Introduction
Cataracts are a common lens disease worldwide and
are one of the main causes of blindness. Ultraviolet B (UVB)
irradiation is considered an important factor leading to the
formation of cataracts by inducing the apoptosis of human lens
epithelial cells (HLECs) (1,2).
The photobiological effects of UVB may lead to reactive oxygen
species (ROS) generation (3), DNA
damage (4) and apoptosis
(5). Experimental evidence has
indicated that oxidative stress caused by free radical accumulation
may serve a crucial role in the pathogenesis of cataracts, and this
process can be prevented and/or ameliorated by antioxidants
(6).
In recent years, the interest in green tea has grown
because of its lack of toxicity and good efficacy in a wide range
of organs, such as retinal, brain and skin (7–9).
Green tea contains >3,000 compounds, of which nearly one-third
are polyphenols that include catechins such as (−)-epicatechin
(EC), (−)-EC gallate (ECG), (−)-epigallocatechin (EGC) and (−)-EGC
gallate (EGCG) (8). The EC
isomers share a similar backbone but have varying locations and
numbers of hydroxyl groups (Fig.
1). As the most abundant catechin derivative, EGCG is also
considered to be the most effective antioxidant (10). The antioxidant activities of EGCG
are caused by the presence of phenolic groups, which are sensitive
to oxidation and can produce quinones. The trihydroxyl structure of
the D-ring in EGCG further improves its antioxidant activity
(Fig. 1) (11). Previous studies have confirmed
that EGCG exerts a mild protective effect against UVB-induced
oxidative damage (12,13); however, the detailed mechanism
remains unclear.
The mitochondria-mediated apoptotic signaling
pathway is a crucial endogenous apoptosis pathway in which
caspase-dependent and caspase-independent mechanisms have
significant roles. Mitochondrial apoptosis-inducing factor (AIF)
and endonuclease G (Endo G) are involved in caspase-independent
apoptosis (14). In the
caspase-dependent apoptosis pathway, the formation of the
apoptosome contributes to mitochondrial permeabilization, which
promotes the activation of caspases and in turn triggers the
production of other apoptosis-related proteins, leading to cell
death (15). Bax and Bcl-2 are
closely related to the mitochondrial apoptosis pathway (16). During apoptosis, the activation of
Bax leads to the release of numerous mitochondria-related proteins,
including the transport of cytochrome c from the
mitochondria to the cytoplasm via Bax, which overcomes the
Bcl-2-mediated regulation of mitochondrial membrane protein
permeability; in addition, cytochrome c, procaspase 9,
activating factor 1 (Apaf-1) and dATP combine to form an apoptotic
complex that activates caspase-9, which can in turn activate
caspase-3, ultimately triggering caspase-dependent apoptosis
(17,18).
UVB can lead to the apoptosis of HLECs through the
caspase-dependent pathway (1),
whereas EGCG may reduce oxidant damage and thus protect HLECs from
apoptosis (19–21). Heo et al (22) first reported in 2008 that EGCG
increased the cell viability and cell count after UVB irradiation
of cultured HLECs, indicating that EGCG may be able to protect
HLECs against UVB damage. However, whether EGCG reduces UVB-induced
oxidative damage to HLECs via caspase signaling remains unclear.
The present study explored the effect of EGCG on the human lens
epithelial B-3 (HLE B-3) cell line, which was treated with or
without UVB irradiation. The findings of the present study may
provide novel insights into the EGCG-mediated protection of HLECs
under UVB irradiation.
Materials and methods
Cell culture and treatment with
EGCG
HLE B-3 cells were obtained from the American Type
Culture Collection (ATCC); this cell line was authenticated by STR.
The cells were cultured in RPMI 1640 medium (HyClone; Cytiva)
containing 10% fetal bovine serum (HyClone; Cytiva) at 37°C in a
humidified environment containing 5% CO2. After reaching
75–80% confluence, the cells were irradiated with 30
mJ/cm2 UVB at room temperature for 2 min or pretreated
with EGCG (MilliporeSigma) for 2 h prior to UVB irradiation at
37°C. At the designated time points, the cells were collected for
different measurements.
UVB exposure
In the present study, UVB exposure was provided by a
UVB lamp (Nanjing Huaqiang Electronic Co., Ltd.). The UVB spectral
range was 290–320 nm and the peak irradiance was 297 nm. To obtain
a good irradiation effect, the distance between the UVB lamp and
the bottom of the culture plate was adjusted, and the central
irradiation intensity was 0.25 mW/cm2. UVB exposure dose
(30 mJ/cm2)=irradiation intensity (0.25
mW/cm2) × irradiation time (120 sec). Before UVB
irradiation, the cells were washed three times with warm
phosphate-buffered saline (PBS; pH 7.4) to remove nonattached cells
and residual serum, and a small amount of PBS was left to cover the
cells. The cells were then exposed to UVB. After irradiation, fresh
medium was added to each well, and the cells were further cultured
until the required time.
Cell viability assay
HLE B-3 cells were seeded into 96-well plates
(8×103 cells/well) and exposed to UVB irradiation (30
mJ/cm2) alone or following pretreatment with various
concentrations of EGCG (0.78, 1.56, 3.125, 6.25, 12.5, 25 or 50 µM)
for 2 h. Subsequently, the cells were cultured for an additional
24, 48 and 72 h. Then, 20 µl MTT solution (5 mg/ml) was added to
each well for MTT detection. After 4 h of incubation at 37°C, the
supernatant was discarded and the formazan in the cells was
dissolved in dimethyl sulfoxide. Finally, the absorbance was
measured at a wavelength of 490 nm (Multimode Plate Reader;
PerkinElmer, Inc.). The cell viability was expressed as a
percentage of the control.
Detection of mitochondrial membrane
potential (Δψm)
HLE B-3 cells were seeded into 6-well plates
(7.5×104 cells/well) overnight, and each well was
exposed to UVB irradiation (30 mJ/cm2) in the presence
or absence of EGCG pretreatment (50 µM) for 2 h. JC-1 (5, 5′, 6,
6′-tetrachloro-1,1′, 3, 3′-tetra-ethylbenzimidazolylcarbocyanine
iodide; Beyotime Institute of Biotechnology) was used as a probe to
detect the changes in Δψm following an additional 12 h of treatment
with EGCG at 37°C. JC-1 monomers emit green fluorescence (488 nm)
when the mitochondria are polarized, whereas JC-1 aggregates emit
red fluorescence under excitation at 585 nm. The red (PI, 585 nm)
and green (FITC-A, 488 nm) fluorescence was measured simultaneously
using a BD FACSVerse™ flow cytometer (BD FACSVerse™ Flow Cytometer;
cat. no. 651154; BD Biosciences). A total of 1×104 cells
were analyzed for each sample.
