Introduction
Chronic heart failure (CHF) is a clinical syndrome
and heart abnormality is usually observed at the structural and
functional levels (1). Cardiac
output, cardiac contractility, filling pressure, wall stress during
systolic and diastolic function and heart rhythm are the basis of
the pathophysiology of CHF (2). A
variety of pathological conditions, such as myocardial infarction,
cardiomyopathy, hemodynamic overload and development of
inflammation, can lead to decompensation of CHF (3,4).
Once the heart is unable to maintain the peripheral blood perfusion
of the whole body, it may cause mortality in patients (4). Due to the complexity of CHF,
standardized and evidence-based treatment is essential (5).
In recent years, due to its special etiology,
diagnosis and treatment systems, Traditional Chinese Medicine has
received extensive attention, especially in the research of the
mechanism of small molecule active ingredients (6,7).
Magnoliaceae schisandraceae has a high medicinal value
(8). Shengmai Yin (SMY), also
known as pulse-activated decoction, is made of Fructus
schisandrae, Radix ginseng and Radix ophiopogonis and is
commonly used in the treatment of acute myocardial infarction,
cardiogenic shock and arrhythmia (9,10).
It is reported that SMY can alleviate the progression of
adriamycin-induced CHF by suppressing pathological changes
(10). Schisandrin A (Sch A) is
one of the main active ingredients of lignans in Schisandrin
and is a recognized monomer with pharmacological activity (11,12). A previous study noted that Sch A
serves roles in the inhibition of inflammation and elimination of
free radicals (13). In addition,
Sch A suppresses lipopolysaccharide-induced inflammation and
oxidative stress in macrophages in vitro (14). Moreover, Sch A can regulate bone
metabolism (15), prevent
cerebral ischemia reperfusion injury (16), attenuate acute liver injury
(17) and acts against cancer
(18). The present study focused
on investigating the role and mechanism of Sch A in CHF.
Research on the mechanism of microRNAs (miRNAs,
miRs) remains a popular and rewarding area of research. A number of
reports have shown that miRNAs are involved in regulating the
expression of specific genes and cellular processes in the process
of CHF. For example, miR-129-5p targets high mobility group box 1
to ameliorate heart function in CHF rats (19), miR-30d regulates cardiac
remodeling (20) and miR-133a
reduces cardiac hypertrophy by inhibiting the expressions of serum
response factor and cyclin D2 (21). Upregulated miR-155 can be observed
in a myocardial ischemia-reperfusion induction model (22) and circulating miR-155 levels may
be a potential marker for the risk of arrhythmia in patients with
CHF (23). miR-155 is encoded and
co-expressed by the gene B-cell integration cluster and a previous
study has indicated that Sch A regulates the proliferation and
invasion of breast cancer cells through miR-155 (24).
Isoproterenol (ISO) is widely used to induce CHF
(25). As such, it was
hypothesized that the miR-155 may act as a downstream regulator of
Sch A and participate in the treatment of CHF. An ISO-induced model
was constructed and Sch A linked with miR-155 to explore the
specific molecular mechanism of Sch A in the course of CHF for the
first time, to the best of the authors' knowledge.
Materials and methods
Animals
A total of 50 male C57BL/6JGpt mice (10 weeks, 22–25
g) were purchased from GemPharmatech Co., Ltd., (cat. no. N000013)
as the experimental subjects. All mice were adaptively reared under
the specific pathogen-free (SPF) conditions of the animal facility
(temperature, 23±2°C; relative humidity, 45~70%; 12-h light/dark
cycles) for 1 week. Mice were given free access to sufficient food
and water.
All animal experiments were approved by the Ethic
Committee of Experimental Animals of Changzhi Medical College
(approval nos. M20200109 and R20200115; Changzhi, China).
Animal modeling
The mice were randomly divided into five groups,
with 10 mice for each group as follows: i) CHF group, mice were
slowly injected with ISO hydrochloride (cat. no. I5627; Merck KGaA)
at a dosage of 30 mg/kg/day by the 2ML2 type ALZET osmotic pressure
pump (DURECT Corporation) for 2 weeks to establish the CHF model
(26); ii) control group, mice
were injected with 0.9% normal saline (cat. no. D123478; The
resources platform of the National standard material) including
0.002% ascorbic acid (cat. no. A92902; Merck KGaA) for 2 weeks;
iii) Sch A-H group, mice were subjected to the procedures of the
control group, except they also received intraperitoneal injections
of Sch A (40 mg/kg/day; cat. no. HY-N0691; MedChemExpress) for 10
days (including modelling) in advance until the completion of
modeling; iv) CHF + Sch A-L or v) CHF + Sch A-H group, mice were
subjected to the procedures of the CHF group, except they also
received intraperitoneal injections of Sch A (20 mg/kg/day or 40
mg/kg/day) for 10 days (including modelling) in advance until the
modeling was completed (12).
Heartbeat index detection
After the completion of modeling, the mice were
anesthetized by isoflurane (0.41 ml/min, at 4 l/min fresh gas flow;
cat. no. 1349003; Merck KGaA) and fixed on the laboratory bench.
The heartbeat indicators of each group of mice were detected by an
echocardiography imaging system (VisualSonics, Inc.). Heartbeat
indicators, including left ventricular end systolic diameter
(LVESD; mm), left ventricular end diastolic diameter (LVEDD; mm),
left ventricular posterior wall thickness (LVPWD; mm),
intra-ventricular septum diastole (IVSD; mm), ejection fraction
(EF; %) and fractional shortening (FS; %), were recorded and
analyzed.
Cardiac physiological index test
The mice were sacrificed by anesthesia
(Pentobarbital solution; cat. no. P-010-1ML; 60 mg/kg; Merck KGaA).
Death was confirmed by observing whether the heartbeat had
completely stopped and pupils were dilated. Afterwards, their body
weight (BW) and tibia length (TL) were measured. Fresh hearts were
quickly excised in a sterile environment and the heart weight (HW)
was measured. Parts of the heart tissues were fixed at 4°C with 4%
paraformaldehyde (PFA; cat. no. 158127; Merck KGaA) for 2 h,
dehydrated with alcohol (cat. no. PHR1070; Merck KGaA) followed by
xylene (cat. no. B83606; Merck KGaA) and embedded in paraffin, and
the remaining parts were ready for the extraction of RNA.
