gao Y, Yang X, Ren
Z, Gao Y, Xiao L, Hu H, Yu Y, et al: Association of lncRNA
SH3PXD2A-AS1 with preeclampsia and its function in invasion and
migration of placental trophoblast cells. Cell Death Dis.
11:5832020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cardenas-Rodriguez M, Irigoín F, Osborn
DP, Gascue C, Katsanis N, Beales PL and Badano JL: The Bardet-Biedl
syndrome-related protein CCDC28B modulates mTORC2 function and
interacts with SIN1 to control cilia length independently of the
mTOR complex. Hum Mol Genet. 22:4031–4042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu X, Zhang J, Fan W, Gong Y, Yan J, Yuan
Z, Wu L, Cui H, Luo H, Kong Q, et al: MAPKAP1 rs10118570
polymorphism is associated with anti-infection and anti-hepatic
fibrogenesis in schistosomiasis japonica. PLoS One. 9:e1059952014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sloan KE, Bohnsack MT and Watkins NJ: The
5S RNP couples p53 homeostasis to ribosome biogenesis and nucleolar
stress. Cell Rep. 5:237–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhong J, Chen YJ, Chen L, Shen YY, Zhang
QH, Yang J, Cao RX, Zu XY and Wen GB: PRMT2β, a C-terminal splice
variant of PRMT2, inhibits the growth of breast cancer cells. Oncol
Rep. 38:1303–1311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Finney AC, Funk SD, Green JM, Yurdagul A
Jr, Rana MA, Pistorius R, Henry M, Yurochko A, Pattillo CB, Traylor
JG, et al: EphA2 expression regulates inflammation and
fibroproliferative remodeling in atherosclerosis. Circulation.
136:566–582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li XQ, Song JY, Lv W, Zhang D and Wu JZ:
Circular circ_0000885 promotes hepatocellular carcinoma
proliferation by epigenetically upregulating caprin1. Eur Rev Med
Pharmacol Sci. 23:7848–7854. 2019.PubMed/NCBI
|
|
44
|
Miller SE, Sahlender DA, Graham SC, Höning
S, Robinson MS, Peden AA and Owen DJ: The molecular basis for the
endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell.
147:1118–1131. 2011. View Article : Google Scholar : PubMed/NCBI
|




