Detection of antioxidants and
oxidants
HLE B-3 cells were seeded into 6-well plates
(5.0×105 cells/well) overnight and were then exposed to
UVB irradiation (30 mJ/cm2) in the presence or absence
of EGCG pretreatment (50 µM) for 2 h, followed by a 24 h culture.
At the specified time point, the cells were harvested, the cell
pellets were collected by centrifugation at 400 × g for 5 min at
4°C, followed by suspension in PBS and ultrasonication on ice at
moderate energy for 5 sec/time for a total of 10 min. Furthermore,
the supernatants were collected after centrifugation at 5,000 × g
for 10 min at 4°C. According to the manufacturer's instructions,
the activities of catalase (CAT), superoxide dismutase (SOD) and
glutathione peroxidase (GSH-Px), as well as the levels of GSH,
H2O2 and hydroxyl free radicals were detected
using the corresponding kits. The following kits were used (all
purchased from Nanjing Jiancheng Bioengineering Institute): CAT
assay kit (cat. no. A007-1-1), SOD assay kit (cat. no. A001-3-2),
GSH-Px assay kit (cat. no. A005-1-2), reduced GSH assay kit (cat.
no. A006-2-1), hydrogen peroxide assay kit (cat. no. A064-1-1),
hydroxyl free radical assay kit (cat. no. A018-1-1). Finally, the
absorbance was measured at a suitable wavelength (Multimode Plate
Reader; PerkinElmer, Inc.).
Apoptosis assay
A total of 16 h after the aforementioned treatments,
the cells were harvested, and apoptosis was detected using an
Annexin V-FITC/PI apoptosis detection kit (Bipec Biopharma
Corporation). The collected cells were stained according to the
manufacturer's protocol. The collected data were analyzed using BD
FACSuite software and a FACSVerse flow cytometer (version 1.0)
(both from BD Biosciences). A total of 1×104 cells were
analyzed for each experiment.
Reverse transcription-quantitative PCT
(RT-qPCR)
A total of 24 h after the aforementioned treatments,
the cells were washed with PBS twice and collected to extract total
RNA using an RNAqueous TM total RNA isolation kit (Thermo Fisher
Scientific, Inc.). The extracted RNA was reverse transcribed into
cDNA using a RevertAid First-Strand cDNA Synthesis Kit (cat. no.
K1621; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Furthermore, qPCR was conducted in a
20 µl reaction using 2X SYBR-Green qPCR Mix (Thermo Fisher
Scientific, Inc.). The PCR reaction was carried out using the
Stratagene Mx3000p sequence detection system (Agilent Technologies,
Inc.) under the following conditions: 95°C for 10 min, followed by
40 cycles at 95°C for 15 sec and 60°C for 1 min. The target genes
Bcl-2, Bax, cytochrome c, caspase-9 and caspase-3 were
amplified, and the GAPDH gene was used as the internal control. The
primer sequences are listed in Table
I. The 2−ΔΔCq formula was used to
calculate the relative mRNA transcription levels (23).
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′-3′) |
|---|
| GAPDH | F:
ACTTTGGTATCGTGGAAGGACTCAT |
|
| R:
GTTTTTCTAGACGGCAGGTCAGG |
| Bcl-2 | F:
ATCGCCCTGTGGATGACTGA |
|
| R:
GAGACAGCCAGGAGAAATCAAAC |
| Bax | F:
TTTTGCTTCAGGGTTTCATCCA |
|
| R:
TGCCACTCGGAAAAAGACCTC |
| Cytochrome
c | F:
CTTGGACTTAGAGAGTGGGGACG |
|
| R:
GTGGCACTGGGAACACTTCATAA |
| Caspase-9 | F:
TGGACATTGGTTCTGGAGGATT |
|
| R:
AGCACCATTTTCTTGGCAGTCA |
| Caspase-3 | F:
TGGAAGCGAATCAATGGACTCT |
|
| R:
TGAATGTTTCCCTGAGGTTTGC |
Western blotting
A total of 24 h after the aforementioned treatments,
the cells were washed with ice-cold PBS and lysed in RIPA buffer
(Beyotime Institute of Biotechnology) containing 10 mM
phenylmethylsulfonyl fluoride. After incubation on ice for 30 min,
the supernatant was collected following centrifugation at 8,000 × g
for 10 min at 4°C, and the target proteins (50 µg) were then
separated by 5–12% SDS-PAGE and transferred to PVDF membranes. The
membranes were blocked in Tris-buffered saline solution (pH 7.6)
containing 0.1% Tween-20 and 5% non-fat dried milk for 1 h at room
temperature and probed with primary antibodies (all from Abcam)
against Bcl-2 (rabbit; cat. no. ab32124; 1:1,000), Bax (rabbit;
cat. no. ab182733; 1:2,000), cytochrome c (rabbit; cat. no.
ab76107; 1:500), caspase-9 (rabbit; cat. no. ab185719; 1:1,000),
caspase-3 (rabbit; cat. no. ab32042; 1:500) and β-actin (rabbit;
cat. no. ab8227; 1:1,000) overnight at 4°C. Subsequently, the
membranes were washed with PBS and probed with HRP-conjugated goat
anti-rabbit IgG secondary antibodies (cat. no. ab205718; 1:5,000;
Abcam) for 2 h at room temperature. Finally, visualization was
performed with an Immobilon Western Chemiluminescent HRP Substrate
(MilliporeSigma) using the FUSION-FX7 imaging system (Vilber
Lourmat) and quantified using the Fusion CAPT software (version
15.06a; Vilber Lourmat). The levels of β-actin were used to
normalize the protein expression levels.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used for statistical
analysis. All of the experiments were repeated three times and data
are presented as the mean ± SD. One-way ANOVA followed by the LSD
post hoc test was used to analyze data containing ≤3 groups. One
way ANOVA followed by Tukey's post hoc test was used to analyze
data containing >3 groups. The effects of time and different
treatments on cell viability were assessed using two-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Protective effect of EGCG against UVB
irradiation-induced HLEC damage
In our previous experiments, it was revealed that 30
mJ/cm2 UVB irradiation reduced the survival rate of HLE
B-3 cells to 50.79±5.34% (20);
therefore, 30 mJ/cm2 UVB irradiation was selected to
perform the relevant experiment in the present study. The present
study investigated the effect of EGCG on the survival rate of HLECs
after UVB irradiation by MTT assay. The results indicated that
following pretreatment with various concentrations of EGCG, the
cell viability after exposure to UVB irradiation was increased in a
time-dependent manner (Fig. 2).