Pathological analysis of cardiac
tissue
The paraffin-embedded heart tissue samples were
sectioned (6 µm), deparaffinized and rehydrated. A
hematoxylin-eosin staining (HE) staining kit (cat. no. AR1180;
Boster Biological Technology) was used for staining. According to
the manufacturer's protocols, at room temperature, the specimens
were covered with hematoxylin staining solution for 3 min, Scott
blue liquid (cat. no. G1865; Beijing Solarbio Science &
Technology Co., Ltd.) for 1 min and eosin staining solution for 40
sec, respectively. Finally, the pathological abnormalities were
observed under an inverted microscope (IXplore Pro; Olympus
Corporation).
Quantification of mRNA level
Reverse transcription-quantitative (RT-q)PCR was
performed to detect mRNA levels of miR-155, atrial natriuretic
peptide (ANP), B-type natriuretic peptide (BNP) and B-myosin heavy
chain (B-MHC). Primer3 Plus (http://www.primer3plus.com/cgi-bin/dev/primer3plus.cgi)
was used to design the primers. According to the manufacturer's
protocol, mouse myocardial tissues or isolated rat ventricular
myocytes (NRVMs, 1×106) were treated with
TRIzol® (cat. no. 15596026; Thermo Fisher Scientific,
Inc.) to extract total RNA. According to the manufacturer's
protocol, reverse transcription of RNA was then performed using a
miRNA reverse transcription kit (cat. no. 4366597; Thermo Fisher
Scientific, Inc.) and PrimeScript RT-PCR kit (cat. no. RR014A;
Takara Bio, Inc.). The cDNA was used as a template and RT-qPCR was
performed on QuantStudio 3D PCR system (Thermo Fisher Scientific,
Inc.) with QuantStudio 3D AnalysisSuite (Thermo Fisher Scientific,
Inc.). qPCR thermocycling conditions were as follows: Denaturation
at 94°C for 5 min, 37 cycles of 95°C for 15 sec, 60°C for 30 sec
and 72°C for 30 sec. All reactions were repeated three times. U6
and GAPDH were the internal controls. The relative expressions of
miR-155, ANP, BNP and B-MHC were expressed as a function of cycle
quantification (Cq) and analyzed by the 2−ΔΔCq method
(27). The primers are listed in
Table I.
 | Table I.Reverse transcription-quantitative
PCR primers. |
Table I.
Reverse transcription-quantitative
PCR primers.
| A, Gene
(mouse) |
|---|
|
|---|
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| miR-155 |
UUAAUGCUAAUUGUGAUAGGGGU |
TGTGACCCGAAAGCTCTA |
| ANP |
AGGCAGTCGATTCTGCTT |
CGTGATAGATGAAGGCAGGAAG |
| BNP |
TAGCCAGTCTCCAGAGCAATTC |
TTGGTCCTTCAAGAGCTGTCTC |
| Β-MHC |
TTGGATGAGCGACTCAAAAA |
GCTCCTTGAGCTTCTTCTGC |
| GAPDH |
TGCACCACCTGCTTAGC |
GGCATGGACTGTGGTCATGAG |
| U6 |
GCTTCGGCAGCACATATACTA |
CGAATTTGCGTGTCATCCTTG |
|
| B, Gene
(rat) |
|
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|
| miR-155 |
AATGCTAATTGTGATAGGGG |
GAACATGTCTGCGTATCTC |
| ANP-rat |
GAGCAAATCCCGTATACAGTGC |
ATCTTCTACCGGCATCTTCTCC |
| BNP-rat |
GCTGCTGGAGCTGATAAGAGAA |
GTTCTTTTGTAGGGCCTTGGTC |
| Β-MHC |
GAGGAGAGGGCGGACATT |
ACTCTTCATTCAGGCCCTTG |
| GAPDH |
GCAAGAGAGAGGCCCTCAG |
TGTGAGGGAGATGCTCAGTG |
| U6 |
GGAACGATACAGAGAAGATTAGC |
GGAACGATACAGAGAAGATTAGC |
Isolation and culture of ventricular
myocytes
Sprague-Dawley (SD) rat pups (~2–3 days, Kaixue
Biotechnology Co., Ltd.) were purchased to isolate NRVMs. After the
rats were sacrificed by anesthesia (pentobarbital solution, 50
mg/kg), fresh hearts were quickly excised in a sterile environment,
followed by being minced and then lysed with 0.25% trypsin (cat.
no. 25200056; Thermo Fisher Scientific, Inc.) until the tissue
almost dissolved. Then, the cell suspension was centrifuged by a
centrifuge (cat. no. TDL5M; Spring Instrument. Co., Ltd.) at 4,840
× g at room temperature for 5 min and the pellet was collected. The
NRVMs were resuspended in DMEM/F12 (cat. no. A4192001; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; AlphaCell) and streptomycin (100 U/ml; cat. no. 85886; Merck
KGaA) and incubated in a humid incubator at 37°C with 5%
CO2 (Heracell 150i CO2 Incubator; cat. no.
51032719; Thermo Fisher Scientific, Inc.) (28).
Cell viability assay
NRVMs (4×104 cells) in logarithmic growth
phase were seeded in each well of a 96-well plate (cat. no.
HY-E0076, MedChemExpress) with culture medium containing 10% FBS
and different concentrations of Sch A (0, 1, 2, 4, 8, 12 and 24 µM)
and incubated at 37°C with 5% CO2 for 30 min. A total of
three sets of parallel experiments were performed in each group in
total. Next, MTT solution (cat. no. HY-15924, MedChemExpress) was
added to each well and incubated with the cells at 37°C for 4 h,
followed by the addition of SDS (cat. no. 2-270-9; Qiguang
Technology Trade). The OD value at an absorbance of 570 nm was
measured in a microplate reader (SpectraMax iD5; Molecular Devices,
LLC).
Cell grouping
The NRVMs in the ISO group were incubated with ISO
(10 µM) only for 24 h at 37°C. For the Sch A-4 group, the NRVMs
were pretreated with Sch A (4 µM) for 30 min and then incubated
with ISO (10 µM) for 24 h at 37°C. For the Sch A-12 group, the
NRVMs were pretreated with Sch A (12 µM) for 30 min and then
incubated with ISO (10 µM) for 24 h at 37°C (29).