Moreover, EGCG pretreatment at concentrations between 0.78 and 50
µM for 2 h could efficiently improve cell viability compared with
the UVB group at 72 h (Fig. 2).
Notably, pretreatment with EGCG at a concentration of 50 µM
exhibited the strongest protective effect on HLECs. Therefore, 50
µM EGCG was selected for subsequent studies.
Effect of EGCG on changes in Δψm
caused by UVB irradiation
The JC-1 distribution results demonstrated that the
UVB group (72.03±3.17%; Fig. 3C)
had a lower Δψm compared with that in the control group
(94.55±1.89%; Fig. 3A), and a
significant improvement in Δψm was observed in the EGCG + UVB group
(82.63±4.49%; Fig. 3D) compared
with that in the UVB group. In the EGCG group, the Δψm was
96.15±1.67% (Fig. 3B), which was
similar to the control group. The relative Δψm values of the groups
compared with the control group are shown in Fig. 3E. These results indicated that
EGCG could protect the Δψm of HLECs under conditions of UVB
irradiation.
Effect of EGCG on the changes in
enzyme activities induced by UVB irradiation
Compared with in the control group, the enzyme
activities of CAT and SOD were significantly reduced by UVB
irradiation (Fig. 4A and B),
whereas GSH-Px activity was not significantly decreased by UVB
irradiation (Fig. 4C); however,
after pretreatment with EGCG, the activities of CAT, SOD and GSH-Px
were significantly enhanced compared with UVB irradiation group
(Fig. 4A-C). These results
indicated that EGCG may enhance the activities of CAT, SOD and
GSH-Px, and thus may serve a protective role against UVB
irradiation-mediated oxidative damage.
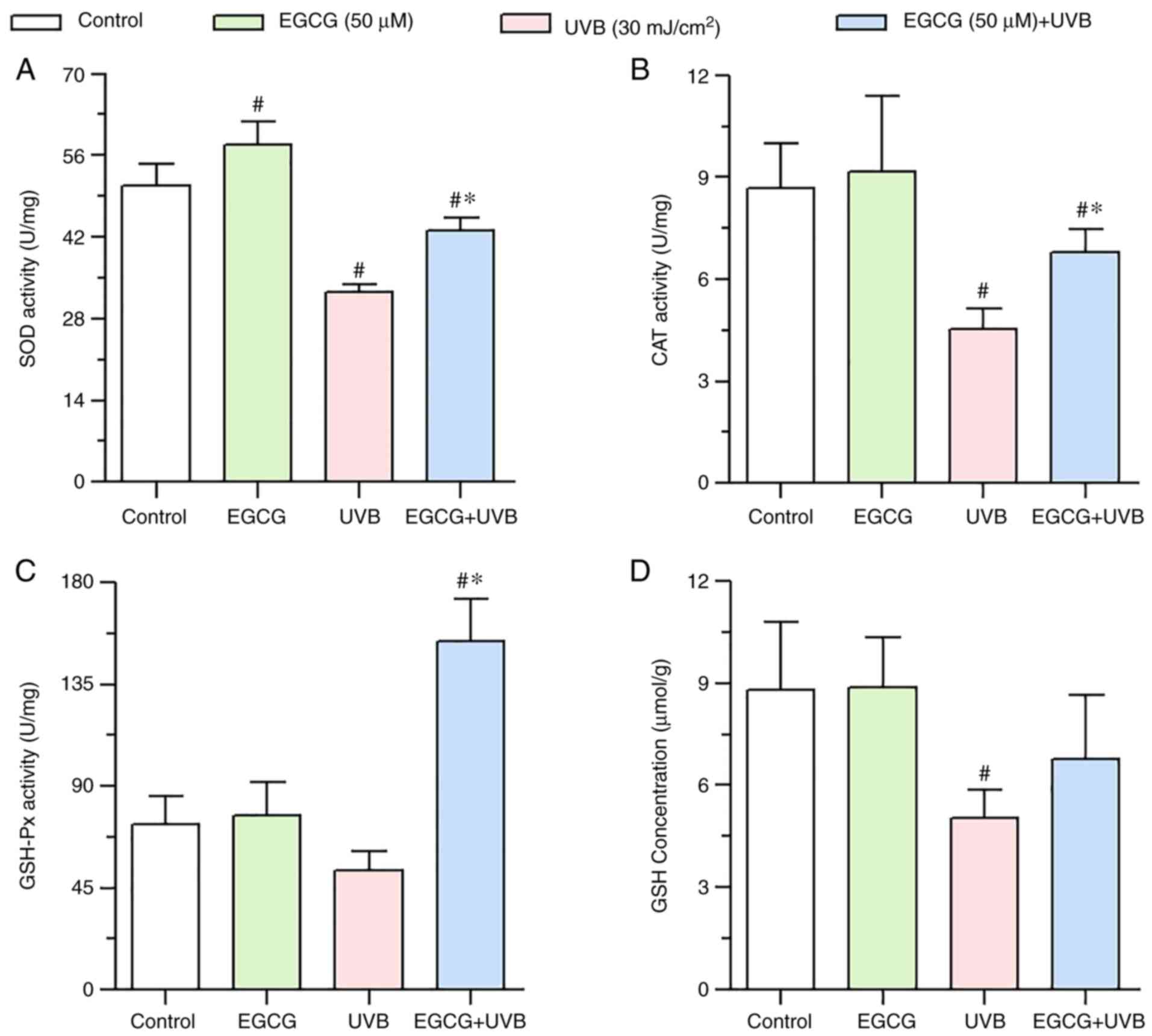 | Figure 4.Effect of EGCG on antioxidant enzyme
activity and GSH concentration under UVB irradiation. (A) SOD
activity, (B) CAT activity, (C) GSH-Px activity and (D) GSH
concentration 24 h after UVB irradiation, with or without EGCG
pretreatment. The results showed that EGCG significantly enhanced
the activities of CAT, SOD and GSH-Px, but did not significantly
increase the levels of GSH. Data are presented as the mean ± SD
(n=3). *P<0.05 vs. the UVB group; #P<0.05 vs. the
control group. CAT, catalase; EGCG, epigallocatechin gallate; GSH,
glutathione; GSH-Px, GSH peroxidase; SOD, superoxide dismutase;
UVB, ultraviolet B. |
Effect of EGCG on changes in GSH
levels induced by UVB irradiation
A significant decrease in GSH levels was observed in
the UVB group (5.04±0.83 µmol/g) compared with in the control group
(8.81±1.99 µmol/g). By contrast, GSH levels were not significantly
increased in HLECs after pretreatment with EGCG before UVB
irradiation (6.77±1.87 µmol/g) compared with in the UVB group
(Fig. 4D).
Effect of EGCG on the levels of
H2O2 and hydroxyl free radicals induced by
UVB irradiation
ROS levels were measured by assessing the production
of intracellular H2O2 and hydroxyl free
radicals. As shown in Fig. 5, a
marked increase in H2O2 levels was observed
in the UVB group (16.85±3.86 mmol/l) compared with in the control
group (7.69±1.20 mmol/l). By contrast, a significant decrease in
the H2O2 levels was observed in HLECs
following pretreatment with EGCG before UVB irradiation (8.40±1.27
mmol/l) compared with in the UVB group (P<0.05).
In addition, the levels of hydroxyl free radicals
were markedly elevated after UVB irradiation (984.53±62.07 mmol/l)
compared with in the control group (879.56±61.36 mmol/l) (Fig. 5). However, a significant reduction
in hydroxyl free radicals was observed in HLECs following
pretreatment with EGCG before UVB irradiation (856.81±66.06 mmol/l)
compared with in the UVB group (Fig.
5).
These results suggested that EGCG may have potent
scavenging activity toward H2O2 and hydroxyl
free radicals, indicating that EGCG may efficiently prevent
UVB-induced intracellular ROS production within HLECs.
Effect of EGCG on apoptosis induced by
UVB irradiation
A prominent increase in the number of early
apoptotic cells (shown in the lower right quadrant) was observed in
the UVB group (14.6±1.57%; Fig.
6C) compared with in the control group (0.39±0.25%; Fig. 6A). Conversely, a significant
decrease in the number of early apoptotic cells was observed in the
EGCG + UVB group (6.77±1.28%; Fig.
6D) compared with in the UVB group. Furthermore, the percentage
of late apoptotic/necrotic cells (shown in the upper right
quadrant) was 18.62±2.63% in the UVB group (Fig. 6C), 0.25±0.03% in the control group
(Fig. 6A) and 2.79±0.15% in the
EGCG group (Fig. 6B). Notably,
following pretreatment with EGCG, a significant decrease in the
number of late apoptotic/necrotic HLECs was detected compared with
in the UVB group (10.34±1.16%; Fig.
6D). UVB irradiation significantly increased the percentage of
the total apoptotic cells (including early and late apoptotic
cells), whereas EGCG intervention significantly decreased the total
apoptosis rate (Fig. 6E). These
observations indicated that EGCG could efficiently decrease the
HLEC apoptosis induced by UVB irradiation.
 | Figure 6.Protective effect of EGCG against
HLECs apoptosis under UVB irradiation. HLECs were treated with 50
µM EGCG for 2 h prior to UVB irradiation (30 mJ/cm2) and
cultured for 16 h. Cell apoptosis was detected by flow cytometry
using Annexin V/PI staining in the (A) control, (B) EGCG, (C) UVB
and (D) EGCG + UVB groups. LL, viable non-stained cells; LR, early
apoptotic Annexin V-FITC-stained cells; UR, late apoptotic/necrotic
Annexin V-FITC and PI-stained cell; UL, dead PI-stained cells. (E)
Proportion of early apoptotic and late apoptotic cells. Data are
presented as the mean ± SD (n=3). *P<0.05 vs. the UVB group;
#P<0.05 vs. the control group. EGCG, epigallocatechin
gallate; LL, lower left; LR, lower right; UL, upper left; UR, upper
right; UVB, ultraviolet B. |
Effect of EGCG on the mRNA expression
levels of Bcl-2, Bax, cytochrome c, caspase-9 and caspase-3 induced
by UVB irradiation
RT-qPCR was used to explore whether EGCG
pretreatment influenced the expression levels of Bcl-2, Bax,
cytochrome c, caspase-9 and caspase-3 in HLECs following UVB
irradiation. The results indicated that pretreatment with EGCG
prior to UVB irradiation (EGCG + UVB group) significantly increased
the expression levels of Bcl-2, but decreased the expression levels
of Bax, cytochrome c, caspase-9 and caspase-3 compared with
in the UVB group (Fig. 7). The
Bcl-2/Bax ratio was significantly decreased after UVB irradiation
compared with the control group, but EGCG treatment significantly
increased this ratio (Fig.
7F).
 | Figure 7.Alterations in the mRNA expression
levels of (A) Bcl-2, (B) Bax, (C) Cyt C, (D) Cas-9 and (E) Cas-3
following pretreatment with EGCG before UVB irradiation were
assessed using RT-qPCR. EGCG inhibited the UVB irradiation-induced
expression of Bax, Cyt C, Cas-9 and Cas-3, and promote Bcl-2
expression in HLECs. (F) RT-qPCR analysis of the Bcl-2/Bax ratio in
HLECs. GAPDH was used as the internal control. *P<0.05 vs. the
UVB group; #P<0.05 vs. the control group. Cas-3,
caspase-3; Cas-9, caspase-9; Cyt C, cytochrome c; EGCG,
epigallocatechin gallate; HLECs, human lens epithelial cells;
RT-qPCR, reverse transcription-quantitative PCR; UVB, ultraviolet
B. |
Effect of EGCG on the protein
expression levels of Bcl-2, Bax, cytochrome c, caspase-9 and
caspase-3 induced by UVB irradiation
The caspase-dependent pathway is an important
pathway for inducing apoptosis, and includes Bcl-2, Bax, cytochrome
c, caspase-9, caspase-3 and other proteins. As shown in
Fig. 8, the western blotting
results indicated that the protein expression levels of Bax,
cytochrome c, caspase-9 and caspase-3 were downregulated in
the EGCG + UVB group compared with those in the UVB group. By
contrast, the expression levels of Bcl-2 were increased in the EGCG
+ UVB group compared with those in the UVB group (Fig. 8). Overall, these findings
suggested that EGCG alleviated the UVB-induced apoptosis of HLECs
by inhibiting the caspase-dependent apoptosis pathway.
Discussion
UVB causes harm to living organisms mainly through
damaging DNA, proteins and cell membranes, as well as inducing
oxidative stress through the generation of ROS, such as hydroxyl
free radicals, hydroxyl peroxide and superoxide, leading to
apoptosis (24–27). It has been well demonstrated that
UVB-induced ROS production mediates cell apoptosis (25,28,29).
The intracellular ROS homeostasis depends on the
dynamic balance between normal cellular aerobic metabolism and the
antioxidant defense system. The antioxidant system includes
enzymatic antioxidants (such as CAT, SOD, GSH-Px and GSH reductase)
and nonenzymatic antioxidants (such as bilirubin, GSH, and vitamins
C and E) (30–33). Oxidative stress arises when the
balance between antioxidant and pro-oxidant levels is disrupted
(34).