Cell transfection
The transfection of miR-155 mimic [M; Sangon Biotech
(Shanghai) Co., Ltd.] or miR-155 inhibitor [I; Sangon Biotech
(Shanghai) Co., Ltd.] regulated the expression of miR-155, with the
establishment of their corresponding control groups (transfection
with non-sense sequence) [M control (MC) and I control (IC)].
miR-155 M or I was mixed with X-tremeGENE 9 DNA Transfection
Reagent (cat. no. 6365787001; Merck KGaA) to configure the
transfection complex, which was incubated at 25°C for 15 min and
added into the cell suspension (1×105) dropwise and
continued to incubate at 37°C for 48 h. The sequences were as
follows: M, 5′-UUCCAAUGCUAAUUGUGAUAGGGGU-3′; I,
5′-ACCCCTATCACAATTAGCATTAA-3′; MC, 5′-AAGAAACCATGCAAAGTAAGGTT-3′;
and IC, 5′-CCTAGGCTCACGTGACGCG-3′.
Immunofluorescence
The NRVMs were fixed at room temperature with 4% PFA
on the glass slide for 15 min and then immersed in 0.5% Triton
X-100 (cat. no. T8200; Beijing Solarbio Science & Technology
Co., Ltd.) to permeate at room temperature for 20 min. Normal goat
serum (cat. no. ab7481; Abcam) was added to block cells at room
temperature for 30 min. Then the antibody against α-smooth muscle
actin (α-SMA; cat. no. 19245, Cell Signaling Technology, Inc.;
1:500) was added and incubated at 4°C overnight. On the second day,
Goat Anti-Rabbit IgG H&L (HRP; cat. no. 4412; Cell Signaling
Technology, Inc.; 1:2,000) was added and incubated at 37°C for 1 h.
The subsequent steps were performed in the dark. DAPI (cat. no.
4083; Cell Signaling Technology, Inc.) was used to incubate at room
temperature for 5 min for nuclear counterstaining. The collected
images were captured under a fluorescence microscope (Dmi8; Leica
Microsystems GmbH) and the results were expressed as average cell
surface area (µm2).
Western blotting
The NRVMs (1×105 cells) were mixed with
the RIPA Lysis buffer (cat. no. 89901; Thermo Fisher Scientific,
Inc.) to lyse protein for 10 min, followed by a centrifugation
(3,000 × g) at 4°C for 5 min. A bicinchoninic acid (BCA) kit (cat.
no. P0011; Beyotime Institute of Biotechnology) was used to measure
protein concentration. Denatured protein was mixed with 1X protein
loading buffer [cat. no. C508320-0001; Sangon Biotech (Shanghai)
Co., Ltd.]. The protein samples (30 µg/lane) were separated on 15%
sodium dodecyl sulfate polyacrylamide gel electrophoresis (Bio-Rad
Laboratories, Inc.) and then transferred to nitrocellulose
membranes (cat. no. 1620090; Bio-Rad Laboratories, Inc.). Membranes
were then blocked with 5% skimmed milk at room temperature for 2 h.
The membranes were incubated with the diluted solution of primary
antibodies, including protein kinase B (AKT; cat. no. ab8805;
Abcam; 1:500; 60 kDa), phosphorylated (p)-AKT (cat. no. ab38449;
Abcam; 1:1,000; 56 kDa), Cyclic AMP response-element binding
protein (CREB; cat. no. ab32515; Abcam; 1:1,000; 40 kDa), p-CREB
(ab32096; cat. no. Abcam; 1:5,00; 37 kDa), GAPDH (cat. no.
ab181602; Abcam; 1:10,000; 36 kDa) at 4°C overnight. The next day,
the secondary antibody Goat Anti-Rabbit IgG H&L (cat. no.
ab205718; Abcam; 1:50,000) was added for further 2 h incubation at
room temperature. GAPDH was used as an internal control.
Chromogenic liquid (cat. no. 34577; Thermo Fisher Scientific, Inc.)
was used for the visualization in the dark, followed by the
detection in an imaging system (Chemi Doc Touch; Bio-Rad
Laboratories, Inc.), and the results were semi-quantified using
ImageJ software (v1.8.0; National Institutes of Health).
Statistical analysis
Statistical analysis was performed by SPSS 20.0 (IBM
Corp.). All experiments were repeated at least three times and the
data are presented as the mean ± standard deviation. One-way
analysis of variance was used for the comparison between multiple
groups followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Sch A can ameliorate the gravimetric
parameters of ISO-induced CHF mice
Following ISO treatment, echocardiography was used
to assess the cardiac function of mice. Compared with normal mice,
the indexes (LVPWD, IVSD, LVEDD and LVESD) of ISO-induced CHF mice
were larger, while EF and FS were reduced (P<0.01; Fig. 1A-F). Meanwhile, it was found that
Sch A treatment had no significant effect on normal mice, but it
could partially reverse these indicators in CHF mice (P<0.05;
Fig. 1A-F). Moreover, Sch A
partially reversed the CHF-induced increased ratios of HW/BW and
HW/TL (P<0.05; Fig. 1G and H).
Pathologically, it could be observed that Sch A treatment
alleviated the myocardial hypertrophy caused by CHF (Fig. 1I) and significantly reduced these
adverse changes, with improved results represented following the
treatment of higher concentration, suggesting that the therapeutic
effect of Sch A was concentration-dependent (Fig. 1A-I).