Previous studies have shown that EGCG, a crucial
component in green tea, exerts protective effects against oxidative
stress in HLE B-3 cells (19,20). Yao et al (19) and our previous study (20) indicated that EGCG could increase
the survival and reduce the apoptosis of HLECs by decreasing the
H2O2- or UVB-induced generation of ROS and
the loss of Δψm. Notably, the present study reached a similar
conclusion that is consistent with those of these previous
studies.
Katiyar et al (35) revealed that the intracellular
levels of H2O2 in normal human epidermal
keratinocytes cultured with EGCG for 24 h did not exhibit a
significant decrease compared with that in the control cells.
Similar results were observed in the present study. The
H2O2 levels in the EGCG group (7.61±1.33
mmol/l) were slightly lower than those in the control group
(7.69±1.20 mmol/l), but the difference was not statistically
significant. Yamamoto et al (36) and Cao et al (37) reported that EGCG reduced the
intracellular ROS levels in normal cells.
H2O2 and hydroxyl free radicals are the main
ROS within cells. In the present study, the hydroxyl free radical
levels in the EGCG group (704.61±65.30 mmol/l) were significantly
lower compared with those in the control group (879.56±61.36
mmol/l). EGCG has been shown to serve a dual role in promoting and
inhibiting the production of H2O2 (38). On the one hand, some studies
suggest EGCG is an antioxidant. For example, EGCG can inhibit
ultraviolet radiation-induced oxidative stress in skin (39,40). On the other hand, other reports
claim that EGCG exerts pro-oxidant actions. EGCG automatically
oxidizes and produces H2O2 in cell culture
media with and without cells (38). At an appropriate concentration,
EGCG can stimulate cells to produce a low concentration of
intracellular ROS, which stimulates the activation of various
signaling pathways to promote cellular protective mechanisms
(40). It was hypothesized that
this may be the reason why EGCG cannot significantly downregulate
the level of H2O2 in normal cells as it can
hydroxyl free radicals; however, the exact mechanism requires
further study.
GSH is known to protect cells from the toxic effects
of lipid peroxidation. Furthermore, GSH is essential to maintain
cellular redox status and its consumption is considered to be an
indicator of oxidative stress (41). GSH-Px can promote the breakdown of
H2O2 and reduce toxic peroxides to nontoxic
hydroxyl compounds, thereby protecting the structure and function
of the cellular membrane from oxide damage. The activity of GSH-Px
is a significant marker of the antioxidant capacity of cells. CAT
defends against free radicals, and is responsible for the catalytic
decomposition of H2O2 into water and
molecular oxygen (42). SOD can
efficaciously scavenge oxygen radicals, protect cells from
oxidative damage and eliminate the oxidative stress caused by
superoxide anion (43). In
addition, SOD stops the free radical chain reaction by transforming
superoxide radicals into H2O2.
H2O2 and reducing GSH are decomposed into
water and oxygen by CAT or GSH reductase (44).
Previous studies have suggested that UVB irradiation
can increase the concentration of H2O2 and
hydroxyl free radicals. UVB irradiation has also been reported to
inhibit the activities of CAT, SOD and GSH-Px, and reduce the
concentration of GSH, thus causing oxidative damage to cells
(24,25,45). Previous studies have revealed that
EGCG can protect against light (12,13), and that it can also reduce the
oxidative damage caused by UVB irradiation by increasing CAT, SOD,
GSH-Px and GSH activities (10,46). In the present study, pretreatment
with 50 µM EGCG exerted a protective effect on HLECs against UVB
irradiation by reducing the production of
H2O2 and hydroxyl free radicals,
significantly promoting the activities of CAT, SOD and GSH-Px, but
not significantly increasing the levels of GSH. Notably, CAT and
SOD activities, and GSH levels were markedly decreased following
treatment with UVB irradiation (GSH-Px activity was not
significantly decreased), whereas the activity of antioxidant
enzymes (such as CAT, SOD and GSH-Px) was enhanced by EGCG
pretreatment, which can remove free radicals in a highly efficient
manner.
Apoptosis serves an important role in maintaining
homeostasis. Changes in mitochondrial structure and function are
associated with cell apoptosis, and these changes are mainly
manifested by the release of proapoptotic factors, abundant
production of ROS and an imbalance in intracytoplasmic calcium
levels (47–49). UVB irradiation-induced cell
apoptosis can be mediated by the mitochondria-initiated apoptotic
pathway (50). In the present
study, Annexin V-FITC/PI staining confirmed that UVB irradiation
induced HLECs apoptosis and that EGCG reduced apoptosis. The
specific mechanism underlying the ability of EGCG to reduce
apoptosis through the mitochondrial pathway requires further study.
It has been reported that mitophagy is critical for maintaining
mitochondrial quality, energy metabolism and organ function
(51). Mitochondrial quality and
mitochondrial mitophagy may also be the possible mechanisms by
which EGCG reduces UVB damage to HLECs. The present study did not
perform the relevant investigations to explore the effect of EGCG
on mitochondrial autophagy and mitochondrial quality; therefore, we
aim to further study the possible mechanism underlying the effects
of EGCG on apoptosis from the aspects of mitochondrial autophagy
and mitochondrial quality in future studies.
The levels of cytochrome c in the cytoplasm
are a sign of mitochondrial damage, which contributes to cell
apoptosis. EGCG has been reported to inhibit the release of
cytochrome c from the mitochondria into the cytoplasm, and
to reduce mitochondria-mediated apoptosis (52). Cytochrome c is released
from the mitochondria into the cytoplasm and binds to Apaf-1 and
pro-caspase-9 to form the apoptotic body, where caspase-9 is
activated. Active caspase-9 is further cleaved and activates
effector caspases, such as caspases-3 and −7, which execute the
death program. Caspase activation is the main initiator of
apoptosis. Zhang et al (53) reported that with the occurrence of
apoptosis, the expression levels of the active form of caspase-3
were increased and caspase-3 activity simultaneously enhanced. EGCG
can reduce the increased caspase-3 activity induced by oxidative
damage and inhibit cell apoptosis (54). The present study demonstrated that
EGCG pretreatment efficiently protected HLECs against UVB-induced
cell apoptosis by inhibiting the mRNA and protein expression levels
of cytochrome c, caspase-9 and caspase-3, suggesting that
EGCG may block the mitochondria-dependent cell death pathway.
However, the present study only analyzed the expression of
caspase-3, which significantly increased following UVB irradiation.