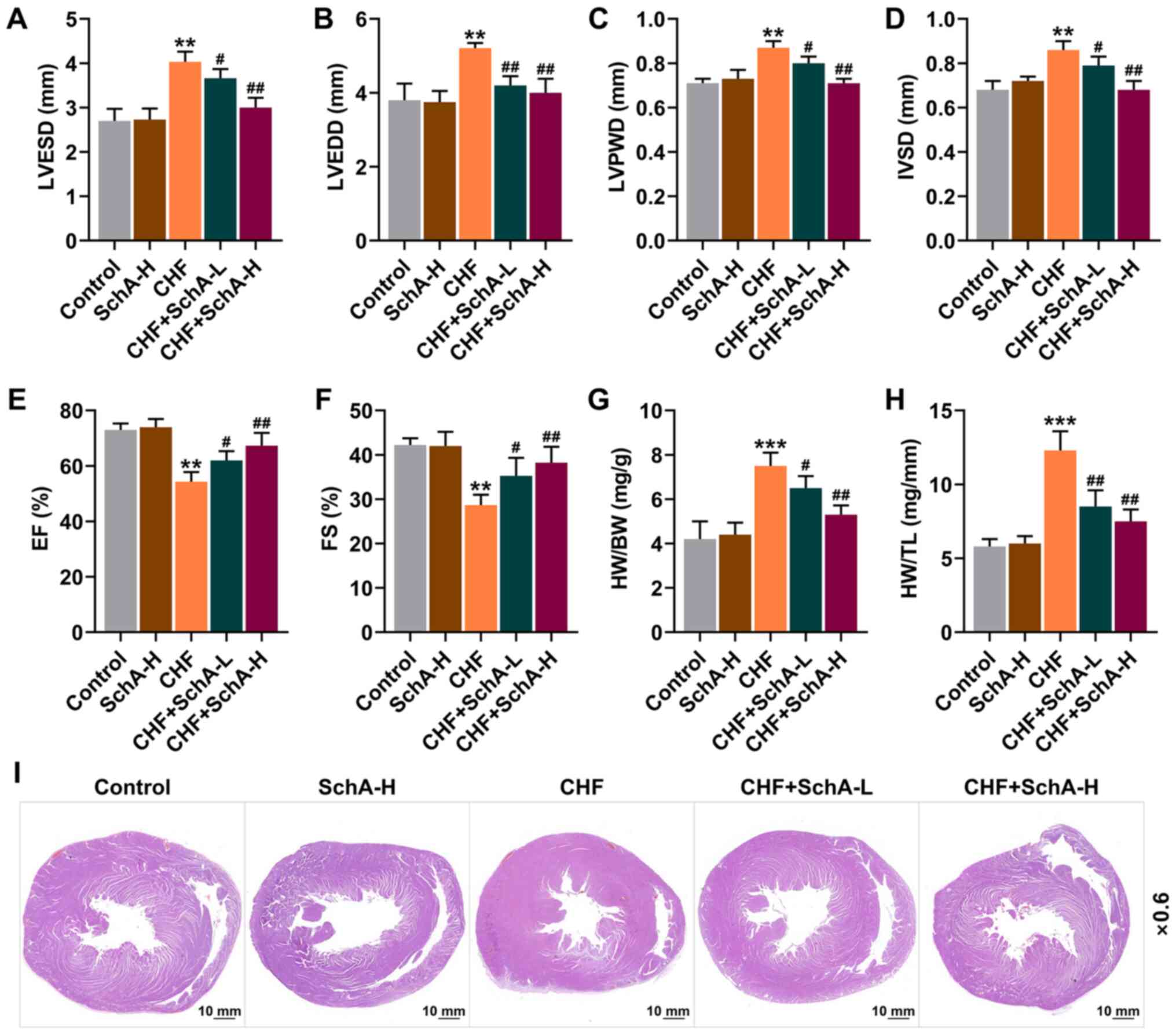 | Figure 1.Sch A reverses the pathological
changes induced by CHF. The levels of (A) LVESD, (B) LVEDD, (C)
LVPWD, (D) IVSD, (E) EF and (F) FS were detected by
echocardiography. (G) The ratio of HW to BW. (H) The ratio of HW to
TL. (I) Pathological hematoxylin-eosin staining stained sections of
heart tissue. Scale bar, 10 mm; magnification, ×0.6. **P<0.01,
***P<0.001 vs. Control; #P<0.05,
##P<0.01 vs. CHF. Sch A, Schisandrin A; CHF, chronic
heart failure; LVESD, left ventricular end systolic diameter;
LVEDD, left ventricular end diastolic diameter; LVPWD, left
ventricular posterior wall thickness; IVSD, intra-ventricular
septum diastole; EF, ejection fraction; FS, fractional shortening;
HW, heart weight; BW, body weight; TL, tibia length; Sch A-H,
injected intraperitoneally with 40 mg/kg/day Sch A; Sch A-L,
injected intraperitoneally with 20 mg/kg/day Sch A. |
Sch A can inhibit the expression of
myocardial hypertrophy markers and miR-155
In the CHF mouse model, the expression levels of
ANP, BNP and Β-MHC were all increased, while Sch A treatment
partially reduced the expression levels of these genes (P<0.01;
Fig. 2A). Notably, Sch A could
inhibit the upregulation of miR-155 in the CHF group (P<0.01;
Fig. 2B). Similarly, high
concentration of Sch A led to more obvious effects (P<0.001;
Fig. 2A and B).
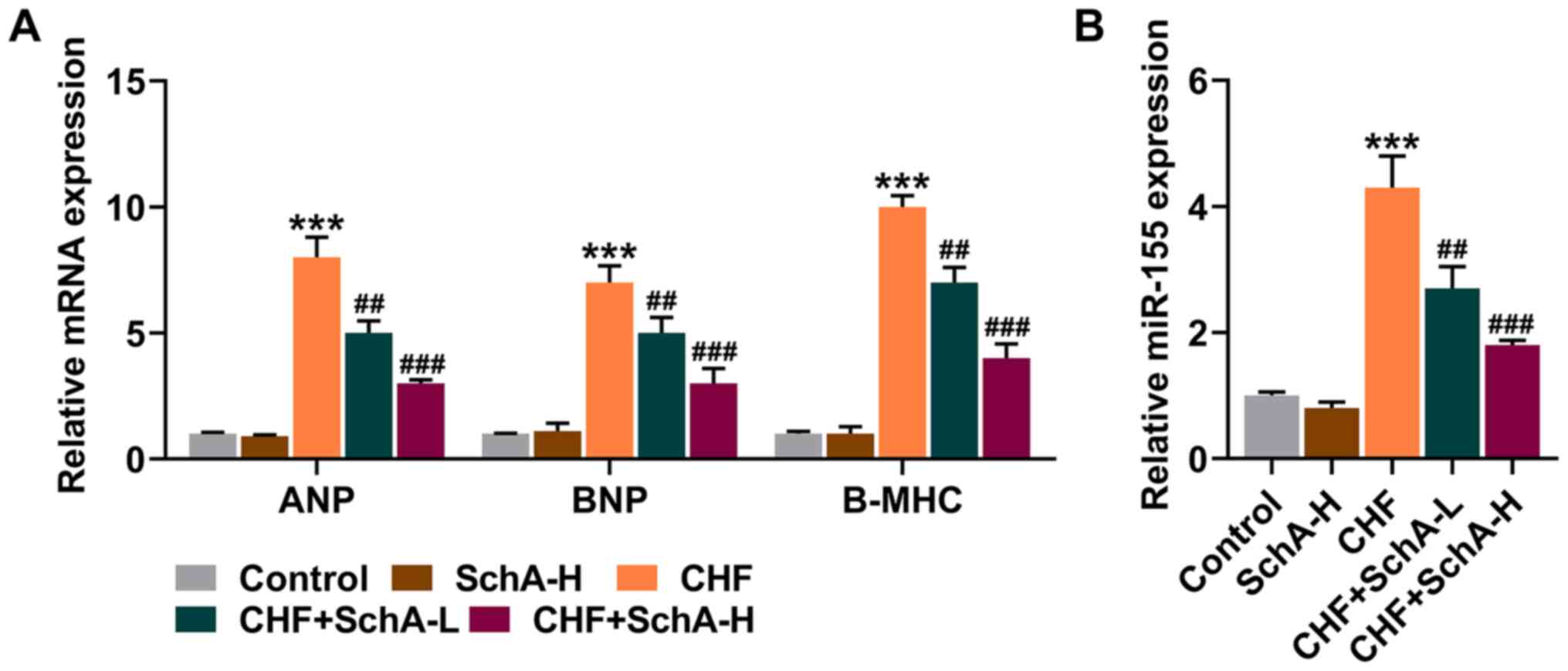 | Figure 2.Sch A downregulates the expression
levels of ANP, BNP, B-MHC and miR-155 induced by CHF. (A) mRNA
expression levels of ANP, BNP and B-MHC in myocardial tissue were
quantified by RT-qPCR. GAPDH was used as a control. (B) The
expression of miR-155 in myocardial tissue was measured via
RT-qPCR. U6 was used as a control. ***P<0.001 vs. Control;
##P<0.01, ###P<0.001 vs. CHF. Sch A,
Schisandrin A; CHF, chronic heart failure; ANP, atrial natriuretic
peptide; BNP, B-type natriuretic peptide; B-MHC, B-myosin heavy
chain; miR, microRNA; RT-qPCR, reverse transcription-quantitative
PCR; Sch A-H, injected intraperitoneally with 40 mg/kg/day Sch A;
Sch A-L, injected intraperitoneally with 20 mg/kg/day Sch A. |
Interaction between Sch A and miR-155
in vitro
To determine the interaction between Sch A and
miR-155, NRVMs were isolated from SD rat pups for cell experiments.
The results of MTT test suggested that under the treatment of
different gradients, Sch A significantly inhibited the cell
viability of NRVMs when the concentration reached 24 µM (P<0.05;
Fig. 3A). Therefore, the
concentrations of 4 and 12 µΜ were chosen for follow-up
experiments. Upregulation of miR-155 was also observed in
ISO-treated cells, the trend of which was reversed by Sch A
(P<0.001; Fig. 3B). As the
inhibitory effect of the SchA-12 group was stronger than that of
the SchA-4 group, the cells were subsequently treated with 12 µM
SchA.
miR-155 M and I were designed to further investigate
the role of miR-155 in CHF and its corresponding mechanism. The
transfection of miR-155 M notably upregulated the expression level
of miR-155, while miR-155 I led to a downregulation (P<0.001;
Fig. 4A). The transfection of I
also effectively attenuated the ISO-induced increase of miR-155
expression, the effects of which were similar to those of Sch A
(P<0.001; Fig. 4B). The
expression of miR-155 was promoted in the ISO + Sch A + M group
when compared with the ISO + Sch A + MC group (P<0.001; Fig. 4B). Furthermore, it was
demonstrated in the results of immunofluorescence that Sch A
attenuated the promotive effects of ISO on the level of α-SMA
(P<0.001; Fig. 4C and D).
Downregulation of miR-155 also could inhibit ISO-induced increase
of α-SMA content in NRVMs, while miR-155 M attenuated the
inhibitory effect of Sch A on the level of α-SMA (P<0.001;
Fig. 4C and D), suggesting that
Sch A might regulate myocardial hypertrophy by inhibiting
miR-155.
 | Figure 4.Sch A relieves fibrosis of neonatal
rat ventricular myocytes via miR-155. (A) The transfection
efficiency of miR-155 mimic or miR-155 inhibitor was evaluated by
RT-qPCR. (B) The expression of miR-155 was determined via RT-qPCR.
U6 was used as a control. (C and D) The level of α-SMA was observed
by immunofluorescence. Scale bar, 50 µm; magnification, ×200.
&&&P<0.001 vs. MC;
ΔΔΔP<0.001 vs. IC; ***P<0.001 vs. Blank;
▲▲▲P<0.001 vs. ISO + IC; ###P<0.001 vs.
ISO; ^^^P<0.001 vs. ISO + Sch A + MC. Sch A,
Schisandrin A; miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR; α-SMA, α-smooth muscle actin; M,
miR-155 mimic; MC, miR-155 mimic control; I, miR-155 inhibitor; IC,
miR-155 inhibitor control; ISO, isoproterenol. |
As for ANP, BNP, Β-MHC, p-AKT and p-CREB, the
transfection of miR-155 I attenuated the promotion of ISO, the
effect of which was similar to that of Sch A and upregulating
miR-155 expression attenuated the therapeutic effect of Sch A
(P<0.001; Fig. 5A-D),
suggesting that higher-expressed miR-155 could promote both the
expressions of ANP, BNP, Β-MHC and the phosphorylation of AKT and
CREB. The above evidence indicated that Sch A ameliorated
ISO-induced hypertrophy in cardiomyocyte and inhibited the
expressions of ANP, BNP, Β-MHC, p-AKT and p-CREB via regulation of
miR-155.
 | Figure 5.Sch A regulates ANP, BNP, B-MHC,
pAKT/AKT and p-CREB/CREB via miR-155. (A) mRNA expression levels of
ANP, BNP and B-MHC were measured using reverse
transcription-quantitative PCR. GAPDH was used as a control. (B)
The protein expression of AKT, p-AKT, CREB and p-CREB was
semi-quantified by western blotting; GAPDH was used as a control.
(C) The ratio of p-AKT to AKT. (D) The ratio of p-CREB to CREB.
***P<0.001 vs. Blank; ▲▲▲P<0.001 vs. ISO + IC;
###P<0.001 vs. ISO; ^^^P<0.001 vs. ISO
+ Sch A + MC. Sch A, Schisandrin A; miR, microRNA; ANP, atrial
natriuretic peptide; BNP, B-type natriuretic peptide; B-MHC,
B-myosin heavy chain; p-, phosphorylated; CREB, cyclic AMP
response-element binding protein; M, miR-155 mimic; MC, miR-155
mimic control; I, miR-155 inhibitor; IC, miR-155 inhibitor control;
ISO, isoproterenol. |
Discussion
Epidemiological data suggests that the prevalence of
CHF is still rising, with high mortality and frequent
hospitalizations (1).