By contrast, following EGCG intervention, a decrease in caspase-3
expression observed. It is hypothesized that the decrease in
caspase-3 may be related to the change in the expression of active
caspase-3. Active caspase-3 (cleaved caspase-3) could stimulate the
apoptotic cascade, and active caspase-3 may reflect the apoptotic
status of target cells (55). In
the present study, only the caspase-3 protein were measured;
therefore, the lack of detection of active caspase-3 expression in
the present study is a limitation and the effect of EGCG on cleaved
caspase-3 (active caspase-3) requires further investigation.
Bcl-2 family members, such as Bax and Bcl-2, are
crucial regulators of various apoptotic pathways. Bax serves a
central role in the execution of mitochondrial apoptosis and an
increase in Bax expression affects mitochondrial membrane
permeability; this change leads to the release of cytochrome
c, which further activates caspases. By contrast,
anti-apoptotic proteins, such as Bcl-2, are known to downregulate
Bax expression, suppressing cytochrome c release and
inhibiting cell apoptosis through the mitochondrial pathway.
Changes in the ratio of anti-apoptotic/pro-apoptotic Bcl-2 family
proteins are essential for determining whether apoptosis is
executed (56). In the present
study, HLECs treated with UVB irradiation exhibited downregulated
expression of Bcl-2, upregulated expression of Bax, and a decreased
Bcl-2/Bax ratio, which are closely associated with HLECs apoptosis.
However, pretreatment with EGCG effectively reversed the expression
levels of Bax and Bcl-2, and increased the Bcl-2/Bax ratio. This
result is identical to the results of previous investigations,
which revealed that UV irradiation could induce apoptosis of lens
epithelial cells by regulating Bax and Bcl-2 expression (57,58) and that EGCG could suppress
downregulation of the Bcl-2/Bax ratio induced by oxidative stress
(59). These observations
indicated that Bcl-2 family proteins may have an important role in
regulating HLECs apoptosis under UVB irradiation and that EGCG may
be able to prevent UVB irradiation-induced HLECs apoptosis by
regulating the expression levels of Bax and Bcl-2.
EGCG has been suggested to protect HLECs from
H2O2-induced apoptosis by regulating the
caspase, MAPK and Akt pathways (19). EGCG may protect against high
glucose-induced HLECs apoptosis by regulating the gene expression
levels of the Bcl-2 family, c-fos, c-myc and p53 (60). EGCG may also block the adverse
effects of UVB radiation by modulating the JNK1/c-Jun pathway in
ARPE19 cells (37). In addition,
it has been reported that EGCG can reduce UVB-induced photodamage
and apoptosis by inhibiting the expression levels of p53, p21 and
c-fos genes in HaCaT cells (61).
In our previous study (20), it
was demonstrated that EGCG reduced UVB-induced apoptosis through
AIF/Endo G signaling pathways. The present study further
supplemented the findings of our previous study. Based on these
results, it was suggested that EGCG could notably attenuate
UVB-induced apoptosis through the caspase-dependent and
caspase-independent mitochondrial apoptosis pathways. As shown in
Fig. 9, it was inferred that EGCG
may scavenge free radicals by enhancing the activities of CAT, SOD
and GSH-Px, and increasing GSH levels, thus increasing the survival
rate of HLECs. EGCG also efficiently prevented UVB
irradiation-induced HLECs apoptosis by modulating the expression of
Bcl-2/Bax, cytochrome c, caspases and AIF/Endo G (20), thereby playing a protective role
in HLECs survival under conditions of UVB irradiation.
In conclusion, the present study investigated the
protective effect of EGCG on HLECs under conditions of UVB
irradiation in vitro. The results indicated that UVB
irradiation could induce HLECs apoptosis, which is driven by
oxidative stress via the mitochondrial signaling pathway. In
addition, EGCG scavenged free radicals, protected HLECs viability,
improved Δψm, and enhanced CAT, SOD and GSH-Px activities, but did
not significantly increase GSH levels. EGCG may also efficiently
prevent UVB irradiation-induced HLECs apoptosis by modulating
Bcl-2, Bax, cytochrome c, caspase-9 and caspase-3
expression, indicating its protective role and potential
application in preventing cataract in clinical practice.
Acknowledgements
The authors would like to thank Dr Wenjun Jiang
(Shandong Provincial Key Laboratory of Integrated Traditional
Chinese, Shandong, China) for kindly providing guidance in the
experiment and statistical analysis.
Funding
This work was supported by the Natural Science Foundation of
Shandong province (grant nos. ZR2017PH002 and ZR2014HL048), the
National Key Research and Development Project (grant nos.
2019YFC1710200 and 2019YFC1710203) and the National Natural Science
Foundation of China (grant no. 82104937).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW wrote the main manuscript. QW, DG, JS, YG, YZ, JG
and XZ carried out the experiments and statistical analysis, and
generated all figures. DG, DL and HB jointly participated in the
research design. DG and HB are the corresponding authors. HB, DG
and QW confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kang LH, Zhang GW, Zhang JF, Qin B and
Guan HJ: Ganoderic acid A protects lens epithelial cells from UVB
irradiation and delays lens opacity. Chin J Nat Med. 18:934–940.
2020.PubMed/NCBI
|
|
2
|
Rong X, Rao J, Li D, Jing Q, Lu Y and Ji
Y: TRIM69 inhibits cataractogenesis by negatively regulating p53.
Redox Biol. 22:1011572019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung I, Hah YS, Ju S, Kim JH, Yoo WS, Cho
HY, Yoo JM, Seo SW, Choi WS and Kim SJ: Ultraviolet B radiation
stimulates the interaction between nuclear factor of activated T
cells 5 (NFAT5) and nuclear factor-kappa B (NF-κB) in human lens
epithelial cells. Curr Eye Res. 42:987–994. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cencer CS, Chintala SK, Townsend TJ,
Feldmann DP, Awrow MA, Putris NA, Geno ME, Donovan MG and Giblin
FJ: PARP-1/PAR activity in cultured human lens epithelial cells
exposed to two levels of UVB light. Photochem Photobiol.
94:126–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piao MJ, Kang KA, Zhen AX, Kang HK, Koh
YS, Kim BS and Hyun JW: Horse oil mitigates oxidative damage to
human HaCaT keratinocytes caused by ultraviolet B irradiation. Int
J Mol Sci. 20:14902019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu X, Liang Y, Zhao B and Wang Y:
Oxyresveratrol protects human lens epithelial cells against
hydrogen peroxide-induced oxidative stress and apoptosis by
activation of Akt/HO-1 pathway. J Pharmacol Sci. 139:166–173. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perdices L, Fuentes-Broto L, Segura F,
Cavero A, Orduna-Hospital E, Insa-Sánchez G, Sánchez-Cano AI,
Fernández-Sánchez L, Cuenca N and Pinilla I: Systemic
epigallocatechin gallate protects against retinal degeneration and
hepatic oxidative stress in the P23H-1 rat. Neural Regen Res.
17:625–631. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Unno K and Nakamura Y: Green tea
suppresses brain aging. Molecules. 26:48972021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu FW, Lv YL, Zhong YF, Xue YN, Wang Y,
Zhang LY, Hu X and Tan WQ: Beneficial effects of green tea EGCG on
skin wound healing: A comprehensive review. Molecules. 26:61232021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Faheem NM and Ali TM: The counteracting
effects of (−)-epigallocatechin-3-gallate on the immobilization
stress-induced adverse reactions in rat pancreas. Cell Stress
Chaperones. 26:159–172. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh BN, Shankar S and Srivastava RK:
Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim E, Hwang K, Lee J, Han SY, Kim EM,
Park J and Cho JY: Skin protective effect of epigallocatechin
gallate. Int J Mol Sci. 19:1732018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen MH, Tsai CF, Hsu YW and Lu FJ:
Epigallocatechin gallate eye drops protect against ultraviolet
B-induced corneal oxidative damage in mice. Mol Vis. 20:153–162.
2014.PubMed/NCBI
|
|
14
|
Kitagawa K and Niikura Y:
Caspase-independent mitotic death (CIMD). Cell Cycle. 7:1001–1005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kiraz Y, Adan A, Kartal Yandim M and Baran
Y: Major apoptotic mechanisms and genes involved in apoptosis.
Tumour Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou YF, Guo B, Ye MJ, Liao RF and Li SL:
Protective effect of rutin against H2O2-induced oxidative stress
and apoptosis in human lens epithelial cells. Curr Eye Res.
41:933–942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JS, Cho IA, Kang KR, Lim H, Kim TH, Yu
SK, Kim HJ, Lee SA, Moon SM, Chun HS, et al: Reversine induces
caspase-dependent apoptosis of human osteosarcoma cells through
extrinsic and intrinsic apoptotic signaling pathways. Genes
Genomics. 41:657–665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi S, Guo L, Yan S, Lee RJ, Yu S and Chen
S: Hypocrellin A-based photodynamic action induces apoptosis in
A549 cells through ROS-mediated mitochondrial signaling pathway.
Acta Pharm Sin B. 9:279–293. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao K, Ye P, Zhang L, Tan J, Tang X and
Zhang Y: Epigallocatechin gallate protects against oxidative
stress-induced mitochondria-dependent apoptosis in human lens
epithelial cells. Mol Vis. 14:217–223. 2008.PubMed/NCBI
|
|
20
|
Wu Q, Li Z, Lu X, Song J, Wang H, Liu D,
Guo D and Bi H: Epigallocatechin gallate protects the human lens
epithelial cell survival against UVB irradiation through AIF/endo G
signalling pathways in vitro. Cutan Ocul Toxicol. 40:187–197. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao J, Liu Y, Wang X, Shen Y, Yuan S, Wan
Y and Jiang Q: UVB radiation induces human lens epithelial cell
migration via NADPH oxidase-mediated generation of reactive oxygen
species and up-regulation of matrix metalloproteinases. Int J Mol
Med. 24:153–159. 2009.PubMed/NCBI
|
|
22
|
Heo J, Lee BR and Koh JW: Protective
effects of epigallocatechin gallate after UV irradiation of
cultured human lens epithelial cells. Korean J Ophthalmol.
22:183–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Q, Guo D, Bi H, Wang D and Du Y: UVB
irradiation-induced dysregulation of plasma membrane calcium
ATPase1 and intracellular calcium homeostasis in human lens
epithelial cells. Mol Cell Biochem. 382:263–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oliveira MM, Ratti BA, Daré RG, Silva SO,
Truiti MD, Ueda-Nakamura T, Auzély-Velty R and Nakamura CV:
Dihydrocaffeic acid prevents UVB-induced oxidative stress leading
to the inhibition of apoptosis and MMP-1 expression via p38
signaling pathway. Oxid Med Cell Longev. 2019:24190962019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pustisek N and Situm M: UV-radiation,
apoptosis and skin. Coll Antropol. 35 (Suppl 2):S339–S341.
2011.PubMed/NCBI
|
|
27
|
Cadet J and Wagner JR: DNA base damage by
reactive oxygen species, oxidizing agents, and UV radiation. Cold
Spring Harb Perspect Biol. 5:a0125592013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin Y, Meng F, Sui C, Jiang Y and Zhang L:
Arsenic enhances cell death and DNA damage induced by ultraviolet B
exposure in mouse epidermal cells through the production of
reactive oxygen species. Clin Exp Dermatol. 44:512–519. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang D, Lu C, Yu Z, Wang X, Yan L, Zhang
J, Li H, Wang J and Wen A: Echinacoside alleviates UVB
irradiation-mediated skin damage via inhibition of oxidative
stress, DNA damage, and apoptosis. Oxid Med Cell Longev.
2017:68514642017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rashikh A, Ahmad SJ, Pillai KK, Kohli K
and Najmi AK: Aliskiren attenuates myocardial apoptosis and
oxidative stress in chronic murine model of cardiomyopathy. Biomed
Pharmacother. 66:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heruye S, Maffofou N LN, Singh NU, Munt D,
Njie-Mbye YF, Ohia SE and Opere CA: Standardization of a new method
for assessing the development of cataract in cultured bovine
lenses. J Pharmacol Toxicol Methods. 98:1065922019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He L, He T, Farrar S, Ji L, Liu T and Ma
X: Antioxidants maintain cellular redox homeostasis by elimination
of reactive oxygen species. Cell Physiol Biochem. 44:532–553. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gopi N, Vijayakumar S, Thaya R,
Govindarajan M, Alharbi NS, Kadaikunnan S, Khaled JM, Al-Anbr MN
and Vaseeharan B: Chronic exposure of oreochromis niloticus to
sub-lethal copper concentrations: Effects on growth, antioxidant,
non-enzymatic antioxidant, oxidative stress and non-specific immune
responses. J Trace Elem Med Biol. 55:170–179. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gokul S, Patil VS, Jailkhani R, Hallikeri
K and Kattappagari KK: Oxidant-antioxidant status in blood and
tumor tissue of oral squamous cell carcinoma patients. Oral Dis.
16:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katiyar SK, Afaq F, Azizuddin K and
Mukhtar H: Inhibition of UVB-induced oxidative stress-mediated
phosphorylation of mitogen-activated protein kinase signaling
pathways in cultured human epidermal keratinocytes by green tea
polyphenol (−)-epigallocatechin-3-gallate. Toxicol Appl Pharmacol.