Echocardiography is the most useful and reliable non-invasive
method for the diagnosis of cardiac insufficiency (3). Decreased myocardial contractility,
abnormal hemodynamics and neuroendocrine activation are the
distinctive features of CHF. In the current study, to obtain more
evidence to further transform the application of Sch A in clinical
patient management, mice were used to construct CHF models for
in vivo and NRVMs isolated from rat pups for in
vitro. Rat and mouse experiments are involved in the previous
studies and, by contrast, the mouse CHF model is more mature, while
the rat cell extraction is easier (30–32). The present study demonstrated the
protective effects of Sch A in CHF via the administration of Sch A.
The results of echocardiography showed that Sch A treatment
alleviated ISO-induced heart damage, which was characterized by the
reversal of LVPWD, IVSD, LVEDD, LVESD, EF and FS. In addition, it
was found that Sch A ameliorated myocardial hypertrophy and reduced
HW/BW, HW/TL in CHF mice. Myocardial hypertrophy, specifically
referring to the increase in volume and weight of myocardial cells,
rather than an increase in number (33), is an important compensation method
for CHF in order to maintain the body's demand for cardiac output
(33,34). The in vivo experiments of
the present study supported the hypothesis that Sch A could prevent
the ISO-induced deterioration in heart function and structure. CHF
is the terminal stage of a number of diseases and neurohumoral
activation is important for its diagnosis and prognosis (35). ANP and BNP belong to the family of
natriuretic peptides and can be secreted by NRVMs (36,37). ANP can promote relaxation of
vascular smooth muscles to stretch the heart wall and reduce blood
pressure, which delays progress of CHF (38,39), while BNP has the effect of
regulating the homeostasis of blood pressure and blood volume,
which is used as a biochemical indicator as its concentration in
plasma is directly proportional to the severity of CHF (40,41). In addition, B-MHC is specifically
expressed in the heart of mammals and is closely related to cardiac
function and myocardial hypertrophy (42,43). The present study observed that Sch
A downregulated the mRNA levels of ANP, BNP and B-MHC in both CHF
mouse model and cell model.
MicroRNA can be used as a biomarker and prognostic
indicator of CHF in clinical applications (44). miR-155 is a conservative and
multifunctional miRNA, which serves a non-negligible role in cancer
(45), fibrotic diseases
(46), lymphocyte homeostasis
(47) and cardiovascular diseases
(48). A study reports that the
overexpression of miR-155 in human cardiomyocyte progenitor cells
is associated with protection from necrotic cell death (49). Another report pointed out that
inhibiting endogenous miR-155 may have a clinical potential to
inhibit cardiac hypertrophy and CHF (50). The data from the present study
demonstrated that Sch A specifically inhibited the ISO-induced
upregulation of miR-155 in vitro and in vivo. α-SMA
is known as a feature that distinguishes myofibroblasts (MF) from
fibroblasts, which indicates the differentiation and maturity of MF
(51). Based on the results of
immunofluorescence, it was shown that following SchA treatment, the
intracellular level of α-SMA in ISO-treated NRVMs was
downregulated, which was restored by inhibiting miR-155. The
occurrence of fibrosis is one of the main factors of physiological
cardiac hypertrophy and pathological cardiac hypertrophy (52,53). The present study revealed that Sch
A could target miR-155 to alleviate pathological cardiac
hypertrophy. In addition, it was found that miR-155 was not only
associated with ANP, BNP and B-MHC, but was also involved in the
activation of AKT and CREB signaling pathways. AKT is essential for
the development of the heart and the insulin-like growth factor
1/PI3K/AKT pathway serves a key role in regulating exercise-induced
physiological cardiac hypertrophy and cardioprotection (54). CREB is an important protein that
regulates gene transcription, the transcription of which is
regarded as a necessary regulator of the hypertrophic response
(55,56). The present study demonstrated that
blocking the activation of AKT/CREB counteracted the ISO-induced
hypertrophy and Sch A inhibited the expression of miR-155, which
thereby suppressed the phosphorylation of AKT and CREB.
Targeted therapy is a trend for precision medicine.
In conclusion, the results of the present study provided evidence
of the protective effect of Sch A on cardiac insufficiency and CHF
remodeling, in addition to the revelation that Sch A-mediated
downregulation of miR-155 was an essential mechanism implicated in
the Sch A-mediated improvement of cardiac function, cardiac
hypertrophy and fibrosis. Furthermore, in vitro experiments
also demonstrated that Sch A inhibited the expression levels of
ANP, BNP and B-MHC and blocked AKT/CREB activation via miR-155.
This article provided a preliminary scientific basis for the
clinical application of Sch A in CHF in the future.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation
of China (grant no. 81902020), Scientific Research Project of
Shanxi Provincial Health Commission (grant no. 2020135), Changzhi
Medical College Doctor Startup Fund (grant no. BS17001) and Shanxi
Province Applied Basic Research Project (grant no.
201901D211469).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG made substantial contributions to conception and
design. TL, SL, ZS, YC and LY performed data acquisition, analysis
and interpretation. LG drafted the article and critically revised
it for important intellectual content. LG and TL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of Experimental Animals of Changzhi Medical College
(approval nos. M20200109 and R20200115; Changzhi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ewen S, Nikolovska A, Zivanovic I,
Kindermann I and Böhm M: Chronic heart failure - new insights.
Dtsch Med Wochenschr. 141:1560–1564. 2016.(In German). PubMed/NCBI
|
|
2
|
Špinar J, Špinarová L and Vítovec J:
Pathophysiology, causes and epidemiology of chronic heart failure.
Vnitr Lek. 64:834–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
King M, Kingery J and Casey B: Diagnosis
and evaluation of heart failure. Am Fam Physician. 85:1161–1168.
2012.PubMed/NCBI
|
|
4
|
Climent M, Viggiani G, Chen YW, Coulis G
and Castaldi A: MicroRNA and ROS crosstalk in cardiac and pulmonary
diseases. Int J Mol Sci. 21:212020. View Article : Google Scholar
|
|
5
|
Dick SA and Epelman S: Chronic heart
failure and inflammation: what do we really know? Circ Res.
119:159–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Wong YK and Liao F: What has
traditional Chinese medicine delivered for modern medicine? Expert
Rev Mol Med. 20:e42018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zang Y, Wan J, Zhang Z, Huang S, Liu X and
Zhang W: An updated role of astragaloside IV in heart failure.
Biomed Pharmacother. 126:1100122020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rybnikář M, Šmejkal K and Žemlička M:
Schisandra chinensis and its phytotherapeutical applications. Ceska
Slov Farm. 68:95–118. 2019.PubMed/NCBI
|
|
9
|
Ma S, Li X, Dong L, Zhu J, Zhang H and Jia
Y: Protective effect of Sheng-Mai Yin, a traditional Chinese
preparation, against doxorubicin-induced cardiac toxicity in rats.
BMC Complement Altern Med. 16:612016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang K, Zhang J, Wang X, Wang L, Pugliese
M, Passantino A and Li J: Cardioprotection of Sheng Mai Yin a
classic formula on adriamycin induced myocardial injury in Wistar
rats. Phytomedicine. 38:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu D, Liu J, Ma H, Guo W, Wang J, Kan X,
Li Y, Gong Q, Cao Y, Cheng J, et al: Schisandrin A protects against
lipopolysaccharide-induced mastitis through activating Nrf2
signaling pathway and inducing autophagy. Int Immunopharmacol.
78:1059832020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhi Y, Jin Y, Pan L, Zhang A and Liu F:
Schisandrin A ameliorates MPTP-induced Parkinson's disease in a
mouse model via regulation of brain autophagy. Arch Pharm Res.
42:1012–1020. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui L, Zhu W, Yang Z, Song X, Xu C, Cui Z
and Xiang L: Evidence of anti-inflammatory activity of Schizandrin
A in animal models of acute inflammation. Naunyn Schmiedebergs Arch
Pharmacol. 393:2221–2229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwon DH, Cha HJ, Choi EO, Leem SH, Kim GY,
Moon SK, Chang YC, Yun SJ, Hwang HJ, Kim BW, et al: Schisandrin A
suppresses lipopolysaccharide-induced inflammation and oxidative
stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and
PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int J Mol
Med. 41:264–274. 2018.PubMed/NCBI
|
|
15
|
Ni S, Qian Z, Yuan Y, Li D, Zhong Z,
Ghorbani F, Zhang X, Zhang F, Zhang Z, Liu Z, et al: Schisandrin A
restrains osteoclastogenesis by inhibiting reactive oxygen species
and activating Nrf2 signalling. Cell Prolif. 53:e128822020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou F, Wang M, Ju J, Wang Y, Liu Z, Zhao
X, Yan Y, Yan S, Luo X and Fang Y: Schizandrin A protects against
cerebral ischemia-reperfusion injury by suppressing inflammation
and oxidative stress and regulating the AMPK/Nrf2 pathway
regulation. Am J Transl Res. 11:199–209. 2019.PubMed/NCBI
|
|
17
|
Lu Y, Wang WJ, Song YZ and Liang ZQ: The
protective mechanism of schisandrin A in d-galactosamine-induced
acute liver injury through activation of autophagy. Pharm Biol.
52:1302–1307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu X, Rajamanicham V, Xu S, Liu Z, Yan T,
Liang G, Guo G, Zhou H and Wang Y: Schisandrin A inhibits triple
negative breast cancer cells by regulating Wnt/ER stress signaling
pathway. Biomed Pharmacother. 115:1089222019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao N, Zhang J, Chen C, Wan Y, Wang N and
Yang J: miR-129-5p improves cardiac function in rats with chronic
heart failure through targeting HMGB1. Mamm Genome. 30:276–288.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Salvador AM, Li G, Valkov N, Ziegler
O, Yeri A, Yang Xiao C, Meechoovet B, Alsop E, Rodosthenous RS, et
al: Mir-30d regulates cardiac remodeling by intracellular and
paracrine signaling. Circ Res. 128:e1–e23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wehbe N, Nasser SA, Pintus G, Badran A,
Eid AH and Baydoun E: Micrornas in cardiac hypertrophy. Int J Mol
Sci. 20:47142019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ge X, Meng Q, Wei L, Liu J, Li M, Liang X,
Lin F, Zhang Y, Li Y, Liu Z, et al: Myocardial ischemia-reperfusion
induced cardiac extracellular vesicles harbour proinflammatory
features and aggravate heart injury. J Extracell Vesicles.
10:e120722021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blanco RR, Austin H, Vest RN III, Valadri
R, Li W, Lassegue B, Song Q, London B, Dudley SC, Bloom HL, et al:
Angiotensin receptor type 1 single nucleotide polymorphism 1166A/C
is associated with malignant arrhythmias and altered circulating
miR-155 levels in patients with chronic heart failure. J Card Fail.
18:717–723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan H and Guo M: Schizandrin A inhibits
cellular phenotypes of breast cancer cells by repressing miR-155.
IUBMB Life. 72:1640–1648. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang SC, Ren S, Rau CD and Wang JJ:
Isoproterenol-induced heart failure mouse model using osmotic pump
implantation. Methods Mol Biol. 1816:207–220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Q, Pan LL, Xue R, Ni G, Duan Y, Bai
Y, Shi C, Ren Z, Wu C, Li G, et al: The anti-microbial peptide
LL-37/CRAMP levels are associated with acute heart failure and can
attenuate cardiac dysfunction in multiple preclinical models of
heart failure. Theranostics. 10:6167–6181. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan X, Li J, Wang X, Chen N, Cai B, Wang
G, Shan H, Dong D, Liu Y, Li X, et al: Tanshinone IIA protects
against cardiac hypertrophy via inhibiting calcineurin/NFATc3
pathway. Int J Biol Sci. 7:383–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Y, Xu C, Tang S and Xia Z:
Interleukin-9 Aggravates isoproterenol-induced heart failure by
activating signal transducer and activator of transcription 3
signalling. Can J Cardiol. 36:1770–1781. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
You X, Guo ZF, Cheng F, Yi B, Yang F, Liu
X, Zhu N, Zhao X, Yan G, Ma XL, et al: Transcriptional
up-regulation of relaxin-3 by Nur77 attenuates β-adrenergic
agonist-induced apoptosis in cardiomyocytes. J Biol Chem.