176:110–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamamoto T, Hsu S, Lewis J, Wataha J,
Dickinson D, Singh B, Bollag WB, Lockwood P, Ueta E, Osaki T and
Schuster G: Green tea polyphenol causes differential oxidative
environments in tumor versus normal epithelial cells. J Pharmacol
Exp Ther. 307:230–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao G, Chen M, Song Q, Liu Y, Xie L, Han
Y, Liu Z, Ji Y and Jiang Q: EGCG protects against UVB-induced
apoptosis via oxidative stress and the JNK1/c-Jun pathway in ARPE19
cells. Mol Med Rep. 5:54–59. 2012.PubMed/NCBI
|
|
38
|
Kim HS, Quon MJ and Kim JA: New insights
into the mechanisms of polyphenols beyond antioxidant properties;
lessons from the green tea polyphenol, epigallocatechin 3-gallate.
Redox Biol. 2:187–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katiyar SK, Afaq F, Perez A and Mukhtar H:
Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of
human skin inhibits ultraviolet radiation-induced oxidative stress.
Carcinogenesis. 22:287–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elbling L, Herbacek I, Weiss RM,
Jantschitsch C, Micksche M, Gerner C, Pangratz H, Grusch M,
Knasmüller S and Berger W: Hydrogen peroxide mediates EGCG-induced
antioxidant protection in human keratinocytes. Free Radic Biol Med.
49:1444–1452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mary Momo CM, Ferdinand N, Omer Bebe NK,
Alexane Marquise MN, Augustave K, Bertin Narcisse V, Herve T and
Joseph T: Oxidative effects of potassium dichromate on biochemical,
hematological characteristics, and hormonal levels in rabbit doe
(oryctolagus cuniculus). Vet Sci. 6:302019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ibrahim IA, Abdulla MA, Hajrezaie M, Bader
A, Shahzad N, Al-Ghamdi SS, Gushash AS and Hasanpourghadi M: The
gastroprotective effects of hydroalcoholic extract of Monolluma
quadrangula against ethanol-induced gastric mucosal injuries in
sprague dawley rats. Drug Des Dev Ther. 10:93–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bhattacharyya A, Chattopadhyay R, Mitra S
and Crowe SE: Oxidative stress: An essential factor in the
pathogenesis of gastrointestinal mucosal diseases. Physiol Rev.
92:329–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kumar J, Khan S, Mandotra SK, Dhar P,
Tayade AB, Verma S, Toppo K, Arora R, Upreti DK and Chaurasia OP:
Nutraceutical profile and evidence of alleviation of oxidative
stress by spirogyra porticalis (Muell.) cleve inhabiting the high
altitude trans-himalayan region. Sci Rep. 9:40912019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Santhakumaran I, Kesavan SS and Arumugam
G: Asperyellone pretreatment protects HaCaT cells from UVB
irradiation induced oxidative damages: Assessment under in vitro
and in vivo conditions and at molecular level. J Cell Biochem.
120:10715–10725. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Filip A, Daicoviciu D, Clichici S, Mocan
T, Muresan A and Postescu ID: Photoprotective effects of two
natural products on ultraviolet B-induced oxidative stress and
apoptosis in SKH-1 mouse skin. J Med Food. 14:761–766. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim SH and Kim H: Inhibitory effect of
astaxanthin on oxidative stress-induced mitochondrial dysfunction-A
mini-review. Nutrients. 10:11372018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cosentino K and García-Sáez AJ:
Mitochondrial alterations in apoptosis. Chem Phys Lipids.
181:62–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jing L, Kumari S, Mendelev N and Li PA:
Coenzyme q10 ameliorates ultraviolet B irradiation induced cell
death through inhibition of mitochondrial intrinsic cell death
pathway. Int J Mol Sci. 12:8302–8315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Onishi M, Yamano K, Sato M, Matsuda N and
Okamoto K: Molecular mechanisms and physiological functions of
mitophagy. EMBO J. 40:e1047052021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Adikesavan G, Vinayagam MM, Abdulrahman LA
and Chinnasamy T: (−)-Epigallocatechin-gallate (EGCG) stabilize the
mitochondrial enzymes and inhibits the apoptosis in cigarette
smoke-induced myocardial dysfunction in rats. Mol Biol Rep.
40:6533–6545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang W, Zhao L, Liu J, Du J, Wang Z, Ruan
C and Dai K: Cisplatin induces platelet apoptosis through the ERK
signaling pathway. Thromb Res. 130:81–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou X, Liang L, Zhao Y and Zhang H:
Epigallocatechin-3-gallate ameliorates angiotensin II-induced
oxidative stress and apoptosis in human umbilical vein endothelial
cells through the activation of Nrf2/caspase-3 signaling. J Vasc
Res. 54:299–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Estrov Z, Thall PF, Talpaz M, Estey EH,
Kantarjian HM, Andreeff M, Harris D, Van Q, Walterscheid M and
Kornblau SM: Caspase 2 and caspase 3 protein levels as predictors
of survival in acute myelogenous leukemia. Blood. 92:3090–3097.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lv J and Xing Y: Effects of UV on
apoptotic factors in lens epithelial cells of an animal model. Exp
Ther Med. 16:2309–2312. 2018.PubMed/NCBI
|
|
58
|
Qiu X, Rong X, Yang J and Lu Y: Evaluation
of the antioxidant effects of different histone deacetylase
inhibitors (HDACis) on human lens epithelial cells (HLECs) after
UVB exposure. BMC Ophthalmol. 19:422019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu S, Sun Z, Chu P, Li H, Ahsan A, Zhou
Z, Zhang Z, Sun B, Wu J, Xi Y, et al: EGCG protects against
homocysteine-induced human umbilical vein endothelial cells
apoptosis by modulating mitochondrial-dependent apoptotic signaling
and PI3K/Akt/eNOS signaling pathways. Apoptosis. 22:672–680. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ye P, Lin K, Li Z, Liu J, Yao K and Xu W:
(−)-Epigallocatechin gallate regulates expression of apoptotic
genes and protects cultured human lens epithelial cells under
hyperglycemia. Mol Biol (Mosk). 47:251–257. 2013.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Luo D, Min W, Lin XF, Wu D, Xu Y and Miao
X: Effect of epigallocatechingallate on ultraviolet B-induced
photo-damage in keratinocyte cell line. Am J Chin Med. 34:911–922.
2006. View Article : Google Scholar : PubMed/NCBI
|