293:14001–14011. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng H, Wu X, Ni G, Wang S, Peng W, Zhang
H, Gao J and Li X: Citri Reticulatae Pericarpium protects
against isoproterenol-induced chronic heart failure via activation
of PPARγ. Ann Transl Med. 8:13962020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laudette M, Coluccia A, Sainte-Marie Y,
Solari A, Fazal L, Sicard P, Silvestri R, Mialet-Perez J, Pons S,
Ghaleh B, et al: Identification of a pharmacological inhibitor of
Epac1 that protects the heart against acute and chronic models of
cardiac stress. Cardiovasc Res. 115:1766–1777. 2019.PubMed/NCBI
|
|
33
|
Gallo S, Vitacolonna A, Bonzano A,
Comoglio P and Crepaldi T: ERK: A key player in the pathophysiology
of cardiac hypertrophy. Int J Mol Sci. 20:202019. View Article : Google Scholar
|
|
34
|
Heinzel FR, Hohendanner F, Jin G, Sedej S
and Edelmann F: Myocardial hypertrophy and its role in heart
failure with preserved ejection fraction. J Appl Physiol 1985.
119:1233–1242. 2015.PubMed/NCBI
|
|
35
|
Hartupee J and Mann DL: Neurohormonal
activation in heart failure with reduced ejection fraction. Nat Rev
Cardiol. 14:30–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raveendran VV, Al-Haffar K, Kunhi M,
Belhaj K, Al-Habeeb W, Al-Buraiki J, Eyjolsson A and Poizat C:
Protein arginine methyltransferase 6 mediates cardiac hypertrophy
by differential regulation of histone H3 arginine methylation.
Heliyon. 6:e038642020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qi Y, Li JJ, Di XH, Zhang Y, Chen JL, Wu
ZX, Man ZY, Bai RY, Lu F, Tong J, et al: Excess sarcoplasmic
reticulum-mitochondria calcium transport induced by
Sphingosine-1-phosphate contributes to cardiomyocyte hypertrophy.
Biochim Biophys Acta Mol Cell Res. 1868:1189702021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Bold AJ: Atrial natriuretic factor: A
hormone produced by the heart. Science. 230:767–770. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Charloux A, Piquard F, Doutreleau S,
Brandenberger G and Geny B: Mechanisms of renal hyporesponsiveness
to ANP in heart failure. Eur J Clin Invest. 33:769–778. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gardner DG, Chen S, Glenn DJ and Grigsby
CL: Molecular biology of the natriuretic peptide system:
Implications for physiology and hypertension. Hypertension.
49:419–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kuwahara K, Nakagawa Y and Nishikimi T:
Cutting Edge of Brain Natriuretic Peptide (BNP) Research - The
Diversity of BNP Immunoreactivity and Its Clinical Relevance. Circ
J. 82:2455–2461. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ingles J, Doolan A, Chiu C, Seidman J,
Seidman C and Semsarian C: Compound and double mutations in
patients with hypertrophic cardiomyopathy: Implications for genetic
testing and counselling. J Med Genet. 42:e592005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hang CT, Yang J, Han P, Cheng HL, Shang C,
Ashley E, Zhou B and Chang CP: Chromatin regulation by Brg1
underlies heart muscle development and disease. Nature. 466:62–67.
2010.Erratum in: Nature 475: 532, 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vegter EL, van der Meer P, de Windt LJ,
Pinto YM and Voors AA: MicroRNAs in heart failure: From biomarker
to target for therapy. Eur J Heart Fail. 18:457–468. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Michaille JJ, Awad H, Fortman EC, Efanov
AA and Tili E: miR-155 expression in antitumor immunity: The higher
the better? Genes Chromosomes Cancer. 58:208–218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eissa MG and Artlett CM: The microRNA
miR-155 is essential in fibrosis. Noncoding RNA. 5:52019.
|
|
47
|
Chen L, Gao D, Shao Z, Zheng Q and Yu Q:
miR-155 indicates the fate of CD4+ T cells. Immunol Lett.
224:40–49. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ding H, Wang Y, Hu L, Xue S, Wang Y, Zhang
L, Zhang Y, Qi H, Yu H, Aung LHH, et al: Combined detection of
miR-21-5p, miR-30a-3p, miR-30a-5p, miR-155-5p, miR-216a and miR-217
for screening of early heart failure diseases. Biosci Rep.
40:BSR201916532020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu J, van Mil A, Vrijsen K, Zhao J, Gao
L, Metz CH, Goumans MJ, Doevendans PA and Sluijter JP: MicroRNA-155
prevents necrotic cell death in human cardiomyocyte progenitor
cells via targeting RIP1. J Cell Mol Med. 15:1474–1482. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Seok HY, Chen J, Kataoka M, Huang ZP, Ding
J, Yan J, Hu X and Wang DZ: Loss of MicroRNA-155 protects the heart
from pathological cardiac hypertrophy. Circ Res. 114:1585–1595.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gabbiani G: The myofibroblast in wound
healing and fibrocontractive diseases. J Pathol. 200:500–503. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rai V, Sharma P, Agrawal S and Agrawal DK:
Relevance of mouse models of cardiac fibrosis and hypertrophy in
cardiac research. Mol Cell Biochem. 424:123–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
McLellan MA, Skelly DA, Dona MSI, Squiers
GT, Farrugia GE, Gaynor TL, Cohen CD, Pandey R, Diep H, Vinh A, et
al: High-Resolution Transcriptomic Profiling of the Heart During
Chronic Stress Reveals Cellular Drivers of Cardiac Fibrosis and
Hypertrophy. Circulation. 142:1448–1463. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Weeks KL, Bernardo BC, Ooi JYY, Patterson
NL and McMullen JR: The IGF1-PI3K-Akt Signaling Pathway in
Mediating Exercise-Induced Cardiac Hypertrophy and Protection. Adv
Exp Med Biol. 1000:187–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang DS, Bian ZY, Zhang Y, Zhang SM, Liu
Y, Zhang R, Chen Y, Yang Q, Zhang XD, Fan GC, et al: Role of
interferon regulatory factor 4 in the regulation of pathological
cardiac hypertrophy. Hypertension. 61:1193–1202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Müller FU, Lewin G, Baba HA, Bokník P,
Fabritz L, Kirchhefer U, Kirchhof P, Loser K, Matus M, Neumann J,
et al: Heart-directed expression of a human cardiac isoform of
cAMP-response element modulator in transgenic mice. J Biol Chem.
280:6906–6914. 2005. View Article : Google Scholar : PubMed/NCBI
|



















